Abstract
Red blood cells (RBC) storage facilitates the supply of RBC to meet the clinical demand for transfusion and to avoid wastage. However, RBC storage is associated with adverse changes in erythrocytes and their preservation medium. These changes are responsible for functional alterations and for the accumulation of potentially injurious bioreactive substances. They also may have clinically harmful effects especially in critically ill patients. The clinical consequences of storage lesions, however, remain a matter of persistent controversy. Multiple retrospective, observational, and single-center studies have reported heterogeneous and conflicting findings about the effect of blood storage duration on morbidity and/or mortality in trauma, cardiac surgery, and intensive care unit patients. Describing the details of this controversy, this review not only summarizes the current literature but also highlights the equipoise that currently exists with regard to the use of short versus current standard (extended) storage duration red cells in critically ill patients and supports the need for large, randomized, controlled trials evaluating the clinical impact of transfusing fresh (short duration of storage) versus older (extended duration of storage) red cells in critically ill patients.
Keywords: Age of red blood cells, Storage lesion, Critically ill patients, Outcome, Transfusion, Anemia, Trauma, Cardiac surgery, Mortality, Cytokines
Introduction
Anemia is common in critically ill patients: up to 90% of patients will be anemic by day 3 of their intensive care unit (ICU) stay [1,2]. Red blood cells (RBC) transfusion rates in critically ill patients are reported between 20% and 40% in ICU [2-4], with a mean of 2 to 5 RBC units transfused per patient [3,4]. Such anemia of critical illness has been associated with a poor prognosis even in the absence of ischemic heart disease [3,5,6]. This association supports the value of RBC transfusion in critically ill patients. Nonetheless, although potentially life-saving for individual patients, RBC transfusion also has been associated with an increased risk of morbidity and/or mortality in critically ill, surgical, and trauma populations [7,8]. In this setting, studies have increasingly focused on the possible deleterious role played by RBC storage duration (so-called age of red cells) [9-12]. In particular, they have raised concerns that prolonged RBC storage may lead to harm once such “older” red cells are transfused into ICU patients.
To avoid wasting RBC units and improve the provision of blood stock, standard practice worldwide consists of transfusing the oldest compatible and available RBC unit. In addition, RBC can be stored up to 42 days maximizing their availability and the likelihood that red cells older than 2 weeks will be transfused into critically ill patients. This RBC storage duration usually up to 42 days has been defined on the bases of 1) a percentage of RBC still present in the circulation 24 hours after transfusion higher than 75%, and 2) hemolysis <1% at the end of the storage period [13]. Despite improved preservation methods, “storage lesions” occur in such cells, because in a way that increases over time erythrocytes develop important biochemical and structural derangements that affect their function and possibly their safety [10,14].
Current practice and concerns with “age of red cells” for ICU patients
The mean age of blood transfused in ICU patients varies from 16 to 21 days and is very similar throughout the world [2,3]. A large range of adverse effects related to RBCs storage have been reported in critically ill patients when RBC stored for 2 to 4 weeks are transfused. These include increased mortality [15-22], nosocomial infections [18,22-27], multiple organ failure [18,28], renal failure [18,22] deep vein thrombosis [20], increase in ICU [15] and hospital length of stay (LOS) [29,30], and in mechanical ventilation duration [18]. Nonetheless, most clinical studies in this area have been observational in nature, retrospective in design, small in size, and subject to bias, leaving this issue unresolved for more than 20 years.
Given the above concerns, further research to determine the effect of storage lesion (age of red cells) on clinical outcome in critically ill patients seems important. If fresh blood is associated with a decrease of mortality in critically ill patients, changes in transfusion policies will be applied. Considering the cost and the potential consequences on blood supply of delivery of fresh blood instead old blood, pivotal trials are required to answer definitively whether age of blood impacts the mortality and or morbidity of critically ill patients.
This review will consider: 1) the nature of storage lesions and why critically ill patients may be especially susceptible to the adverse effects of prolonged RBC storage; 2) the experimental studies that have explored the impact of blood storage on tissue oxygenation parameters; 3) the clinical studies of the effect of prolonged storage in adult trauma, postcardiac surgery, or ICU patients; and 4) the need for randomized, controlled studies in this field.
RBC storage lesions
During storage, RBC and their preservative medium suffer metabolic, biochemical, and molecular changes commonly referred to as “storage lesion” (Figure 1; Table 1) [10,31]. Structural RBC changes include depletion of adenosine triphosphate (ATP) and of 2,3-diphosphoglycerate (2,3-DPG); membrane phospholipid vesiculation; protein oxidation; and lipid peroxidation of the cell membrane [10,31,32]. Over time, RBC shape changes with increased osmotic fragility and loss of deformability [31,33]. Decreased membrane flexibility may compromise the effect of RBC on microcirculatory flow and participates in increasing red cell-endothelial cell interaction, with activation of inflammatory pathways. Furthermore, bioreactive substances accumulate in storage medium. These include 1) lipids that prime recipient neutrophils and have been implicated in transfusion-related acute lung injury [34]; 2) cytokines; and 3) free iron from hemolysis [35].
Figure 1.
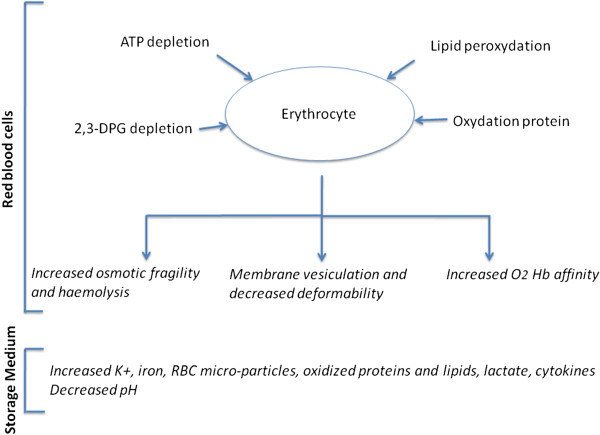
Changes occurring in red blood cells and storage medium over the storage time. ATP adenosine triphosphate; 2,3-DPG 2,3-diphosphoglycerate; RBC red blood cells.
Table 1.
Main biochemical changes in RBC storage medium and in loss of RBC deformability over the storage period [ [10,67]]
| Variables | Length of storage | |||
|---|---|---|---|---|
| |
Day 1 |
Days 7 |
Days 15 |
Day 42 |
| K (mmol/L) |
3.9 ± 0.6 |
13.6 ± 1.7 |
24.5 ± 2.1 |
46.6 ± 4.1 |
| pH |
6.8 ± 0.03 |
6.74 ± 0.03 |
6.64 ± 0.02 |
6.37 ± 0.04 |
| Lactate (mmol/L) |
3.6 ± 0.4 |
7.8 ± 0.7 |
17.2 ± 2.5 |
34.5 ± 4.4 |
| Iron (μmol/L) |
3.8 ± 0.9 |
6.8 ± 2.9 |
7.6 ± 1.6 |
14.2 ± 2.9 |
| Free hemoglobin (g/L) |
1.3 ± 0.5 |
1.5 ± 0.8 |
1.7 ± 0.5 |
3.0 ± 2.1 |
| Percentage of irreversible deformed RBCs | - | 8.4 ± 1.6 | 14.7 ± 2.6 | 29.9 ± 4.0 |
Recently, a study conducted in healthy volunteers reported the presence of higher extravascular hemolysis after older RBC transfusion (storage of 40–42 days) compared with fresh blood (storage of 3–7 days) illustrating the possible harmful effect of iron delivery [36]. This RBC “storage lesion” also alters oxygen delivery (because of the higher affinity of hemoglobin to oxygen secondary to a decrease in 2,3-DPG) and pH and, in addition, it increases cell lysis [10]. Free hemoglobin interacts with nitric oxide (NO) leading to endothelial dysfunction and contributing to intravascular thrombosis, vasoconstriction, and leukocyte adhesion [37]. Finally, white blood cells present in the transfused RBC can increase hemolysis and potassium release, liberate oxygen radical, and increase erythrocyte alterations [14,31].
ICU patients may be especially susceptible to transfusion of older RBC
The severity of illness of blood transfusion recipients may increase their susceptibility to the deleterious effects of RBC storage in several ways, making these concerns particularly relevant to critically ill patients. First, critically ill patients frequently have disease states that lead to impaired microcirculatory blood flow [38]. Second, their neutrophils may be primed by a trigger event (e.g., sepsis or trauma) and subsequent exposure to bioreactive substances of RBC unit may initiate enhanced activation of adherent leucocytes [39]. This hypothetical “two-hit” model was supported by a study that compared the transfusion of fresh or aged RBC into healthy rats with lipopolysaccharide pretreated rats. Transfusion of aged erythrocytes caused mild pulmonary inflammation but no coagulopathy in healthy rats, while it augmented lung injury by inducing coagulopathy in lipopolysaccharide pretreated rats. This difference was not found with the transfusion of fresh blood [39]. ICU patients are likely to undergo invasive mechanical ventilation (MV). Experimental data report an increase of transfusion related acute lung injury in mice receiving MV compared with those without MV. MV, particularly with injurious ventilator setting, induces lung injury and increase susceptibility of TRALI [40].
Finally, a “dose-effect” may exist and, because critically ill patients are especially susceptible to receiving multiple transfusions, this effect may increase their risk of developing adverse events [20,41]. In addition, receiving multiple transfusions will increase the risk that at least one of the RBC units transfused will be “old.” In a retrospective study, Weinberg et al. found an independent increase in the risk of death when trauma patients received 6 or more RBC units with at least one RBC stored for ≥14 days [16]. The same authors, in 1,647 trauma patients transfused within the first 24 hours postinjury, reported a higher independent risk of death only in patients who received 3 or more units stored for ≥14 days (RR = 1.57, 95% CI 1.14-2.15, p = 0.01) [41].
RBC storage and tissue oxygenation parameters
In critically ill patients, RBC transfusion is commonly used to restore or increase tissue oxygenation. Different surrogate markers of tissue oxygenation have been used to explore the relationship between duration of RBC storage and their efficacy in restoring optimal tissue oxygenation. In 1993, Marik et al. were the first to report a harmful effect of duration of RBC storage on systemic and tissue oxygenation. In 23 mechanically ventilated septic patients, they prospectively demonstrated an inverse association between change in gastric intramucosal pH (reflecting oxygen uptake) and age of blood [42]. Nonetheless, this was a post-hoc analysis and such a relationship was not confirmed in three subsequent studies [43-45]. One of these negative studies was a double-blind, randomized trial that compared the effect of RBC transfusion on tonometric indexes when RBC units were stored less than 5 days versus more than 20 days. This study did not show any difference between the study groups. Furthermore, there was no benefit from transfusing RBC whatever the age of blood possibly because of the defined transfusion target (80–90 g/L) or because the poor sensitivity of the tonometric technique [44]. More recently, Sakr et al. did not find any impact of storage time on sublingual microvascular perfusion in a prospective, single-center, observational study conducted in 35 patients with severe sepsis and septic shock [45]. In the setting of severe traumatic brain injury, the impact of RBC storage duration on cerebral oxygenation assessed by brain tissue oxygen pressure (PtiO2) was recently studied in an observational prospective study [46]. RBC stored for more than 19 days were unable to increase brain oxygenation, whereas fresh blood (<19 days) was effective [46]. In light of the contradictory findings of these five in vivo studies, it remains controversial whether storage lesions affect the ability of RBC to modulate tissue oxygenation.
RBC storage and clinical outcome - Review
We searched with the PubMed database studies comparing clinical outcomes of critically ill patients receiving fresh or old blood. Excluding non-English language reports and studies conducted in pediatric patients, we identified 32 studies that examined the clinical effect of blood storage in trauma patients, ICU patients, and patients undergoing cardiac surgery or those with acute heart disease [15-30,41,47-59]. Eighteen of these studies reported a deleterious effect of increasing duration of RBC storage on clinically relevant outcomes [15-30,41] (Table 2), whereas 14 of these studies did not demonstrate any effect of prolonged RBC storage (Table 3) [47-59].
Table 2.
Studies reporting a clinically harmful effect of prolonged RBC storage
| Authors | Year | Setting | N | Study design | Main confounders used for adjustment | Leukodepletion | Outcome and main results |
|---|---|---|---|---|---|---|---|
| Purdy et al.
[16] |
1997 |
ICU, severe sepsis or septic shock |
31 |
Retrospective single-center |
No |
No |
Median of RBC storage was lower in survivors (17 days) than in nonsurvivors (median 25 days) (p < 0.0001) |
| Zallen et al.
[28] |
1999 |
Trauma, ≥6 RBCs units in the first 12 hours post injury |
63 |
Retrospective single-center |
Patient age, serum lactate, base deficit |
No |
Mean age of RBC >14 days associated with MOF (OR 1.16, CI 95%, 1.01-1.34, p = 0.03) |
| Vamvakas et al.
[23] |
1999 |
Post-CABG |
416 |
Retrospective single-center |
Chronic systemic illness, CABG surgery type, IABP, intubation, impaired consciousness, patient age, bypass time, chest tube drainage, admission WBC count |
No |
Oldest blood was associated with a higher risk of pneumonia and/or wound infection compared with fresh blood (median of the mean age of the oldest and second oldest RBC units = 21.6 (range: 4–41) days vs. 13 (range: 2–39) days, p = 0.0002) |
| Offner et al.
[26] |
2002 |
Trauma, ≥ 6 RBC units in the first 12 hours postinjury |
61 |
Prospective single-center observational |
Patient age, ISS, gender, mechanism of injury |
No |
Risk of major infectious complications increased with the number of RBC units >14 days (OR = 1.13, 95% CI, 1.01-1.26, p = 0.03) |
| Keller et al.
[29] |
2002 |
Trauma with up to 4 RBC units in the first 48 hours post injury |
86 |
Retrospective single-center |
ISS, requirement for surgery, volume of RBC, patient age |
No |
Association between the number of RBC >14 days and hospital LOS |
| Leal-Noval et al.
[24] |
2003 |
Post-CABG or valve surgery |
585 |
Prospective single-center observational |
Re-intubation, central nervous system dysfunction, Apache II score, MV duration |
No |
Association between older RBC (>28 days) and the risk of pneumonia (OR = 1.06, 95% CI, 1.01-1.11, p = 0.018) No association with mortality |
| Murrell et al.
[30] |
2005 |
Trauma |
275 |
Retrospective single-center |
Patient age, ISS, leukodepletion volume of RBC |
95% |
Association between older blood and longer ICU and hospital LOS (RR = 1.15, 95% CI, 1.11-1.2) No association with mortality |
| Koch et al.
[18] |
2008 |
Post-CABG or valve surgery |
6002 |
Retrospective single-center |
Baseline characteristics |
Mixed |
Old blood >14 days was associated with mortality, MV duration, renal failure, infections and MOF |
| Weinberg et al.
[17] |
2008 |
Trauma, ≥ 1 RBC unit in the first 24 hours post injury |
1813 |
Retrospective single-center |
Patient age, gender, ISS, mechanism of injury, volume of RBC, hospital LOS |
Yes |
Transfusion ≥6 RBC units of RBC older ≥14 days was associated with higher mortality |
| Weinberg et al.
[22] |
2008 |
Trauma without RBC transfusion in the first 48 hours post injury |
430 |
Retrospective single-center |
Patient age, gender, ISS, presence of thoracic injury, MV, volume of RBC |
Yes |
RBC ≥14 days was associated with mortality (OR = 1.12, 95% CI: 1.02 to 1.23), renal failure (OR = 1.18, 95% CI, 1.07-1.29) and pneumonia (OR = 1.10, 95% CI, 1.04-1.17) Not with ARDS |
| Weinberg et al.
[41] |
2010 |
Trauma, ≥1 RBC unit for the first 24 hours |
1647 |
Retrospective single-center |
Patient age, gender, ISS, mechanism of injury, volume of RBC,FFP and platelets, presence of head injury |
Yes |
3 or more RBC ≥14 days increased risk of death (RR = 1.57, 95% CI, 1.14-2.15, p = 0.01) |
| Spinella et al.
[20] |
2009 |
Trauma, ≥5 RBC units |
202 |
Retrospective single-center |
Patient age, cryoprecipitate, Glasgow coma score, ISS |
Mixed |
Association between RBC >21 days and DVT occurrence Association between RBC >28 days and mortality (OR = 4, 95% CI, 1.34-11.61) |
| Vandrome et al.
[27] |
2009 |
Trauma |
487 |
Retrospective single-center |
Patient age, gender, ISS, mechanism of injury and MV time |
Yes |
Risk of pneumonia higher in patients transfused with RBC ≥14 days (RR = 1.42, 95% CI, 1.01-2.02) |
| Robinson et al.
[21] |
2010 |
Post-percutaneous coronary intervention |
909 |
Retrospective multi center |
Volume of RBC, procedures details, demographic characteristics |
NG |
Increased in age of the youngest RBC was associated with 30-day mortality (HR = 1.02, 95% CI, 1.18-1.34, p < 0.001) |
| Eikelboom et al.
[19] |
2010 |
Acute cardiovascular disease |
4933 |
Prospective single-center observational |
Demographic characteristics, comorbidities, clinical characteristics, patient ABO group |
Yes |
Hospital mortality higher when the oldest RBC >31 days compared with RBC <10 days (RR = 1.48, 95% CI, 1.07-2.05) |
| Andreasen et al.
[25] |
2011 |
Post-CABG or valve surgery |
1748 |
Retrospective multicenter |
Place of surgery, patient age gender, BMI, preoperative Hb, diabetes, reoperation due to bleeding, use of cardiopulmonary bypass, concomitant valve surgery, comorbidities, volume of RBC and platelets units, ABO blood group |
Mixed |
Higher risk of severe postoperative infections (OR = 2.5, 95% CI, 1.2-4.2) in patients with RBC exclusively ≥14 days |
| Pettila et al.
[15] |
2011 |
ICU |
757 |
Prospective multicenter observational |
Apache III score, leukodepletion status, pre-ICU transfusion, cardiac surgery, other transfused blood components, pretransfusion Hb preceding the first transfusion, centers |
80% |
Oldest RBC associated with longer LOS and higher mortality |
| Juffermans et al. [61] | 2012 | Trauma | 196 | Retrospective single-center | ISS, head trauma, surgery, use for SDD, volume of RBC and of platelets | Yes | Patients with infections received more old blood (>14 days) than patients without infections (8 RBC units (range: 2–16) versus 4 RBC units (range: 2–8), p = 0.02) |
RBC red blood cells; ICU intensive care unit; LOS length of stay; MV mechanical ventilation; DVT deep vein thrombosis; ARDS acute respiratory distress syndrome; CABG coronary artery bypass graft; MOF multi organ failure; IABP intra-aortic balloon pump; WBC White blood cells; ISS injury severity score; APACHE II score Acute Physiology and Chronic Health Evaluation II score; RR relative risk; BMI body mass index; Hb hemoglobin; FFP fresh frozen plasma; SDD selective digestive decontamination, NG not given.
Table 3.
Studies reporting no clinical effect of prolonged RBC storage
| Author | Year | Setting | N | Study design | Adjustment for confounders | Leukodepletion | Outcome and main results |
|---|---|---|---|---|---|---|---|
| Wasser et al.
[47] |
1989 |
Post- CABG |
237 |
Single-center randomized Cases: RBC<12 hours; controls: RBC stored for 2 to 5 days |
NA |
No |
No difference in bleeding and RBC transfusion requirement, nonetheless the platelets counts and thrombotest were significantly less altered in the study arm |
| Schulman et al.
[48] |
2002 |
Trauma, ≥2 RBC units |
17 |
Single-center randomized pilot study “Fresh group”: RBC<11 days; “Old group”: RBC >20 days |
NA |
Yes |
Mortality, infectious complications, respiratory failure |
| Vamvakas et al.
[49] |
2000 |
Post- CABG |
268 |
Retrospective single-center |
Gender, patient age, comorbidities, type of CABG, IABP, duration of anaesthesia, time on bypass, other surgery, repeated surgery, chest tube drainage volume |
No |
Post-operative ICU LOS, hospital LOS and MV duration |
| Gajic et al.
[50] |
2004 |
ICU patients with MV |
181 |
Retrospective single-center |
APACHE III score, Tidal volume, thrombocytopenia, massive transfusion |
70% |
Median storage duration of the oldest RBC unit = 20.3 days (range: 16–31) in absence of ALI versus 20.1 days (range: 16–27) in presence of ALI |
| Hebert et al.
[51] |
2005 |
ICU |
57 |
Double-blind multicenter, randomized pilot study |
Comorbidities, major diagnostic grouping, center |
Yes |
Composite outcome (mortality, nosocomial infections, thrombotic events, ischemic stroke) |
| Van de Watering et al.
[52] |
2006 |
Post- CABG RBC given during surgery and for 3 days post-surgery |
2732 |
Retrospective single-center study Cases: RBC <18 days Controls: standard cares |
Year of surgery, volume of transfusion, duration of surgery, previous CABG, number of distal anastomoses, patient age, gender, Hb at admission |
No |
30-day survival, hospital and ICU LOS |
| Taylor et al.
[53] |
2006 |
ICU |
449 |
Prospective single-center observational |
Patient age, survival probability |
Mixed |
Nosocomial infection, mortality, ICU and hospital LOS |
| Gajic et al.
[54] |
2007 |
ICU with ALI |
74 |
Prospective single-center case–control study |
Patients characteristic, transfusion factors |
NG |
Patients with ALI (median of average RBC storage = 22.9 days (range: 17–31) versus 22.9 days (range: 15–30) in controls (p = 0.801) |
| Yap et al.
[55] |
2008 |
Post-CABG and valve surgery, ≥2 RBC units |
670 |
Retrospective single-center |
Pre-operative risk profile, volume of RBC |
<5% |
Mortality, renal failure, nosocomial pneumonia, ICU LOS, MV duration |
| Van Buskirk et al.
[56] |
2010 |
ICU |
298 |
Retrospective single-center |
Volume of RBC, patient age, gender, severity at ICU admission, admission diagnosis |
NG |
Transfusion complications, change in SOFA score, ICU LOS, mortality |
| Katsios et al.
[57] |
2011 |
ICU |
126 |
Prospective single-center observational |
History of previous DVT, chronic dialysis, platelets transfusion, requirement of vasopressors |
No |
DVT |
| *Mckenny et al.
[58] |
2011 |
Post-cardiac surgery |
1153 |
Retrospective single-center |
Volume of RBC, baseline and patient characteristics |
Yes |
Early post-operative mortality, post-operative MV >72h, renal failure, infections, 30 day mortality, hospital mortality, prolonged MV, new renal failure, infectious complications and ICU LOS |
| Van Straten et al.
[59] |
2011 |
Post-CABG, ≤10 RBC units |
3475 |
Retrospective single-center |
Patient age, comorbidities, redo cardiac surgery, pre-operative Hb, emergency operation, perioperative MI, Re-exploration, year of operation, volume of RBC, FFP and platelets |
Yes |
Mortality |
| Kor et al. [62] | 2012 | ICU patients with MV | 100 | Double-blind randomized single-center Cases: one fresh RBC unit (<5 days) Control: one RBC unit of standard practices | NA | Yes | Change in PaO2/FiO2 ratio, in peak and plateau airway pressures, in markers of immune status and in coagulation |
NA not applicable; RBC red blood cell; ICU intensive care unit; LOS length of stay; MV mechanical ventilation; DVT deep vein thrombosis; ALI acute lung injury; CABG coronary artery bypass graft, APACHE III score Acute Physiology and Chronic Health Evaluation III score; Hb hemoglobin; MI myocardial infarction; FFP fresh-frozen plasma; SOFA Sequential Organ Failure Assessment; NG not given.
*Blood stored up 35 days and not 42 days.
Positive studies
ICU patients
Two of the 18 positive studies were conducted in ICU patients [15,16]. The first was retrospective and underpowered and was conducted in 31 patients with severe sepsis or septic shock, and without adjustment for confounding factors [16]. The second was a recent, prospective, multicenter, observational study conducted in 47 Australian and New Zealand ICUs. This study enrolled 757 patients and found a significant increase in ICU LOS and in-hospital mortality rate in patients receiving the oldest blood (median of age = 17.6 days, range: 12.9-24.0) versus the freshest (median of age = 7.5 days, range: 5.7-9.0; OR = 2.01, 95% confidence interval [CI]: 1.07-3.77). This effect was seen after adjustment for severity of illness (APACHE III score), number of transfusions, pre-ICU transfusions, fresh-frozen plasma and platelet transfusions, leukodepletion status, pretransfusion hemoglobin concentration, clustering of study sites, and cardiac surgery [15]. Figure 2 illustrates the association between hospital mortality and maximum age of RBC found by these authors. However, in this observational study, patients received a mix of blood of different age (e.g., fresh and old units) and the statistical analysis could only use a surrogate of age of blood (fresh or old) received by the patients, by using the age of the oldest RBC unit as the unit as representative of age of red cell transfused for statistical assessment of their effect.
Figure 2.

Hospital mortality (%, 95% confidence interval) according to maximum age of red blood cells (days) from Pettila et al.[15]with permission.
Cardiovascular patients
In the setting of cardiac surgery or acute cardiovascular heart diseases, six positive studies have been published. Of these, two were prospective [19,24] and four were retrospective [18,21,23,25]. Their methods and their primary results are summarized in Table 2. Three of them have reported an increased incidence of postoperative infections in patients who were transfused with the oldest blood [23-25]. The largest study was conducted by Koch et al. and included 6,002 patients undergoing coronary artery bypass graft (CABG) and/or valve surgery [18]. Patients who received exclusively “oldest” blood defined by a storage duration longer than 14 days had longer duration of mechanical ventilation (9.7% vs. 5.6%, p < 0.001), an increased incidence of sepsis (4% vs. 2.8%, p = 0.01) and a higher 1-year mortality rate (11% vs. 7.4%, p < 0.001). Transfusion of older RBC also was independently associated with an increased risk-adjusted rate of a composite of serious adverse events (25.9% vs. 22.4%, p = 0.001) [18]. Despite a large sample size, this study suffered important limitations, including a retrospective and single-center design, the use of an arbitrary cutoff point to define fresh and old blood, and the absence of adjustment for some important confounding factors [60]. Two other reports, however, considered patients with heart disease also found an impact of RBC storage on mortality [19,21]. One, a retrospective multicenter study reported that the age of the youngest RBC transfused within 10 days of percutaneous coronary intervention was significantly associated with 30-day mortality after adjustment for confounders (HR = 1.02, 95% CI 1.18-1.34, p < 0.001) [21]. The other found a linear relationship between the age of blood and mortality in 4,933 cardiovascular disease patients in acute care facilities [19].
Trauma patients
In trauma patients, ten studies have reported an impact of the storage duration on mortality and/or morbidity [17,20,22,26-30,41]. All were retrospective, single-center studies. Trauma patients transfused with blood older than 14 days appeared to have a higher risk of developing postinjury multiorgan failure [28], infectious complications [22,26,27,61], renal dysfunction [22], greater LOS [29], and higher mortality [17,22,41]. Regardless of the arbitrary cutoff of 14 days to define fresh and old blood, other studies have found an association between blood storage duration and hospital LOS [30], occurrence of deep vein thrombosis [20], and mortality [20,30]. Trauma patients are more likely to receive a “massive” transfusion and, as already mentioned, a dose effect of oldest blood may exist [17,20,27,41].
Despite these positive observational studies, the evidence for a harmful effect of blood storage remains uncertain. This is because few of these reports adjusted for key confounding factors, including leukodepletion, volume of RBC transfused, the year of transfusion, and ABO type, and very few analyzed RBC age as a continuous variable [60]. None adjusted for all key confounders.
Negative studies
In contrast to the above-mentioned investigations, 14 studies did not find any relationship between blood storage duration and mortality, ICU and hospital LOS, duration of mechanical ventilation, acute lung injury, nosocomial infection, renal failure, or deep vein thrombosis occurrence (Table 3) [47-59,62].
ICU patients
One of the seven studies conducted in ICU patients was a double-blind, multicenter, randomized pilot study that enrolled 57 patients and compared a composite outcome, including mortality, between patients who received only blood of less than 8 days of age versus standard care. The small sample size (n = 57) likely contributed to an inconclusive result [51]. Taylor et al. reported an independent increase of nosocomial infection after blood transfusion in a prospective, observational study of 449 ICU patients but did not find any effect of age of RBC [53]. Three reports have especially studied the impact of RBC age on acute lung injury (ALI) or short-term pulmonary function in critically ill patients and did not find any difference [50,54,62]. Similarly, no effect of age of blood on deep vein thrombosis, ICU LOS, and mortality was found in two recent studies of ICU patients [56,57]. All of these studies were small and likely underpowered.
Cardiovascular patients
In postcardiac surgery patients, one of the six negative studies conducted more than 20 years ago was a single-center, randomized, controlled, blind trial that compared post-CABG bleeding in 237 patients who received either 2 units of freshly collected whole blood (fresher than 12 hours) followed by blood stored between 2 and 5 days or blood aged between 2 and 5 days. In this nonpragmatic study, there was no difference for bleeding and RBC transfusion requirements [47].
All the other negative, postcardiac surgery studies were retrospective and single center in design. Nonetheless, some had a large sample size [52,58,59]. For example, in a 2,732 patient cohort, Van de Watering et al. did not show any difference in 30-day survival, ICU, and hospital LOS between patients receiving fresh blood versus old blood, whatever the criteria to estimate the storage duration (only blood < or > 18 days, the mean RBC storage time for each patient, the storage time of the youngest RBC transfused per patient, and the storage time of the oldest RBC transfused per patient) [52]. Similar results were reported by Yap et al. who did not find any association between RBC storage duration estimated by the mean age of RBC per patient, the oldest RBC unit and RBC stored longer than 30 days, and early postoperative mortality or morbidity in 670 post cardiac surgery patients [55]. Van Straten et al. also reported no association between the risk of early and late mortality and the age or the number of RBC units transfused during or within the 5 first days post-CABG [59].
Trauma patients
The only negative study in trauma patients was an underpowered (n = 17) single-center, randomized trial that was conducted between 2000 and 2001. The study design required 15 compatible RBC units of both study arms to be available at randomization, which impacted the study feasibility [48].
Need for randomized, controlled trials
As outlined earlier, studies that evaluated the clinical impact of RBC storage in critically ill patients were 1) heterogeneous in outcomes, study design, population, threshold to differentiate fresh and old blood, and blood characteristics (leukodepleted or not, different storage medium, or storage medium not documented) and 2) often of insufficient quality in methodology (retrospective, observational, small sample size, no or limited inclusion of key confounding factors, and arbitrary cutoff for age of blood).
Their inherent limitations do not allow confirmation of the potential impact of duration of RBC storage on adverse effects or justify a change in current clinical transfusion practice. Two of the four randomized, controlled trials (RCTs) that evaluated clinical outcomes were very small and underpowered [48,51]. The third had a protocol based on the hypothesis that extremely fresh blood (<12 hours) may contain functionally active platelets and coagulation factors and decrease postsurgery bleeding [47], and the most recent RCT only focused on the age and effect of a single RBC unit with mainly pulmonary complications as outcome [62].
Previous systematic reviews [9-11,13] and a meta-analysis conducted in critically ill patients [63] have been inconclusive. Recently, in a meta-analysis, including 21 studies, Wang et al. concluded that older stored blood was associated with an estimated OR for death of 1.16 (95% CI 1.07-1.24). However, some studies did not adjust the risk of death for important confounders , making their conclusions open to challenge [12]. In addition, definition of fresh and old blood remains based on observational studies and therefore unknown because of the heterogeneity of their results (Figures 3a and b).
Figure 3.
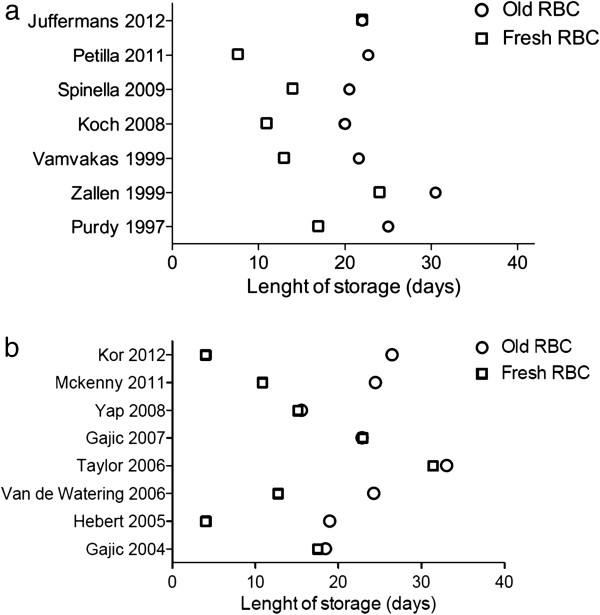
Length of blood storage for positive (a[15,16,18,23,27,28,61]) and negative (b,[50-55,58,62]) studies. The age of blood is expressed as the mean age or the median age of all RBC units, or in some studies as the median of the oldest (or oldest and second oldest) RBC units. The figures give the age of blood for each study comparing the primary study outcome. For instance, if the study outcome is TRALI, the figure shows the age of blood in the group of patients with TRALI and in the group of patients without TRALI.
The above observations make a large, multicenter, RCT in critically ill patients of the greatest priority [64]. In this regard, several such multicenter RCTs are currently underway in adult critically ill patients (Table 4) [65].
Table 4.
Multicenter, randomized, clinical trials about blood storage in critically ill adults
| Authors or study name | Population | Sample size | Case criteria | Controlled criteria | Outcome | Status |
|---|---|---|---|---|---|---|
| Hebert et al.
[51] |
ICU |
57 |
<8 days |
Standard practices |
Composite outcome* (pilot study) |
Achieved |
| Aubron et al.
[67] |
ICU |
51 |
Freshest compatible available RBC |
Standard practices |
Feasibility (pilot study) |
Achieved |
| **RECESS (NCT00991341) |
Post cardiac surgery |
1434 |
≤10 days |
≥21 days |
Change in MODS |
In progress |
| **ABLE (ISRCTN44878718) |
ICU |
2510 |
<8 days |
Standard practices |
90-day mortality |
In progress |
| **TRANSFUSE (ACTRN12612000453886) | ICU excluding postcardiac surgery | 5000 | Freshest compatible available RBC | Standard practices | 90-day mortality | In progress |
RECESS, Red Cell Storage Duration Study; ABLE, Age of Blood Evaluation; TRANSFUSE, STandaRd Issue TrANsfusion versuS Fresher red blood cell Use in intenSive carE; MODS multiorgan dysfunction score; **Indicates the trial is in progress.
*Included hospital mortality, serious nosocomial infections, thrombotic events with myocardial infarction and acute ischemic stroke.
The first such trial is the RECESS (Red Cell Storage Duration Study [RECESS]: NCT00991341) study. RECESS will randomize 1,434 cardiac surgical patients to receive either RBC stored 10 days or less or 21 days or more. However, its results could not be generalizable to any heterogeneous ICU population.
The Canadian ABLE study (Age of Blood Evaluation [ABLE] trial of the resuscitation of critically ill patients: ISRCTN44878718) is currently being conducted in ICU patients. It will enroll a total of 2,510 patients in Canada, France, and the United Kingdom [66]. The ABLE study will compare 90-day mortality between patients transfused with fresh RBC (defined as a storage duration <8 days) and patients transfused in accordance with standard practices [66]. Potential limitations of ABLE include 1) a sample size based on an estimated 25% relative risk reduction in the primary endpoint and 2) a design (fresh blood always fresher than 8 days) that seems unlikely to be reproducible always in future clinical practice.
The TRANSFUSE trial (ACTRN12612000453886) commences in 2012 in Australia and New Zealand. TRANSFUSE is a large (5,000 patients) pivotal, multicenter, randomized, controlled trial in critically ill patients to determine whether, compared with standard care, transfusion of the freshest available RBC decreases patient mortality. Completion is expected in 2015 and its findings are likely to guide blood transfusion policy in ICU patients.
Conclusions
Blood transfusion is a common therapeutic intervention in critically ill patients. Much scientific evidence, however, supports the occurrence of alterations in red cells over their storage time, and observational studies suggest that transfusion of older RBC may have important adverse clinical consequences, including mortality in this population. Nonetheless, making structural changes in transfusion policy to deliver only fresh red cells to critically ill patients would have far-reaching logistics. The possibility that a clinically significant risk of older RBC transfusion exists and the possible benefits of making adjustments to transfusion policy can only be resolved by supporting and completing the current, pivotal, multicenter, double-blind RCTs. Until such trials are reported, any clinical practice change is premature.
Abbreviations
ICU: Intensive care unit; RBC: Red blood cells; LOS: Length of stay; ATP: Adenosine triphosphate; 2,3-DPG: 2,3-diphosphoglycerate; APACHE III: Acute Physiology Age Chronic Health Evaluation; CABG: Coronary artery bypass graft; RCT: Randomized controlled trials; ALI: Acute lung injury.
Competing interests
The authors declare they have no competing interests.
Authors’ contribution
CA and RB conceived the review. CA executed the necessary search and wrote the first draft. RB, AN, and JC reviewed and modified the draft. CA and RB reviewed and completed the final version. All authors read and approved the final manuscript.
Contributor Information
Cécile Aubron, Email: cecile.aubron@monash.edu.
Alistair Nichol, Email: alistair.nichol@monash.edu.
D Jamie Cooper, Email: jamie.cooper@monash.edu.
Rinaldo Bellomo, Email: Rinaldo.BELLOMO@austin.org.au.
References
- Corwin HL, Surgenor SD, Gettinger A. Transfusion practice in the critically ill. Crit Care Med. 2003;31:S668–S671. doi: 10.1097/01.CCM.0000099348.99451.84. [DOI] [PubMed] [Google Scholar]
- Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: Anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- Westbrook A, Pettilä V, Nichol A, Bailey MJ, Syres G, Murray L, Bellomo R, Wood E, Phillips LE, Street A, French C, Orford N, Santamaria J, Cooper DJ. Blood Observational Study Investigators of ANZICS-Clinical Trials Group. Transfusion practice and guidelines in Australian and New Zealand intensive care units. Intensive Care Med. 2010;36:1138–1146. doi: 10.1007/s00134-010-1867-8. [DOI] [PubMed] [Google Scholar]
- Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM. et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci. 2010;43:95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384–1394. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31:S687–S697. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- Pettila V, Westbrook AJ, Nichol AD, Bailey MJ, Wood EM, Syres G, Phillips LE, Street A, French C, Murray L. et al. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15:R116. doi: 10.1186/cc10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G Jr, Griffin RL, Huynh VQ, Cherry SA 3rd, Marques MB, Reiff DA, Kerby JD, Rue LW 3rd. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Cook RJ, Liu Y, Heddle NM. Duration of red cell storage before transfusion and in-hospital mortality. Am Heart J. 2010;159:737–743. doi: 10.1016/j.ahj.2009.12.045. e731. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Janssen C, Fretz EB, Berry B, Chase AJ, Siega AD, Carere RG, Fung A, Simkus G, Klinke WP, Hilton JD. Red blood cell storage duration and mortality in patients undergoing percutaneous coronary intervention. Am Heart J. 2010;159:876–881. doi: 10.1016/j.ahj.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G Jr, Marques MB, Cherry SA 3rd, Reiff DA, Kerby JD, Rue LW 3rd. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, Marin-Niebla A, Herruzo-Aviles A, Camacho-Larana P, Loscertales J. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98:815–822. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- Andreasen JJ, Dethlefsen C, Modrau IS, Baech J, Schonheyder HC, Moeller JK, Johnsen SP. Storage time of allogeneic red blood cells is associated with risk of severe postoperative infection after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2011;39:329–334. doi: 10.1016/j.ejcts.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–716. doi: 10.1001/archsurg.137.6.711. discussion 716–717. [DOI] [PubMed] [Google Scholar]
- Vandromme MJ, McGwin G Jr, Marques MB, Kerby JD, Rue LW 3rd, Weinberg JA. Transfusion and pneumonia in the trauma intensive care unit: an examination of the temporal relationship. J Trauma. 2009;67:97–101. doi: 10.1097/TA.0b013e3181a5a8f9. [DOI] [PubMed] [Google Scholar]
- Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/S0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- Keller ME, Jean R, LaMorte WW, Millham F, Hirsch E. Effects of age of transfused blood on length of stay in trauma patients: a preliminary report. J Trauma. 2002;53:1023–1025. doi: 10.1097/00005373-200211000-00037. [DOI] [PubMed] [Google Scholar]
- Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71:781–785. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- Chin-Yee I, Arya N, d'Almeida MS. The red cell storage lesion and its implication for transfusion. Transfus Sci. 1997;18:447–458. doi: 10.1016/S0955-3886(97)00043-X. [DOI] [PubMed] [Google Scholar]
- Wolfe LC. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985;25:185–203. doi: 10.1046/j.1537-2995.1985.25385219897.x. [DOI] [PubMed] [Google Scholar]
- Card RT, Mohandas N, Perkins HA, Shohet SB. Deformability of stored red blood cells. Relationship to degree of packing. Transfusion. 1982;22:96–101. doi: 10.1046/j.1537-2995.1982.22282177134.x. [DOI] [PubMed] [Google Scholar]
- Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–2554. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam O, Tucci M, Toledano BJ, Robitaille N, Cousineau J, Thibault L, Lacroix J, Le Deist F. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion. 2009;49:2326–2334. doi: 10.1111/j.1537-2995.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S. et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Gladwin MT. Bad blood: the risks of red cell storage. Nat Med. 2010;16:381–382. doi: 10.1038/nm0410-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chierego M, Verdant C, De Backer D. Microcirculatory alterations in critically ill patients. Minerva Anesthesiol. 2006;72:199–205. [PubMed] [Google Scholar]
- Vlaar AP, Hofstra JJ, Levi M, Kulik W, Nieuwland R, Tool AT, Schultz MJ, de Korte D, Juffermans NP. Supernatant of aged erythrocytes causes lung inflammation and coagulopathy in a "two-hit" in vivo syngeneic transfusion model. Anesthesiology. 2010;113:92–103. doi: 10.1097/ALN.0b013e3181de6f25. [DOI] [PubMed] [Google Scholar]
- Vlaar AP, Wolthuis EK, Hofstra JJ, Roelofs JJ, Boon L, Schultz MJ, Lutter R, Juffermans NP. Mechanical ventilation aggravates transfusion-related acute lung injury induced by MHC-I class antibodies. Intensive Care Med. 2010;36:879–887. doi: 10.1007/s00134-010-1802-z. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW 3rd. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–1432. doi: 10.1097/TA.0b013e3181fa0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. doi: 10.1001/jama.1993.03500230106037. [DOI] [PubMed] [Google Scholar]
- Fernandes CJ Jr, Akamine N, De Marco FV, De Souza JA, Lagudis S, Knobel E. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Crit Care. 2001;5:362–367. doi: 10.1186/cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, McClelland DB. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, Creteur J, Gullo A, Vincent JL, De Backer D. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35:1639–1644. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, Marin-Caballos A, Amaya-Villar R, Marin A, Puppo-Moreno A, Ferrandiz-Millon C, Flores-Cordero JM, Murillo-Cabezas F. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- Wasser MN, Houbiers JG, D'Amaro J, Hermans J, Huysmans HA, van Konijnenburg GC, Brand A. The effect of fresh versus stored blood on post-operative bleeding after coronary bypass surgery: a prospective randomized study. Br J Haematol. 1989;72:81–84. doi: 10.1111/j.1365-2141.1989.tb07656.x. [DOI] [PubMed] [Google Scholar]
- Schulman CI, Nathe K, Brown M, Cohn SM. Impact of age of transfused blood in the trauma patient. J Trauma. 2002;52:1224–1225. doi: 10.1097/00005373-200206000-00036. [DOI] [PubMed] [Google Scholar]
- Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion. 2000;40:101–109. doi: 10.1046/j.1537-2995.2000.40010101.x. [DOI] [PubMed] [Google Scholar]
- Gajic O, Rana R, Mendez JL, Rickman OB, Lymp JF, Hubmayr RD, Moore SB. Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion. 2004;44:1468–1474. doi: 10.1111/j.1537-2995.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Chin-Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, Olberg B. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–1438. doi: 10.1213/01.ANE.0000148690.48803.27. table of contents. [DOI] [PubMed] [Google Scholar]
- Van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–1718. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- Taylor RW, O'Brien J, Trottier SJ, Manganaro L, Cytron M, Lesko MF, Arnzen K, Cappadoro C, Fu M, Plisco MS. et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302–2308. doi: 10.1097/01.CCM.0000234034.51040.7F. [DOI] [PubMed] [Google Scholar]
- Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, Evenson LK, Malinchoc M, DeGoey SR. et al. Transfusion-related acute lung injury in the critically ill: prospective nested case–control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Van Buskirk CM, Thakur SJ, Murray DL, Bryant SC, Winters JL, Stubbs JR, Gajic O. Red blood cell storage age has no impact on clinical outcome in critically ill patients. Transfusion. 2009;49 SupplementS3. [Google Scholar]
- Katsios C, Griffith L, Spinella P, Lacroix J, Crowther M, Hebert P, Meade M, Geerts W, Rabbat C, Cook D. Red blood cell transfusion and increased length of storage are not associated with deep vein thrombosis in medical and surgical critically ill patients: a prospective observational cohort study. Crit Care. 2011;15:R263. doi: 10.1186/cc10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenny M, Ryan T, Tate H, Graham B, Young VK, Dowd N. Age of transfused blood is not associated with increased postoperative adverse outcome after cardiac surgery. Br J Anaesth. 2011;106:643–649. doi: 10.1093/bja/aer029. [DOI] [PubMed] [Google Scholar]
- Van Straten AH, Soliman Hamad MA, van Zundert AA, Martens EJ, ter Woorst JF, de Wolf AM, Scharnhorst V. Effect of duration of red blood cell storage on early and late mortality after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141:231–237. doi: 10.1016/j.jtcvs.2010.02.059. [DOI] [PubMed] [Google Scholar]
- Van de Watering L. Pitfalls in the current published observational literature on the effects of red blood cell storage. Transfusion. 2011;51:1847–1854. doi: 10.1111/j.1537-2995.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- Juffermans NP, Vlaar AP, Prins DJ, Goslings JC, Binnekade JM. The age of red blood cells is associated with bacterial infections in critically ill trauma patients. Blood Transfus. 2012;10:290–295. doi: 10.2450/2012.0068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor DJ, Gajic O. Blood product transfusion in the critical care setting. Curr Opin Crit Care. 2012;16:309–316. doi: 10.1097/MCC.0b013e32833bc4a4. [DOI] [PubMed] [Google Scholar]
- Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of "old" (versus "fresh") red blood cells: are we at equipoise? Transfusion. 2010;50:600–610. doi: 10.1111/j.1537-2995.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- Aubron C, Carteaux G, Cooper DJ. Why we must "TRANSFUSE.". Crit Care Resusc. 2011;13:67–68. [PubMed] [Google Scholar]
- Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, Triulzi D, Stowell CP. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. pp. 107–116. [DOI] [PMC free article] [PubMed]
- Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Aubron C, Syres G, Nichol A, Bailey M, Board J, Magrin G, Murray L, Presneill J, Sutton J, Vallance S, Morrison S, Bellomo B, Cooper DJ. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion. 2012;52:1196–1202. doi: 10.1111/j.1537-2995.2011.03437.x. [DOI] [PubMed] [Google Scholar]


