Abstract
Background
Reports from studies of twins, disease aggregation in families, animal models for periodontal disease, and various genetic-analysis studies have determined that genetics plays a role in the susceptibility to periodontal disease. The purpose of this pilot study was to evaluate the effect of genetics on periodontal disease by evaluating the heritability of alveolar bone loss in a captive baboon population.
Methods
A collection of baboon skulls from a pedigreed colony (for which scientists and veterinarians maintain complete genealogical and veterinary records) were obtained from the Southwest National Primate Research Center and used in this pilot study. Measurements of alveolar bone loss were performed on 390 dry baboon skulls. A periodontal probe was used to measure alveolar bone loss. Maximum likelihood methods (designed to handle complex genealogies) were used to determine the heritability of alveolar bone loss. This software utilized known pedigrees in the captive baboon sample and tested the relationship between pairwise kinship and alveolar bone loss data to determine the heritability of alveolar bone loss from periodontal disease.
Results
Genetic data were available for 347 of the 390 specimens. Using age and sex as covariates, genetic analysis indicated a heritability of 35% (standard error=20%, p=0.01). While sex was not a significant factor in periodontal disease (p=0.96), age was highly significantly associated with periodontal disease (p<0.0001).
Conclusion
In this pilot study, analysis of alveolar bone loss measurements from captive baboons indicates that bone loss increases with age and that a portion of periodontal disease risk may be due to genetic variance. These findings provide evidence that periodontal disease is heritable in captive baboons and indicate that a larger, more-detailed study is warranted.
Keywords: periodontitis, alveolar bone, genetics, baboons
Introduction
Chronic periodontal disease has been reported to affect 48% of adults in the United States.1 Periodontal disease is primarily caused by microorganisms in the bacterial plaque, which accumulates on tooth surfaces. Bacteria and their byproducts cause a host immune response - resulting in inflammation of tooth supporting structures with resultant attachment and bone loss. Many different factors (including microbial, environmental, behavioral, and systemic) may play roles in determining the risk for periodontal disease.2 Variation in the host response to bacterial challenge explains why some people develop disease and others do not. In a study of the natural history of periodontal disease in humans, it was found that disease susceptibility in Sri Lankan tea workers with poor oral hygiene and little or no access to dental care was different from that of other groups.3 Disease variation was thought to be due either to an unrecognized environmental factor or to genetic susceptibility to periodontal disease.
Genetic variants have been shown to play a significant role in the susceptibility to periodontal disease. There are many reports in the literature on familial aggregation of early-onset forms of periodontitis which are now referred to as aggressive periodontitis. The current theory on the genetics of aggressive periodontitis is that prepubertal periodontitis, localized aggressive periodontitis, and generalized aggressive periodontitis are most likely due to a major gene locus transmitted in an autosomal manner.4 Twin studies have been valuable in studying genetics in periodontal disease. A study of 4908 twin pairs concluded that heritable factors were important in the reported periodontal health.5 Estimates were also made of the genetic and environmental variances and heritabilities for gingivitis and chronic periodontitis in 117 adult twin pairs.6 Although there was no evidence for heritability for gingivitis, chronic periodontitis was estimated to have approximately 50% heritability. Many studies have evaluated associations of genetic polymorphisms with periodontal disease, and it was reported that a specific genotype of the polymorphic IL-1 gene cluster was associated with the severity of periodontitis in non-smokers and distinguished individuals with severe periodontitis from those with mild disease.7 The additive effect of multiple genes may be a determinant of disease susceptibility in chronic periodontitis. The current models of complex diseases, such as chronic periodontitis, suggest that 5 to 10 different genes may be important determinants of susceptibility. These diseases may result from the combined effect of multiple functional genetic polymorphisms interacting with each other and with environmental factors.8
Over 30 years ago, baboons were investigated as a possible model for studying periodontal disease in humans and several observations were made.9 The periodontal disease presentation in baboons was strikingly similar, both histologically and clinically, to that in humans. The pattern of disease was similar with horizontal and vertical osseous defects, clinical pocket formation with severe gingival inflammation, and bilateral furcation involvement on the posterior teeth. Young and middle-aged baboons had generalized plaque and calculus accumulations. The animals of the population had been captive for various lengths of time and both captive and recently, wild-caught baboons had similar amounts of disease. Although baboons are large-bodied, terrestrial omnivores adapted to a wide range of environmental habitats from semi-desert to woodland and require large amounts of food, they have long lives (up to 30 years or more in captivity) and similar patterns of dental diseases to humans.9,10 In one study, periodontal examinations were performed on 116 baboons and significant increases with age were found in mean probing depths and mean attachment levels, with the conclusion being that the baboon may be a suitable model for studies of human periodontal disease.10
Baboons also provide a model for genetic studies because for common diseases they exhibit the same physiological characteristics as humans. A genetic similarity exists between baboons and humans that is evident at the level of overall DNA sequence, the sequences of specific genes, and the arrangement of genetic loci on chromosomes.11
The purpose of this pilot study was to test the hypothesis that genetic differences among individual baboons are partly responsible for individual differences in susceptibility to periodontal disease. This hypothesis was tested by means of a quantitative genetic analysis of alveolar bone loss in a captive baboon population and would determine if a more detailed study of a larger sample would be warranted.
Materials and Methods
The Southwest Foundation for Biomedical Research12 is the site of the Southwest National Primate Research Center and the world's largest colony of baboons for biomedical research. Approximately 1,200 living baboons are part of a pedigreed colony for which the researchers and veterinarians have maintained demographic, genealogical, and clinical veterinary records. This invaluable information allows researchers to conduct various types of genetic analyses, including estimation of heritability (proportion of variation attributable to genes) of phenotypes related to numerous common human diseases. The animals are housed in small social groups consisting of one breeding male, multiple breeding females, and their offspring. This results in the inclusion of large numbers of half-sibships in the pedigree, and this minimizes the genotype-environment covariance in the population. The pedigree is several generations deep, and this results in a wide range of degrees of relatedness among individuals within and among generations. After necropsy, baboon heads are collected and sent to Washington University School of Medicine where they are macerated and curated.
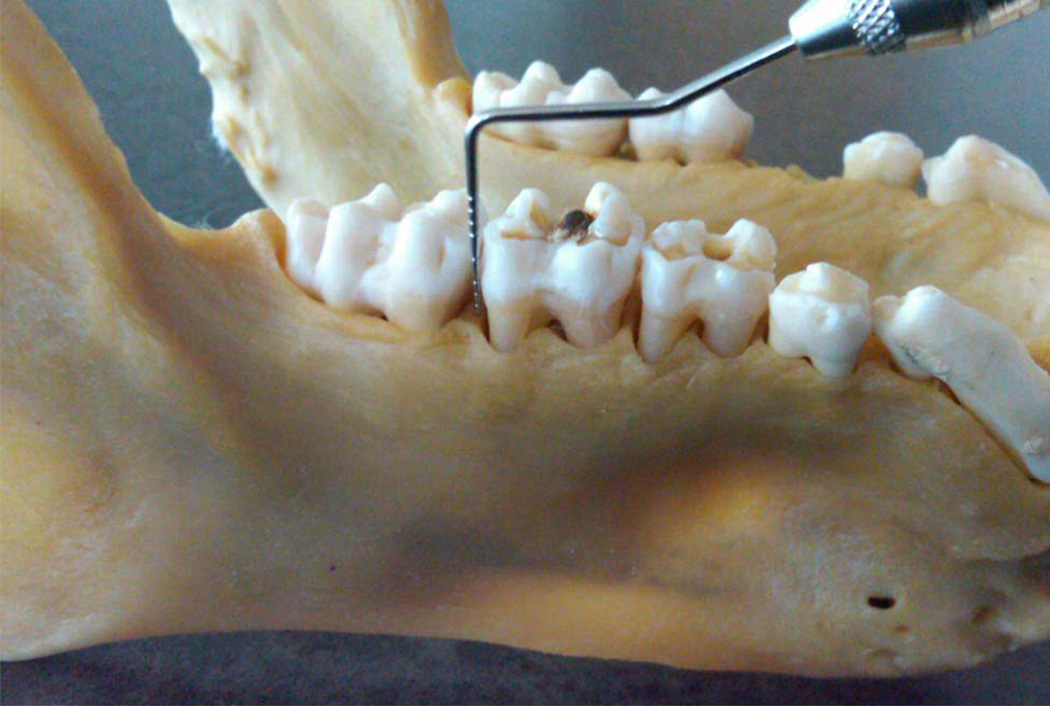
For this pilot study, the mandibular right first and second molars of 390 dry baboon skulls were chosen as the sites for estimating periodontal disease severity. A Williams periodontal probe was used to measure alveolar bone loss at the distobuccal regions of tooth numbers 30 and 31. The probe was held at an angle parallel to the long axis of the tooth against the buccal side of the interproximal contact and the distance from the cementoenamel junction (CEJ) to the alveolar crest was measured to the nearest millimeter. Interdental osseous defects were measured by orienting the probe against the buccal side of the interproximal contact to the centermost deepest extent of the defect (Figure 1). Osseous interdental defects were chosen because they were the most consistent among specimens while buccal alveolar bone loss was inconsistent due to thin facial bone and frequently, but not always, large facial dehiscences.
Figure 1. Measurement of interdental osseous defects.
Measurements were recorded by a person other than the single examiner who measured all specimens. Reliability was determined from repeat measurements of ten skulls. Measurement reliability was assessed with an analysis of variance [ANOVA] and a variance of components analysis, which were performed with statistical analysis software#.
For this study, the severity of clinical attachment loss was characterized in the same way as in humans: severe = 5 mm or greater; moderate = 3 to 4 mm; and slight = 1 to 2 mm. Because bone loss was evaluated on dry skulls devoid of soft tissue, 2 mm was added to each disease severity category to account for the biologic width. The biologic width is defined as the dimension of the junctional epithelium and connective tissue attachment which was determined to be approximately 2 mm.13 Severe periodontal disease was classified as alveolar bone crest (ABC) to CEJ distances of 7 mm and greater. Moderate periodontal disease was classified as ABC-CEJ distances of 5 to 6 mm. Slight periodontal disease was classified as ABC-CEJ distances of 3 to 4 mm. No disease was classified as ABC-CEJ distances of 1 to 2 mm.
Statistical Analysis
Each baboon was categorized according to the extent of disease and assigned a code from −2 to 1. Specimens with no disease were categorized as −2, while slight, moderate, and severe periodontal disease groups corresponded to groups −1, 0, and 1, respectively. For the genetic analysis, baboons with scores of −2 and −1 (ABC-CEJ distances of 1 to 4 mm) were combined as were baboons with scores of 0 and 1 (ABC-CEJ distances of 5 to ≥7 mm). Age distributions for the two periodontal disease groups were tested for normality using the Shapiro-Wilk W test, and means and 95% confidence intervals were calculated.
Quantitative Genetic Analysis
Quantitative genetic analysis of the categorical alveolar loss data was implemented using the SOLAR** (Sequential Oligogenic Linkage Analysis Routines) suite of computer programs.14 SOLAR uses maximum likelihood methods and a variance decomposition approach to handle complex genealogies. The program estimates additive genetic (σ2G) and environmental (σ2E) components of phenotypic variance (σ2P). The model underlying the maximum likelihood framework writes the phenotypic covariance matrix among individuals in a pedigree (Ω) as Ω = 2Θ σ2G + I σ2E, where Θ is the kinship matrix and I is an identity matrix of the same dimensions of Θ and Ω. Narrow-sense heritability (h2) was estimated as the proportion of σ2P accounted for by σ2G. The analysis of the categorical alveolar bone loss data used a liability threshold model that treats the discrete states of the alveolar bone loss data as being a function of an individual’s score on a latent continuous liability variable, with expression or non-expression of a given character state being the result of whether or not an individual’s score on the liability scale exceeds a threshold.15
The effects of potentially confounding covariates of sex, age, and a sex by age interaction were tested and controlled for when found to be significant at the p < 0.10 level. The significance of the effects of covariates was assessed through likelihood ratio tests comparing the estimated likelihoods of models that contained the effects of the covariates and those that did not. For this analysis, baboons were dichotomized into those with no or slight periodontal disease (scores of −2 or −1) and those with moderate or severe periodontal disease (scores of 0 or 1).
Results
Examinations were performed on 390 baboon skulls. The average age at death was 18.9 years. The age range was 2.9 to 33.7 years. The oldest male was 27.0 years and the oldest female was 33.7 years. Two teeth were measured in each specimen for a total of 780 teeth. The goal for reliability was to have greater than 90% of variance in the measurements to occur among baboon skull specimens. Greater than 99% of the variance was due to variance among animals, so measurements were repeatable.
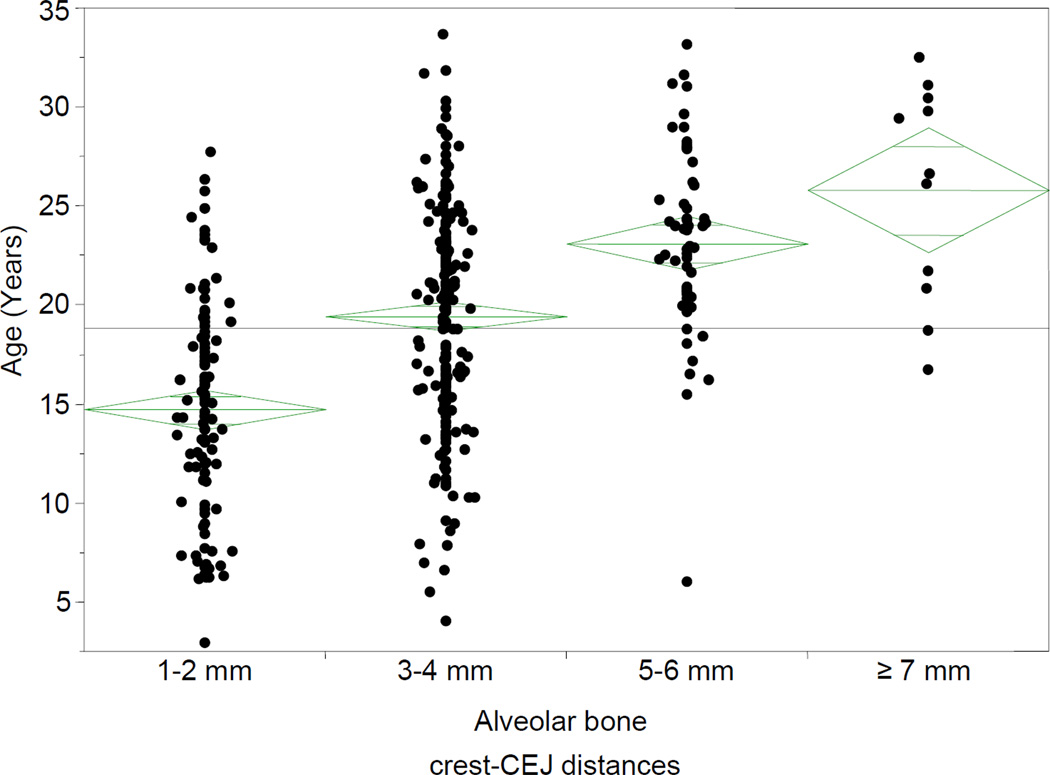
Females had slight to moderate periodontal disease with an average score of −0.5. Males had no disease to slight periodontal disease with an average score of −1.5. Age distributions were normal for the two periodontal disease groups (Shapiro-Wilk W test, p>0.05). Mean age for the no and slight disease groups was 214.3 months (95% confidence limits 206.54 to 222.05). The mean age of the moderate and severe periodontal disease groups was 282.8 months (95% confidence limits 269.1 to 296.4). (Figure 2) (Table 1)
Figure 2. Age distribution for the two groups.
The horizontal lines across the diamonds represent the group means, and the vertical spans of the diamonds represent 95% confidence intervals. The horizontal spans of the diamonds are proportional to the sample sizes of the groups.
Table 1.
Cross-tabulation of sex and alveolar bone crest – CEJ measurements.
| 1–2 mm ABC-CEJ |
3–4 mm ABC-CEJ |
5–6 mm ABC-CEJ |
≥7mm ABC-CEJ |
Total | |
|---|---|---|---|---|---|
| Female | 83 | 138 | 48 | 9 | 278 |
| Male | 24 | 74 | 12 | 2 | 112 |
| Total | 107 | 212 | 60 | 11 | 390 |
ABC = Alveolar Bone Crest
CEJ = Cementoenamel Junction
Three hundred and forty-seven of 390 specimens were part of the baboon pedigree and used for quantitative genetic analysis. Alveolar bone loss was significantly heritable (h2 = 0.35, std. error = 0.2, p = 0.012) indicating that individual differences in the extent of periodontal disease are heritable. Age had a significant effect on individual differences in the phenotypes (p = 0.002, Kullback-Leibner R2 = 0.15) while sex (p = 1.0) and the sex by age interaction (p = 0.62) did not have significant effects on alveolar bone loss. As a result, age was retained as a covariate in the final analysis. This means that in the final genetic analysis, the level of periodontal disease for individual animals was determined relative to animals of the same age.
Discussion
The captive baboon model is unique because of the high degree of control over the animals in a captive colony. The diet and environment of the baboons are tightly controlled. The baboons all eat the same food, do not take any medication, and live in the same type of enclosure. They perform no personal oral hygiene and are not exposed to cigarette smoke, alcohol, or other substances that may complicate similar studies in humans.
Consistent with previous findings,10 gender was not associated with periodontal disease in this baboon population. Females had slightly elevated levels of disease and were older compared with males, but gender was not significant after adjusting for age. The number of females and males in the baboon sample was not equal; there were 2.5 times the number of females as males because of the way that the breeding population is managed. In humans, gender differences are thought to be due to fewer preventive practices, poorer oral hygiene, and a less positive attitude toward oral health in males.16 In this baboon study, these factors were not present.
In our study, age was significantly associated with periodontal disease. In an earlier study of baboons from a large population, periodontal disease was a consistent finding in older animals and rare in young animals.9 The effect of age may simply be that older individuals have been exposed longer to destructive processes such as plaque-induced periodontitis and trauma, which have cumulative effects on attachment loss. Among humans, age has been found to be a determinant/background factor for periodontitis and not a risk factor.17 In humans, an odds ratio of 20.52 for periodontitis was reported in individuals with poor oral hygiene and an odds ratio of 1.24 for age; this indicates that age has a small effect compared with plaque control.18 As baboons do not have oral-hygiene practices, age may be a more significant risk factor in baboons as most aged baboons had periodontal disease in our sample.
The heritability estimate obtained in this study compares well with several other studies in humans, for which heritability estimates ranged from 14–50%. In a study of twins, it was determined that half of the variance in disease was due to genetics.6 Similar results were found in a Swedish twin study for which heritability was 33% in males and 39% in females, with non-shared environmental factors, such as smoking, accounting for the remaining variation.19 A major portion of antibody to periodontitis-associated bacteria is of the IgG2 subclass. The heritability of IgG2 levels was estimated to be 38% in a study of aggressive-periodontitis patients in 60 families.20 A recent study used quantitative measures of periodontitis in a sample of 373 aggressive-periodontitis patients and 237 periodontally-healthy subjects and reported that heritabilities ranged from 13.7% to 30.0% with the highest values obtained for the first molars.21
Although this study utilized a baboon model, it substantiates previous studies that have reported a significant genetic contribution to the pathogenesis of periodontal disease. Periodontal disease is a multifactorial disease influenced by the variation in multiple genes and their interactions with multiple environmental factors. Determining which genes are involved is no easy task, in that many genes with small effects are likely to be involved. One of the most useful and powerful methods that may identify suspect genes involves gene mapping. With an increase in sample size, it will be possible to map genes affecting periodontal disease in this population. Approximately 900 of the animals from the pedigreed part of the colony have been scored for a genome-wide panel of microsatellite marker loci. Once such variants have been discovered, researchers can learn much more about the origins of illnesses and about ways to prevent, diagnose, and treat those illnesses. Medical treatments could be customized, based on a patient's genetic make-up, to maximize effectiveness and minimize side effects. Genetic variants contributing to resistance to disease could be identified, leading to new therapies with widespread benefits.
This was a pilot study to determine whether or not a larger more comprehensive study would be warranted. A limitation of the study is that only two site measurements were used to estimate full-mouth bone loss. Although it has been demonstrated that linear measurements of alveolar bone loss of posterior teeth account for up to 90% of variation that occurs in full-mouth bone loss measures,22 bone loss patterns in the mandibular right molar area of baboons mandibles may be independent of bone loss patterns of the entire baboon dentition and inappropriate for assessments of periodontal-disease heritability. Detailed analyses of full–mouth, bone-loss measurements and assessments of computed tomography (CT) images are needed on a large number of baboon skulls (~900 skulls and CT images are archived at Washington University School of Medicine) to validate the results of our current study.
This pilot study found that approximately 35% of the variance in periodontal disease was heritable in a captive baboon population. Periodontal disease was highly associated with age, as nearly all older animals were affected with periodontitis. A more detailed study of a larger sample of skulls is warranted.
ACKNOWLEDGMENTS
This study used the collection of baboon skulls originally derived from animals at the Southwest National Primate Research Center and now maintained by Dr. James Cheverud at Washington University School of Medicine. Development of this archived collection of skulls was made possible by NSF grants 096881 (Dr. Rogers), BCS-0523305 and BCS-0725068 (Dr. Cheverud) as well as NIH-NCRR grants P51-RR013986, C06-RR013556, C06-RR015456 and C06-RR014578 to the Southwest Foundation for Biomedical Research.
Footnotes
CONFLICTS OF INTEREST
The authors report no financial relationships related to any products involved in this study.
JMP, SAS Inc, Cary, NC
Southwest Foundation for Biomedical Research, San Antonio, TX
REFERENCES
- 1.Albandar JM. Epidemiology and risk factors of periodontal diseases. Dent Clin North Am. 2005;49:517–532. doi: 10.1016/j.cden.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Page RC, Beck JD. Risk assessment for periodontal diseases. Int Dent J. 1997;47:61–87. doi: 10.1111/j.1875-595x.1997.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 3.Loe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate, and no loss of attachment in Sri Lankan laborers 14–46 years of age. J Clin Periodontol. 1986;13(5):431–435. doi: 10.1111/j.1600-051x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 4.Kinane DF, Shiba H, Hart TC. The genetic basis of periodontitis. Periodontology 2000. 2005;39:91–117. doi: 10.1111/j.1600-0757.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 5.Corey LA, Nance WE, Hofstede P, Schenkein HA. Self-reported periodontal disease in a Virginia twin population. J Periodontol. 1993;64:1205–1208. doi: 10.1902/jop.1993.64.12.1205. [DOI] [PubMed] [Google Scholar]
- 6.Michalowicz BS, Diehl SR, Gunsolley JC, et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000;71(11):1699–1707. doi: 10.1902/jop.2000.71.11.1699. [DOI] [PubMed] [Google Scholar]
- 7.Kornman KS, Crane A, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997 Jan;24(1):72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 8.Academy Report. Informational paper. Implications of genetic technology for the management of periodontal diseases. J Periodontol. 2005;76:850–857. doi: 10.1902/jop.2005.76.5.850. [DOI] [PubMed] [Google Scholar]
- 9.Avery BE, Simpson DM. The baboon as a model system for the study of periodontal disease; clinical and light microscopic observations. J Periodontol. 1973;44:675–686. doi: 10.1902/jop.1973.44.11.675. [DOI] [PubMed] [Google Scholar]
- 10.Miller DR, Aufdemorte TB, Fox WC, Waldrop TC, Mealey BL, Brunsvold MA. Periodontitis in the baboon: a potential model for human disease. J Periodontal Res. 1995 Nov;30(6):404–409. doi: 10.1111/j.1600-0765.1995.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 11.Rogers J, Hixson JE. Insights from model systems. Baboons as an animal model for genetic studies of common human disease. Am J Hum Genet. 1997;61:489–493. doi: 10.1086/515527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Southwest National Primate Research Center. [Accessed April 2, 2010]; Available at: http://sfbr.org. [Google Scholar]
- 13.Gargiulo AW. Dimensions and relations of the dentogingival junction in humans. J Periodontol. 1961;32:261–267. [Google Scholar]
- 14.Almasy L, Blangero J. Multipoint quantitative trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duggirala R, Williams JT, Williams-Blangero S, Blangero J. A variance component approach to dichotomous trait linkage analysis using a threshold model. Genet Epidemiol. 1997;14:987–992. doi: 10.1002/(SICI)1098-2272(1997)14:6<987::AID-GEPI71>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Periodontology: Position paper. Epidemiology of periodontal diseases. J Periodontol. 2005:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 17.Novak KF, Novak MJ. Risk Assessment. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza’s Clinical Periodontology. vol. 10. St. Louis: Saunder’s Elsevier; 2006. pp. 602–608. [Google Scholar]
- 18.Abdellatif HM, Burt BA. An epidemiological investigation into the relative importance of age and oral hygiene status as determinants of periodontitis. J Dent Res. 1987;66(1):13–18. doi: 10.1177/00220345870660010201. [DOI] [PubMed] [Google Scholar]
- 19.Mucci LA, Bjorkman L, Douglass CW, Pedersen NL. Environmental and heritable factors in the etiology of oral diseases-A population-based study of Swedish twins. J Dental Res. 2005;84(9):800–805. doi: 10.1177/154405910508400904. [DOI] [PubMed] [Google Scholar]
- 20.Diehl SR, Wu T, Burmeister JA, et al. Evidence of a substantial genetic basis for IgG2 levels in families with aggressive periodontitis. J Dent Res. 2003 Sep;82(9):708–712. doi: 10.1177/154405910308200910. [DOI] [PubMed] [Google Scholar]
- 21.Diehl SR, Wu T, Michalowicz BS, et al. Quantitative measures of aggressive periodontitis show substantial heritability and consistency with traditional diagnoses. J Periodontol. 2005;76:279–288. doi: 10.1902/jop.2005.76.2.279. [DOI] [PubMed] [Google Scholar]
- 22.Shrout MK, Hildebolt CF, Vannier MW, et al. Periodontal diease quantification. I. Optimal selection of teeth for periodontal bone loss surveys. J Periodontol. 1990;61:618–622. doi: 10.1902/jop.1990.61.10.618. [DOI] [PubMed] [Google Scholar]