Abstract
Transfer of DNA between different compartments of the plant cell, i.e., plastid, mitochondrion and nucleus, is a well-known phenomenon in plant evolution. Six directions of inter-compartmental DNA migration are possible in theory, however only four of them have been previously reported. These include frequent cases of mitochondrion and plastid to nucleus transfer, plastid to mitochondrion transfer, and rare nucleus to mitochondrion migrations. The connection between the plastid and mitochondrial genomes in flowering plants has been viewed as a one way road. Contrary to these observations we found that a sequence widespread in the carrot mitochondrial genome, designated as DcMP, was transferred to the plastid genome of a carrot ancestor. Interestingly, DcMP was integrated into a tRNA promoter of the plastid trnV gene, replacing the original promoter sequence. The rearrangement of the plastid genome is specific for carrot and closely related species belonging Scandiceae clade. The structure of the sequence and the presence of a 6 nt target site duplication led us to speculate that the transfer was a result of a transposition event of a non-LTR retrotransposon. These findings open interesting questions about the evolution of organellar genomes and mobile genetic elements and provide a useful plastid marker to phylogenetically delineate species relationships within the Scandiceae clade.
Keywords: Daucus carota, inter-compartmental DNA migration, plastid and mitochondrial genome, retrotransposon
Introduction
Inter-compartmental DNA migration is a well-known phenomenon in plant cell evolution and has contributed significantly to genome evolution by relocating and refashioning genes and consequently contributing to genetic diversity. DNA transfer among the plastid, mitochondrial and nuclear genomes resulted in a functional relocation of organellar genes early in organelle evolution.1-6 In contrast, almost all reported recent inter-compartmental DNA transfers gave rise to non-coding sequences or pseudogenes.1 Six directions of the inter-compartmental DNA migration are possible in theory, and four of them have been previously reported in angiosperms. Transfers of DNA from the mitochondria and from the plastid to the nucleus are the most common reported transfers.7,8 Transfers of DNA from the plastid to the mitochondrion have also been frequently observed. Cases of nuclear DNA transfer to the exceptionally large mitochondrial genomes of Fabaceae and Cucurbitaceae were also reported.9,10 In contrast, no evidence of DNA transfer from the nucleus or the mitochondrion to the plastid has been reported in angiosperms. Smith11 investigated evidence for the presence of mitochondrial DNA in the plastid genome of 42 species including 11 angiosperms and did not find any mtDNA-like sequences. Forces driving inter-comparmental DNA transfer are still unclear. Plant mitochondria actively import DNA via a permeability transition pore complex.12 In contrast, the relative absence of nuclear and mitochondrial DNA in the plastome could reflect the lack of a DNA uptake system, due to the integrity of the plastid membrane. Escape of DNA from organellar genomes to the nuclear genome seems to coincide with the degradation of organelle DNA.1 Both mobile elements and integration of DNA by nonhomologous end-joining (NHEJ) repair of double-strand breaks (DBSs) are important components of the DNA transfer machinery. In our recent paper13 we provided evidence that a sequence designated DcMP (D. carota Mitochondrial-Plastid), was transferred from the mitochondrial genome to the plastid genome of the carrot ancestor. Here we discuss a hypothetical mechanism explaining this event. To date, this represents the first evidence for this direction of DNA transfer. Given the large amount of available angiosperm sequence data, this strongly suggests that this is a rare event. However, the increasing availability of new organellar and nuclear genome sequences could reveal similar events in other species, and this could elucidate the mechanism that makes the uptake of DNA by the plastid genome such a rare event.
Daucus carota Mitochondrial-Plastid (DcMP) Region
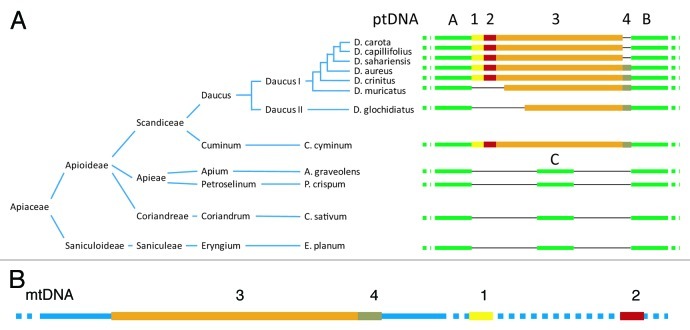
Following the evidence provided by Goremykin and colleagues14 that a region in the Vitis vinifera mitochondrial genome was similar to a fragment of the carrot plastid genome, we identified that this region was part of a DNA segment designated as DcMP that was likely transferred from the mitochondrial to the plastid genome of carrot.13 This was the first time that DNA transfer from the mitochondrion to the plastid was reported in flowering plants. Our evidence indicated that the transferred segment was at least 1,452 nt-long. Currently, it resides in mitochondrial genomes of a wide range of Apiaceae species, but its presence in the plastid genome is restricted to the genus Daucus and their close relatives including cumin (Cuminum cyminum), indicating that the transfer took place in their common ancestor. This observation suggests that DcMP could be a characteristic of species belonging to the tribe Scandiceae15,16 (Fig. 1A). The insertion into the plastid genome was accompanied by a deletion of 339 nt sequence (Fig. 1A, segment C), which was present in the empty insertion site of all examined non-Scandiceae species. The DcMP region was apparently transferred as a contiguous sequence from the mitochondria to the plastid but at present it is highly rearranged in the investigated mitochondrial genomes (Fig. 1B). The position of the DcMP segments 3 and 4 is conserved across the mitochondrial genomes of all investigated Apiaceae species. In the carrot mitochondrial genome segments 1 and 2 are physically separated from 3 and 4 and located ca. 5kb and over 80 kb downstream, respectively, relative to DcMP 3 and 4 (Fig. 1B). Notably, only DcMP segment 2 has homology to other plant mitochondrial genomes - it is a fragment of the cox1 gene. It is therefore likely that the presence of the DcMP segments 1, 3 and 4 in the carrot mitochondrial genome resulted from a DNA transfer of unknown origin, common to Apiaceae, which predated the transfer from the mitochondria to the plastid. The observed fragmentation of the DcMP region in mtDNA can be explained by a high level of recombination activity often observed in plant mitochondrial genomes, and resulting in the intragenomic reshuffling of DNA segments.
Figure 1. Presence of DcMP insertion variants in the plastid genome of Apiaceaeous species according to the reconstructed phylogeny.15,16 (A) Yellow, red, orange and brown segments 1 to 4 represents the region of plastid (pt) DNA transferred from the mitochondrial genome (dubbed DcMP). It has likely been transferred in a single event with the present organization of the transferred sequences in the carrot mitochondrial genome depicted in (B); Green lines A and B denote conserved plastid sequences flanking the insertion; green line C represents the 339 nt region that have been deleted in the Scandiceae plastid genomes; thin black lines denote missing regions. (B) Blue lines denote mitochondrial sequences of carrot; colored segments represent the DcMP regions 1–4; dashed lines represent mtDNA segments separating regions 1–4. The scheme is not drawn to scale.
Functional Replacement of a tRNAVal Initiation Site
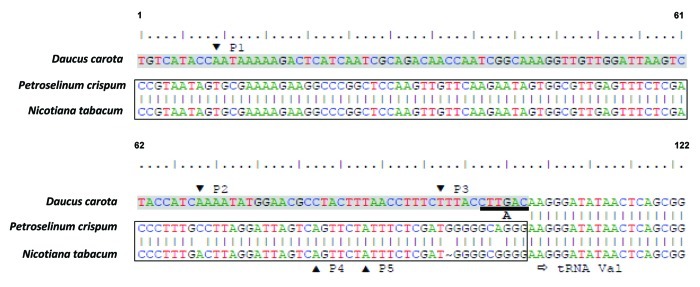
The DcMP sequence integrated into the plastid genome just upstream of the tRNAVal gene (trnV), located in the large inverted repeat. Interestingly, Manna et al.17 investigated the expression level of trnV during carrot embryogenesis and identified three putative plastid trnV promoters: P1, P2 and P3 (Fig. 2). They observed that the three promoters mapped to a region of minor conservation when compared with the tobacco and maize plastid genomes, where two putative trnV promoters P4 and P5 were previously identified18 (Fig. 2). Promoters P1, P2 and P3 are located 105, 41 and 16 nt upstream of trnV, respectively. They suggested that these three promoters were responsible for the differential expression of trnV during embryogenesis. Our findings revealed that all three promoters were in fact a part of the integrated DcMP sequence (Fig. 2, shaded sequence), while P4 and P5 were located at the 3′ end of the 339 nt DNA fragment that was replaced by DcMP in the carrot plastid genome (Fig. 2, rectangular box; Fig. 1A, segment C). A comparison of the carrot, tobacco (Nicotiana tabacum), and parsley (Petroselium crispum) plastid genomes in this region revealed that P4 and P5 are conserved in parsley. This strongly suggests that with the integration of DcMP into the plastid genome, the conserved promoter region upstream of a trnV18 was replaced with a functional substitute, resulting in the modified expression pattern of trnV.
Figure 2. Nucleotide sequence upstream of the plastid trnV gene in D. carota, P. crispum, and N. tabacum. Shading indicates the 3′ end of DcMP plastid region present only in D. carota. The rectangular box represents the 3′ end of the 339 nt DNA fragment replaced by DcMP in the D. carota plastid genome. P1, P2, P3: putative promoters of the D. carota trnV.17 P4, P5: putative promoters of the P. crispum and N. tabacum trnV.18 Horizontal arrow indicates the 5′ end of trnV. Underlined sequence A represents the unit of the DcMP direct repeat.
Sequence Analysis of the Plastid DcMP Variant and a Possible Mechanism for the DcMP Transfer
Analysis of the plastid DcMP sequence, the structure of which likely represents the organization of the transferred contiguous sequence, could suggest its possible mode of integration. Annotation of DcMP sequence was performed using Open Reading Frame Finder http://www.ncbi.nlm.nih.gov/gorf/gorf.html website. Investigation of repeat sequences was carried using blastn. In order to detect sequences flanking the plastid DcMP region with homology to sequences flanking the mitochondrial DcMP region, the sequence of the plastid genome was queried against a local database containing the carrot mitochondrial genome.
Sequence annotation identified three putative ORFs (Open Reading Frame), ORFa and ORFb of 153 and 408 nt respectively (Fig. 3) both with similarity to a gag domain annotated in Vitis vinifera, and the third ORF, ORFc of 144 nt (Fig. 3) with moderate similarity to a reverse transcriptase annotated in Medicago truncatula. Further characterization of the DcMP region in the plastid revealed the presence of a 6 nt direct repeat (CTTGAC), immediately flanking DcMP (Fig. 3, blue vertical lines), i.e., the repeats were present directly upstream of DcMP1 and directly downstream of DcMP4. These characteristics suggest that the DcMP might be a non-LTR retrotransposon and the direct repeats represent target site duplication (TSD)19 created as a result of the DcMP integration following its mobilization from a donor site localized in the mitochondrial genome. It has been shown that some non-LTR elements may specifically target RNA polymerase III dependent genes, such as tRNA genes. Recently, Wenke et al.20 described a group of non-LTR retrotransposons targeting tRNA genes in Dictyostelium discoideum. Integration of non-LTR elements can alter genome structure and function and has been associated with genome rearrangements,21 deletions22,23 and in some cases it resulted in modified expression of adjacent genes.24,25 Alternative mechanisms could have involved homologous recombination by microhomology or transfer of a group I intron. Homologous recombination would require that regions of plastome have homology to the recombinant DNA fragment. However, since we found no homology between sequences flanking the mitochondrial DcMP and the plastid genome13 we find this possibility unlikely. In addition, homologous recombination is an unlikely mechanism for replacing a functional promoter. A second alternative mechanism could have involved group I introns which catalyze their own splicing and function as mobile elements, such as those found in the tRNALeu genes of ptDNA.26 Also group I introns are present in the mithocondrial cox1 gene and are involved in horizontal gene transfer. The structure of DcMP does not support this hypothesis. In fact DcMP has almost no internal homology (11 nt maximum), excluding the possibility of the characteristic folding exhibited by group I introns. Interestingly, the D. carota mt genome lacks the cox1 group I intron as reported by Sanchez-Puerta et al.27 for many other angiosperm species.

Figure 3. Structure of the plastid D. carota DcMP sequence. ORF were detected using Open Reading Frame Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The sequence was oriented according to 5′-3′ (indicated by arrows) ORF orientation is in opposite direction as related to other figures. Thick vertical blue lines indicate target site duplication (TSD). Thin red vertical lines indicate relative position of P1, P2 and P3 tnrV promoters. Red box indicate the region comprising partial sequence of cox1 gene. The scheme is drawn to scale.
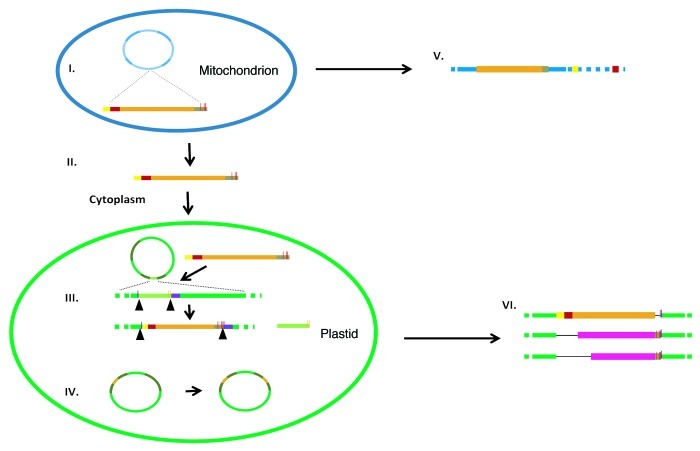
Considering the structure of the DcMP region and the functionality of its sequence proposed by Manna et al.,17 we speculate that it represents a non-LTR element, and that a retrotransposition event likely caused its integration into the mitochondrial genome from an unknown source, which was then followed by the reported transfer to the plastid genome. At present, it is difficult to speculate about the exact mechanism accounting for the transfer and integration of DcMP into the organellar genomes. If the current non-contiguous organization of the DcMP region in the carrot mitochondrial genome is shared by many Apiaceae, this migration event likely was much older than the transfer to the plastid genome, which has been restricted to a much narrower group of closely related species. Moreover, the fragmentation of DcMP in the mitochondria likely predated the transfer into plastids based upon its apparent rearrangements observed in the mitochondrial genome and described above. However, the presence of the cox1 gene fragment in the region that migrated into the plastid genome suggests that the donor sequence must have originated in the mitochondrial genome. A possible scenario could have involved retrotransposition into the mitochondrial genome, mtDNA re-arrangements and acquirement of the cox1 sequence transcription from a chimeric mitochondrial donor sequence and reverse transcription, and finally transfer into the plastid and integration of the new copy comprising both retroelement-specific and mitochondrial sequences (Fig. 4). The scenario involving retrotransposition gains some support from the fact that a retroelement-related ORF coding for reverse transcriptase had already been found in the plastome of green alga.28
Figure 4. A schematic representation of the hypothetical mitochondrial to plastid genome transfer of DcMP in the carrot ancestor. (I) Transcription of a chimeric segment (DcMP) comprising non-LTR fragments and Mt-specific regions (including cox1, red segments); (II) Migration of DcMP transcript to the cytoplasm and into the plastid; (III) Integration of DcMP and deletion of the ancestral Pt-specific segment (light green segment) including creation of a target site duplication (blue vertical lines) and replacement of the conserved promoters P4 and P5 (yellow vertical lines) with P1, P2 and P3 (red vertical lines) upstream of trnV (violet segment), the reverse transcription step is not shown; (IV) Copy correction in the inverted repeat (IR, dark green segments); (V) Mt genome reshuffling and deletions of DcMP; (VI) Accumulation of lineage-specific deletions in the DcMP region. The scheme is not drawn to scale.
In our scenario, following transcription, retrotransposon RNA migrates into the cytoplasm and proliferates via a ‘copy and paste’ mechanism requiring an RNA intermediate. Here the proteins required for transposition are produced (Fig. 4, I). Although the mechanism of this migration is not clear, it is worth mentioning that cases of out-of-mitochondria transcript translocation have been well evidenced for animal cells.29-31 A horizontal transfer scenario would require that the RNA copy was transported into the plastid (Fig. 4, II). The mechanism leading to this event is unknown, but previous studies revealed that stress conditions such as heat shock could cause pore formation in the plastid envelope and allow entry of DNA.32 Related research also demonstrated that plastids were actively able to import mRNA.33 We observed that, in the plastid genome DcMP integrated upstream of the trnV creating a deletion of the ancestral plastid sequence and a target site duplication (TSD) (Fig. 4, III). It was not possible to identify the integration region in the mitochondrial genome, due to extensive rearrangements it underwent subsequent to insertion (Fig. 4, V). The functional replacement of promoter activity upstream of the trnV avoided negative selection and we speculate that its transfer into the inverted repeat (IR), followed by a copy correction mechanism, that duplicated the introduced DcMP into the other inverted repeat (Fig. 4, IV), resulted in a stable integration into the plastid genome. After the integration, deletion events could have occurred within other species of Daucus or Cuminum, both members of the Scandiceae clade (Figs. 1A and 4, VI).
Significance and Applications
The data discussed here provide new insights in the dynamics of inter-compartmental DNA migration. The structure of the DcMP sequence, along with its functional aspects, provides a plausible scenario for the evolution of organellar genomes, including integration of mobile genetic elements, and suggests a mechanism for that process. As revealed by studies in plants34 and other organisms,25 transposable elements are very powerful markers to trace ancestor history. Considering the stability of the plastid genome, the presence of the DcMP region in members of the Scandiceae makes it a candidate marker to delineate relationships in this clade.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/23088
References
- 1.Kleine T, Maier UG, Leister D. DNA transfer from organelles to the nucleus: the idiosyncratic genetics of endosymbiosis. Annu Rev Plant Biol. 2009;60:115–38. doi: 10.1146/annurev.arplant.043008.092119. [DOI] [PubMed] [Google Scholar]
- 2.Woloszynska M. Heteroplasmy and stoichiometric complexity of plant mitochondrial genomes--though this be madness, yet there’s method in’t. J Exp Bot. 2010;61:657–71. doi: 10.1093/jxb/erp361. [DOI] [PubMed] [Google Scholar]
- 3.Davila JI, Arrieta-Montiel MP, Wamboldt Y, Cao J, Hagmann J, Shedge V, et al. Double-strand break repair processes drive evolution of the mitochondrial genome in Arabidopsis. BMC Biol. 2011;9:64. doi: 10.1186/1741-7007-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–81. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 5.Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 2004;5:123–35. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 6.Archibald JM. The puzzle of plastid evolution. Curr Biol. 2009;19:R81–8. doi: 10.1016/j.cub.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 7.Gantt JS, Baldauf SL, Calie PJ, Weeden NF, Palmer JD. Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron. EMBO J. 1991;10:3073–8. doi: 10.1002/j.1460-2075.1991.tb07859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millen RS, Olmstead RG, Adams KL, Palmer JD, Lao NT, Heggie L, et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell. 2001;13:645–58. doi: 10.1105/tpc.13.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alverson AJ, Zhuo S, Rice DW, Sloan DB, Palmer JD. The mitochondrial genome of the legume Vigna radiata and the analysis of recombination across short mitochondrial repeats. PLoS One. 2011;6:e16404. doi: 10.1371/journal.pone.0016404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alverson AJ, Wei X, Rice DW, Stern DB, Barry K, Palmer JD. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae) Mol Biol Evol. 2010;27:1436–48. doi: 10.1093/molbev/msq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DR. Extending the limited transfer window hypothesis to inter-organelle DNA migration. Genome Biol Evol. 2011;3:743–8. doi: 10.1093/gbe/evr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koulintchenko M, Konstantinov Y, Dietrich A. Plant mitochondria actively import DNA via the permeability transition pore complex. EMBO J. 2003;22:1245–54. doi: 10.1093/emboj/cdg128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iorizzo M, Senalik D, Szklarczyk M, Grzebelus D, Spooner D, Simon PW. De novo assembly of the carrot mitochondrial genome using next generation sequencing of whole genomic DNA provides first evidence of DNA transfer into an angiosperm plastid genome. BMC Plant Biol. 2012;12:61. doi: 10.1186/1471-2229-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goremykin VV, Salamini F, Velasco R, Viola R. Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Mol Biol Evol. 2009;26:99–110. doi: 10.1093/molbev/msn226. [DOI] [PubMed] [Google Scholar]
- 15.Spalik K, Downie SR. Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): an appraisal using molecular data. J Biogeogr. 2007;34:2039–54. doi: 10.1111/j.1365-2699.2007.01752.x. [DOI] [Google Scholar]
- 16.Grzebelus D, Barański R, Spalik K, Allender C, Simon PW. Daucus. In: Kole C, ed. Wild Crop Relatives: Genomic and Breeding Resources. Berlin-Heidelberg, Springer-Verlag, 2011:91-113. [Google Scholar]
- 17.Manna F, Massardo DR, Wolf K, Luccarini G, Carlomagno MS, Rivellini F, et al. A tRNA gene mapping within the chloroplast rDNA cluster is differentially expressed during the development of Daucus carota. Nucleic Acids Res. 1994;22:1712–8. doi: 10.1093/nar/22.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tohdoh N, Shinozaki K, Sugiura M. Sequence of a putative promoter region for the rRNA genes of tobacco chloroplast DNA. Nucleic Acids Res. 1981;9:5399–406. doi: 10.1093/nar/9.20.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JS. Non-long terminal repeat (non-LTR) retrotransposons: mechanisms, recent developments, and unanswered questions. Mob DNA. 2010;1:15. doi: 10.1186/1759-8753-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenke T, Döbel T, Sörensen TR, Junghans H, Weisshaar B, Schmidt T. Targeted identification of short interspersed nuclear element families shows their widespread existence and extreme heterogeneity in plant genomes. Plant Cell. 2011;23:3117–28. doi: 10.1105/tpc.111.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burwinkel B, Kilimann MW. Unequal homologous recombination between LINE-1 elements as a mutational mechanism in human genetic disease. J Mol Biol. 1998;277:513–7. doi: 10.1006/jmbi.1998.1641. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert N, Lutz-Prigge S, Moran JV. Genomic deletions created upon LINE-1 retrotransposition. Cell. 2002;110:315–25. doi: 10.1016/S0092-8674(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 23.Symer DE, Connelly C, Szak ST, Caputo EM, Cost GJ, Parmigiani G, et al. Human l1 retrotransposition is associated with genetic instability in vivo. Cell. 2002;110:327–38. doi: 10.1016/S0092-8674(02)00839-5. [DOI] [PubMed] [Google Scholar]
- 24.Speek M. Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes. Mol Cell Biol. 2001;21:1973–85. doi: 10.1128/MCB.21.6.1973-1985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordaux R, Batzer MA. The impact of retrotransposon on human genome evolution. Nature. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Puerta MV, Cho Y, Mower JP, Alverson AJ, Palmer JD. Frequent, phylogenetically local horizontal transfer of the cox1 group I Intron in flowering plant mitochondria. Mol Biol Evol. 2008;25:1762–77. doi: 10.1093/molbev/msn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saldanha R, Mohr G, Belfort M, Lambowitz AM. Group I and group II introns. FASEB J. 1993;7:15–24. doi: 10.1096/fasebj.7.1.8422962. [DOI] [PubMed] [Google Scholar]
- 28.Kück U. The intron of a plastid gene from a green alga contains an open reading frame for a reverse transcriptase-like enzyme. Mol Gen Genet. 1989;218:257–65. doi: 10.1007/BF00331276. [DOI] [PubMed] [Google Scholar]
- 29.Villegas J, Araya P, Bustos-Obregon E, Burzio LO. Localization of the 16S mitochondrial rRNA in the nucleus of mammalian spermatogenic cells. Mol Hum Reprod. 2002;8:977–83. doi: 10.1093/molehr/8.11.977. [DOI] [PubMed] [Google Scholar]
- 30.Ninomiya Y, Ichinose S. Subcellular distribution of mitochondrial ribosomal RNA in the mouse oocyte and zygote. PLoS One. 2007;2:e1241. doi: 10.1371/journal.pone.0001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landerer E, Villegas J, Burzio VA, Oliveira L, Villota C, Lopez C, et al. Nuclear localization of the mitochondrial ncRNAs in normal and cancer cells. Cell Oncol (Dordr) 2011;34:297–305. doi: 10.1007/s13402-011-0018-8. [DOI] [PubMed] [Google Scholar]
- 32.Cerutti HD, Jagendorf A. Movement of DNA across the chloroplast envelop: implications for the transfer of promiscuous DNA. Photosynth Res. 1995;46:329–37. doi: 10.1007/BF00020448. [DOI] [PubMed] [Google Scholar]
- 33.Gómez G, Pallás V. Noncoding RNA mediated traffic of foreign mRNA into chloroplasts reveals a novel signaling mechanism in plants. PLoS One. 2010;5:e12269. doi: 10.1371/journal.pone.0012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawkins JS, Hu G, Rapp RA, Grafenberg JL, Wendel JF. Phylogenetic determination of the pace of transposable element proliferation in plants: copia and LINE-like elements in Gossypium. Genome. 2008;51:11–8. doi: 10.1139/G07-099. [DOI] [PubMed] [Google Scholar]