Abstract
SCAND3 and KRBA2 are two mammalian proteins originally described as “cellular-integrases” due to sharing of a similar DDE-type integrase domain whose origin and relationship with other recombinases remain unclear. Here we perform phylogenetic analyses of 341 integrase/transposase sequences to reveal that the integrase domain of SCAND3 and KRBA2 derives from the same clade of GINGER2, a superfamily of cut-and-paste transposons widely distributed in insects and other protostomes, but seemingly absent or extinct in vertebrates. Finally, we integrate the results of phylogenetic analyses to the taxonomic distribution of SCAND3 and KRBA2 and their transposon relatives to discuss some of the processes that promoted the emergence of these two chimeric genes during mammalian evolution.
Keywords: GINGER2, horizontal transfer, domestication, chimerism
Results
Mobile genetic elements (MGEs) are abundant selfish components of living organisms that can sometimes provide new substrate for the enrichment of host genome complexity by co-option of their coding or non-coding components (see refs. 1 and 2). The process of co-option, also known as exaptation or domestication, is not always straightforward to investigate as it frequently involves events of recombination, gene fusion and/or exonization (the creation of a new exon as a result of mutations in intronic sequences),3-5 making particularly difficult to annotate and interpret these types of gene structures in sequencing projects. Along these lines, in 2005, two groups of DDE-like cellular integrases (C-INTs) were described in diverse eukaryotes.6 Due to the similarity of these sequences with the INTs of LTR retroelements, initially it was hypothesized that they evolved from the latter to serve cellular functions. Further studies revealed that one of these two C-INT groups defines a new type of transposases (TPases) encoded by a previously unrecognized subclass of eukaryotic MGEs called Mavericks7,8 or Polintons.9 As for the other C-INT group, genomic annotations subsequently disclosed two single copy gene variants (restricted to humans and eutherian mammals) formally called SCAND3 and KRBA2 in GenBank.10 The predicted products of these two genes share an INT core but can be distinguished from one another by several features. In the predicted SCAND3 protein (also known as ZNF452), the INT core is flanked at the N-terminus by a SCAN zinc-finger domain,11 and, at the C-terminus, by a TPase-derived hATd dimerization module similar to that of the hobo-Activator-Tam3 (hAT) superfamily.12,13 In the KRBA2 protein, a distinct zinc-finger Krüppel-associated box (KRAB) domain14 is predicted to precede the INT domain, while hATd and SCAN domains are not detected. The availability of multiple ESTs for the two SCAND3 and KRBA2 gene variants, and strong selective constraints acting on their coding sequence,6 suggests that they are functional genes with an unknown cellular role. The two N-terminal zinc-finger domains (i.e., SCAN and KRAB) of their encoded products are typically associated with transcriptional regulation of gene expression. It is known that the KRAB domain acts as a platform to recruit transcriptional repressor complexes (including histone deacetylases) involved in maintenance of the nucleolus, cell differentiation, cell proliferation, apoptosis and neoplastic transformation.14-16 In addition, recent studies have identified KRAB enzymes acting in transcriptional repression of exogenous and endogenous retroviruses.17,18 No precise cellular function has been directly assigned to SCAND3 but its SCAN N-terminus domain is known to play a role in transcriptional regulation of genes involved in metabolism, cell survival and differentiation.11,19 The SCAN domain often co-exists with KRAB in diverse transcription factors,19 and it is thought to be evolutionarily derived from the gag-like proteins encoded by LTR retroelements.20,21 In this article we analyze the transposon-derived origins of the C-INT group constituted by SCAND3 and KRBA2, which based on their respective N-terminal domains were formally classified as putative transcription factors along with other mammalian proteins (for more detailed review, see ref. 19). Taking this into primary consideration, the term SCAN/KRAB C-INTs will be thus used throughout this article when collectively referring to SCAND3 and KRBA2. The largest component of SCAN/KRAB C-INTs is their common INT domain thought to be related to LTR retroelements INTs and/or to Maverick/Polinton TPases.6,7 However, in a previous effort to annotate LTR retrotransposons in the pea aphid genome,22,23 we found high similarity (e-value > 1e-60 in Blast searches) between SCAN/KRAB C-INTs and a group of poorly characterized TPases distantly related to LTR retroelement INTs and the Maverick/Polinton TPases. This preliminary observation prompted us to investigate in more details the evolutionary history of SCAN/KRAB C-INTs and clarify their relationship to other INTs and TPases (IN/TPases).
Phylogenetic analysis based on all known to date IN/TPase groups related to the INTPase domain of SCAND3 and KRBA2 C-INTs (Suppl. Materials 1 and 2) using the Maximum likelihood (ML) method24 resulted in a tree, provided as Supplementary Material 3. Overall the phylogenetic relationship of the major INT/TPase groups was consistent with what was previously known (see refs. 25 and 26 or visit the GyDB Project27 for more detailed information). Moreover, the phylogeny places with high statistical support the INT/TPase cores of SCAND3 and KRBA2 as two sibling clades within the major group of GINGER2 TPases. Fob1p (a fungal host protein) and Tdd-like elements (amoebozoan transposons) also fall within the GINGER2 branch but both appear to be more distantly related to GINGER2 TPases. Figure 1A provides further support to this scenario through a ML phylogenetic tree based only on the GINGER2 branch and their relatives including SCAND3 and KRBA2. In this analysis, the latter form two sister clades clustering with diverse GINGER2 IN/TPases from hemipteran insects such as A. pisum and Rhodnius prolixus, and from colepteran and lepidopteran insects such as Tribolium castaneum and Bombyx mori. Consistent with these results, comparative analyses based on tBLASTn searches28 against the whole-genome shotgun (WGS) (and other) databases of NCBI revealed strong levels of similarity between SCAND3 and KRBA2 and the full-length translated protein of insect GINGER2 transposons (e-values as significant as 10e−90, see also alignment in Figure 2B). In summary, and contrary to previous hypotheses,6,7 the IN/TPase core domain of SCAN/KRAB genes does not appear to be derived from either Maverick or a LTR elements but from the domestication of a full-length GINGER2 transposase probably recruited by horizontally transfer from insect to mammal (see below).
Figure 1. GINGER2 origin of SCAND3 and KRBA2 based on their IN/TPase domain. (A) Inferred phylogenetic ML tree based on GINGER2, SCAN/KRAB, Fob1 and Tdd-like IN/TPases using the latter as an outgroup clade. To the left, the name or acronym of each analyzed sequence is detailed, and accompanied by host genome information. Boostrap values up to 60 are detailed in the figure. (B) Multiple alignment showing the strong similarity between GINGER2 and SCAN/KRAB IN/TPases. The typical INT core shared with other IN/TPases is delimited by arrows.
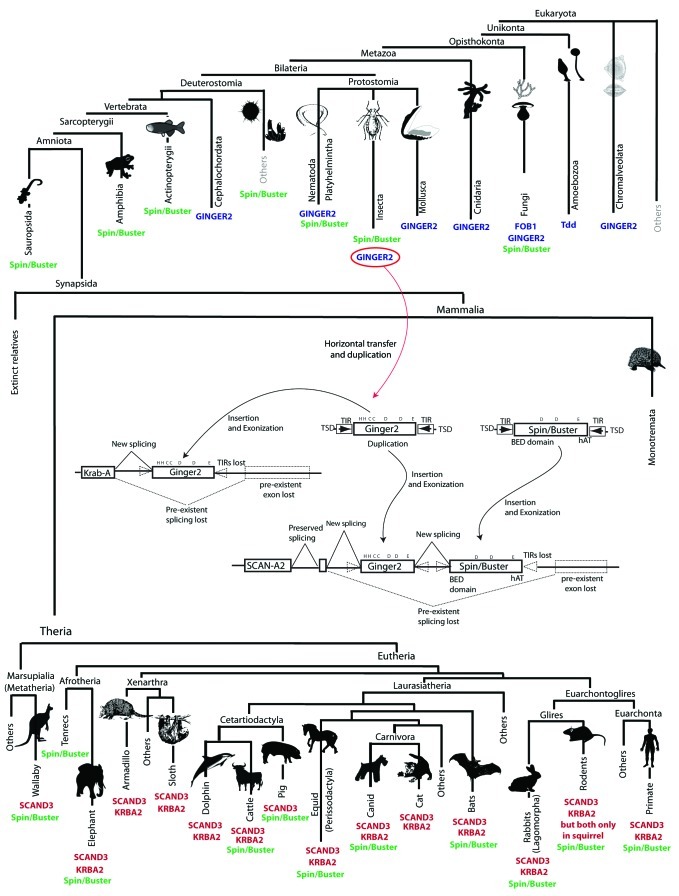
Figure 2. Taxonomic host distribution of SCAN/KRAB cellular-integrases and their related transposons integrated in a tree of life simplified representation. Branches are not to scale. GINGER2 and related elements are summarized in blue, while those of Buster elements are in green and SCAND3 and KRBA2 are red. To implement information about Spin/Buster transposons based on the survey of distinct works29,39-43 or performed distinct BLASTp or tBLASTn searches28 to the NCBI10 non-redundant (NR), genomic, WGS and high throughput genomic (HTGS) databases.
SCAND3 is not only derived from a GINGER2 transposon but from components of two additional MGEs. The first MGE-derived component is the N-terminal SCAN domain, which recently has been demonstrated to have evolved from the gag gene of LTR retroelements.20,21 In addition, the C-terminus of SCAND3 is derived from a distinct transposase (Charlie10) of the hAT superfamily most closely related to the Spin/Buster subgroup29 In an attempt thus to discuss the timing and mechanisms of assembly of SCAND3 and KRBA2 in mammals, we integrated the results of our phylogenetic analysis and the distribution of these two genes onto a simplified tree of life (Fig. 2). According to this reconstruction, the presence of SCAND3 and KRBA2 in almost all eutherian genomes (including the basal mammalian subgroups, afrotherians and xenarthans) and the detection of SCAND3 in at least the wallaby, suggest that SCAN/KRAB C-INTs (or at least SCAND3) might have originated prior to the eutherian-metatherian split (145–65 million years ago30,31). Thus, SCAND3 and KRBA2 are restricted to therian mammals (eutherians and metatherians) and their respective phylogenies follow that expected for single copy genes vertically inherited from a therian ancestor. We found no trace of either genes in Mus musculus and Rattus norvegicus which suggests that they were both lost in the lineage of murine rodents. Additional screenings performed on Ensembl databases suggest that while Cavia porcellus (guinea pig) apparently lack both genes, Ictidomys tridecemlineatus (the squirrel) has preserved both genes (according to the assembly spetri2 accessions JH393724.1 and and JH393405.1 for SCAND3 and KRBA2, respectively). These observations suggest that while the Sciuridae (represented by the squirrel) preserve intact copies of both genes, the Muroidea (rats, mouses etc) and apparently the Hystricomorpha (the guinea pig) lost SCAND3 and KRBA2 probably after the Rodentia split into their diverse suborders. Interestingly, the draft genome sequence of Loxodonta africana (the elephant) contains a single copy of KRBA2 but at least three SCAND3 copies distributed in three different genomic scaffolds (for more details see methods) thus suggesting lineage-specific triplication of this gene. The situation in metatherians (marsupials) is less clear because draft genome sequences are available for only two species; Monodelphis domestica (opossum) and Macropus eugenii (wallaby). The former apparently lacks both genes, while we detected a copy of SCAND3 (still incomplete, as the information is based on a truncated sequence containing the SCAN domain and the INT/TPase central core) in the wallaby. Therefore, it remains unclear whether both genes are truly missing in opossum and whether the SCAND3-like sequence from wallaby represents an ortholog of the gene seen in eutherians. Further genomic data for marsupials will help to determine with more precision the evolutionary origin of both genes in mammals, for example whether SCAND3 is older than KRBA2. Regarding the disclosed transposon counterpart of the SCAND3 SCAN/KRAB IN/TPase, database searches suggest that GINGER2 transposons are common in prostostomes and cnidarians and that they also occur in some basal marine deuterostomes and a few protists. We did not detect any GINGER2 transposon sequence in any vertebrate taxa (except the SCAN/KRAB IN/TPase domain). In turn, Spin/Buster elements are found in a wide range of metazoans although Charlie10 is by now a Spin/Buster element specific of mammals. In similar terms, the distribution of SCAN and KRAB domains, is known to be restricted to vertebrates.19 Together, these observations suggest that the common SCAN/KRAB IN/TPase might derive from an ancient horizontal transfer of a GINGER2 transposon, most likely acquired from insect, to the common therian ancestor, while the SCAN and KRAB domains as well as the hAT Buster TPase of SCAND3 were contributed by the mammalian host genome. From that point on, we speculate about a plausible gene duplication of the ancestral GINGER2 precursor (note that the two SCAND3 and KRBA2 IN/TPase domains are siblings) triggered a subsequent process of exonization. Supporting our hypothesis, annotations of KRBA2 in distinct genomes usually reveal two exons. The first includes the KRAB domain and the second encodes for the whole IN/TPase domain. Similarly, SCAND3 is arranged in four exons where exon 1 and exon 2 contain the SCAN domain, while exon 3 contains the entire IN/TPase domain and exon 4 the full-length Spin/Buster TPase (for a representation, see Suppl. Material 3). Indeed, exonization of MGEs has been shown to be an interesting mechanism for the enrichment of several mammalian genomes that might be involved, or at least correlated, with diverse events of speciation. Along these lines, the processess of transposon exonization that apparently conducted to the emergence of SCAN/KRAB C-INTs constitute an interesting example of how nature has been capable to shape the complexity of mammals during evolution by co-opting and mixing full-length MGEs from distinct sources to acquire new functionalities. The question concerning SCAND3 and KRBA2 is, which functionalities?
The fact that both SCAN and KRAB domains can be typically associated with transcriptional regulation of gene expression suggests that SCAND3 and KRBA2 are transcription factors. However, SCAN and KRAB are only the N-terminal domains of SCAND3 and KRBA2 which are mainly defined by a common GINGER2 core, and in the case of SCAND3, by an additional Spin/Buster core. The high degree of preservation of these transposase cores suggests that SCAN/KRAB C-INTs are indeed functional genes. Such a functionality does not appear to be however based on conventional “cut-and-paste” transposable activity, as in that case we would expect to find more than one SCAND3 and KRBA2 copy per host genome. Therefore, the so called term of C-INTs coined by Gao and Voytas (ref. 6) is appropiate when talking about SCAND3 and KRBA2. Among the distinct cellular functions we might assign to these enzymes the most attractive one is perhaps a defensive role against the recombinant activities of other mobile elements. On one side, this hypothesis is supported by previous works revealing that DDE enzymes can play diverse roles in acquired and innate immunity (see for instance ref. 32). On the other hand, it has been shown that several KRAB carrying proteins play a role in transcriptional regulation of exogenous and endogenous retroviruses.17,18 Moreover, a recent study33 exposed the existence of positive correlation between the number of LTR retroelements and the number of tandems of zinc-finger genes (most of these are carriers of KRAB and/or SCAN domains) across vertebrate genomes. The idea of a defensive role against retroviral infections is indeed plausible and has also been proposed when speculating with the existence in vertebrates of other single-copy GINGER1-like genes such as GIN1,34 GIN2,26,35 and cGIN1.36 There is no evidence as to whether the two genes examined herein might have similar role in mammals. Along similar lines, domesticated transposases such as Metnase/SETMAR37 and Fob138 have been shown to play distinct roles in DNA repair in the genomes of primates and yeasts, respectively. The former has no relationship with SCAN/KRAB C-INTs but Fob1 not only share evolutionary history with SCAN/KRAB C-INTs and GINGER2 transposons but that it is phylogenetically close to them in the INT/TPase tree (see, Suplementary Material 3 and ref. 6). Further experimental studies on SCAN/KRAB C-INTs and other INT/TPases such as GIN1, GIN-2, and CGIN1 will be important to unravel novel and yet unknown biological details concerning the co-evolution of the mobile DNA and the genomic complexity of their hosts.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research has been partly supported by Grants IDI-20100007 from CDTI (Centro de Desarrollo Tecnológico Industrial), IMIDTA/2009/118 from IMPIVA and SAF2009-13032-C02-01 and SAF2012-31187 from ERDF (European Regional Development Fund), and Torres-Quevedo Grants PTQ-09-01-00020 and PTQ-10‑03552 from MICINN (Ministerio de Ciencia e Innovación) and Prometeo/2009/092 from Generalitat Valenciana.
Glossary
Abbreviations:
- C-INT
cellular integrase
- CDD
conserved domain database
- HTGS
high throughput genomic sequencing
- HSPs
high-scoring segment pairs
- INT
integrase
- IN/TPase
Integrase-transposase
- JTT
Jones, Taylor and Thornton
- KRAB
Krüppel-associated box
- ML
naximum likelihood
- MRC
majority rule consensus
- MGEs
mobile genetic elements
- NR
non-redundant
- TIR
terminal inverted repeat
- TPase
transposase
- TSDs
target site duplications
- WGS
whole-genome shotgun
Supplemental Materials
Supplemental materials may be found here: http://www.landesbioscience.com/journals/mge/article/22914/
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/22914
References
- 1.Volff JN. Turning junk into gold: domestication of transposable elements and the creation of new genes in eukaryotes. Bioessays. 2006;28:913–22. doi: 10.1002/bies.20452. [DOI] [PubMed] [Google Scholar]
- 2.Rebollo R, Horard B, Hubert B, Vieira C. Jumping genes and epigenetics: Towards new species. Gene. 2010;454:1–7. doi: 10.1016/j.gene.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Kister KP, Eckert WA. Characterization of an authentic intermediate in the self-splicing process of ribosomal precursor RNA in macronuclei of Tetrahymena thermophila. Nucleic Acids Res. 1987;15:1905–20. doi: 10.1093/nar/15.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sela N, Mersch B, Hotz-Wagenblatt A, Ast G. Characteristics of transposable element exonization within human and mouse. PLoS One. 2010;5:e10907. doi: 10.1371/journal.pone.0010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorek R. The birth of new exons: mechanisms and evolutionary consequences. RNA. 2007;13:1603–8. doi: 10.1261/rna.682507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Voytas DF. A eukaryotic gene family related to retroelement integrases. Trends Genet. 2005;21:133–7. doi: 10.1016/j.tig.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Feschotte C, Pritham EJ. Non-mammalian c-integrases are encoded by giant transposable elements. Trends Genet. 2005;21:551–2. doi: 10.1016/j.tig.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Pritham EJ, Putliwala T, Feschotte C. Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene. 2007;390:3–17. doi: 10.1016/j.gene.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Kapitonov VV, Jurka J. Self-synthesizing DNA transposons in eukaryotes. Proc Natl Acad Sci U S A. 2006;103:4540–5. doi: 10.1073/pnas.0600833103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2010;38(Database issue):D46–51. doi: 10.1093/nar/gkp1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sander TL, Stringer KF, Maki JL, Szauter P, Stone JR, Collins T. The SCAN domain defines a large family of zinc finger transcription factors. Gene. 2003;310:29–38. doi: 10.1016/S0378-1119(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 12.Rubin E, Lithwick G, Levy AA. Structure and evolution of the hAT transposon superfamily. Genetics. 2001;158:949–57. doi: 10.1093/genetics/158.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman AB, Perez ZN, Zhou L, Musingarimi P, Ghirlando R, Hinshaw JE, et al. Molecular architecture of a eukaryotic DNA transposase. Nat Struct Mol Biol. 2005;12:715–21. doi: 10.1038/nsmb970. [DOI] [PubMed] [Google Scholar]
- 14.Bellefroid EJ, Poncelet DA, Lecocq PJ, Revelant O, Martial JA. The evolutionarily conserved Krüppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc Natl Acad Sci U S A. 1991;88:3608–12. doi: 10.1073/pnas.88.9.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 16.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 17.Groner AC, Meylan S, Ciuffi A, Zangger N, Ambrosini G, Dénervaud N, et al. KRAB-zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet. 2010;6:e1000869. doi: 10.1371/journal.pgen.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe HM, Trono D. Dynamic control of endogenous retroviruses during development. Virology. 2011;411:273–87. doi: 10.1016/j.virol.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Edelstein LC, Collins T. The SCAN domain family of zinc finger transcription factors. Gene. 2005;359:1–17. doi: 10.1016/j.gene.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Emerson RO, Thomas JH. Gypsy and the birth of the SCAN domain. J Virol. 2011;85:12043–52. doi: 10.1128/JVI.00867-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov D, Stone JR, Maki JL, Collins T, Wagner G. Mammalian SCAN domain dimer is a domain-swapped homolog of the HIV capsid C-terminal domain. Mol Cell. 2005;17:137–43. doi: 10.1016/j.molcel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 22.International Aphid Genomics Consortium Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8:e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernet GP, Muñoz-Pomer A, Domínguez-Escribá L, Covelli L, Bernad L, Ramasamy S, et al. GyDB mobilomics: LTR retroelements and integrase-related transposons of the pea aphid Acyrthosiphon pisum genome. Mob Genet Elements. 2011;1:97–102. doi: 10.4161/mge.1.2.17635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldrich JRA. Fischer and the making of Maximum Likelihood 1912-1922. Stat Sci. 1997;12:162–76. doi: 10.1214/ss/1030037906. [DOI] [Google Scholar]
- 25.Llorens C, Muñoz-Pomer A, Bernad L, Botella H, Moya A. Network dynamics of eukaryotic LTR retroelements beyond phylogenetic trees. Biol Direct. 2009;4:41. doi: 10.1186/1745-6150-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao W, Kapitonov VV, Jurka J. Ginger DNA transposons in eukaryotes and their evolutionary relationships with long terminal repeat retrotransposons. Mob DNA. 2010;1:3. doi: 10.1186/1759-8753-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llorens C, Futami R, Covelli L, Domínguez-Escribá L, Viu JM, Tamarit D, et al. The Gypsy Database (GyDB) of mobile genetic elements: release 2.0. Nucleic Acids Res. 2011;39(Database issue):D70–4. doi: 10.1093/nar/gkq1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arensburger P, Hice RH, Zhou L, Smith RC, Tom AC, Wright JA, et al. Phylogenetic and functional characterization of the hAT transposon superfamily. Genetics. 2011;188:45–57. doi: 10.1534/genetics.111.126813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wible JR, Rougier GW, Novacek MJ, Asher RJ. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature. 2007;447:1003–6. doi: 10.1038/nature05854. [DOI] [PubMed] [Google Scholar]
- 31.Cifelli RL, Gordon CL. Evolutionary biology: re-crowning mammals. Nature. 2007;447:918–20. doi: 10.1038/447918a. [DOI] [PubMed] [Google Scholar]
- 32.Dreyfus DH. The DDE recombinases: diverse roles in acquired and innate immunity. Ann Allergy Asthma Immunol. 2006;97:567–76, quiz 576-8, 602. doi: 10.1016/S1081-1206(10)61083-6. [DOI] [PubMed] [Google Scholar]
- 33.Thomas JH, Schneider S. Coevolution of retroelements and tandem zinc finger genes. Genome Res. 2011;21:1800–12. doi: 10.1101/gr.121749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloréns C, Marín I. A mammalian gene evolved from the integrase domain of an LTR retrotransposon. Mol Biol Evol. 2001;18:1597–600. doi: 10.1093/oxfordjournals.molbev.a003947. [DOI] [PubMed] [Google Scholar]
- 35.Marín I. GIN transposons: genetic elements linking retrotransposons and genes. Mol Biol Evol. 2010;27:1903–11. doi: 10.1093/molbev/msq072. [DOI] [PubMed] [Google Scholar]
- 36.Marco A, Marín I. CGIN1: a retroviral contribution to mammalian genomes. Mol Biol Evol. 2009;26:2167–70. doi: 10.1093/molbev/msp127. [DOI] [PubMed] [Google Scholar]
- 37.Shaheen M, Williamson E, Nickoloff J, Lee SH, Hromas R. Metnase/SETMAR: a domesticated primate transposase that enhances DNA repair, replication, and decatenation. Genetica. 2010;138:559–66. doi: 10.1007/s10709-010-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dlakić M. A model of the replication fork blocking protein Fob1p based on the catalytic core domain of retroviral integrases. Protein Sci. 2002;11:1274–7. doi: 10.1110/ps.4470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert C, Pace JK, Feschotte C. Horizontal SPINning of transposons. Commun Integr Biol. 2009;2:117–9. doi: 10.4161/cib.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pace JK, 2nd, Gilbert C, Clark MS, Feschotte C. Repeated horizontal transfer of a DNA transposon in mammals and other tetrapods. Proc Natl Acad Sci U S A. 2008;105:17023–8. doi: 10.1073/pnas.0806548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilbert C, Schaack S, Pace JK, 2nd, Brindley PJ, Feschotte C. A role for host-parasite interactions in the horizontal transfer of transposons across phyla. Nature. 2010;464:1347–50. doi: 10.1038/nature08939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feschotte C. Opinion: Transposable elements and evolution of regulatory networks. Nat Rev Genet. 2008;95:397–405. doi: 10.1038/nrg2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pace JK, 2nd, Feschotte C. The evolutionary history of human DNA transposons: evidence for intense activity in the primate lineage. Genome Res. 2007;17:422–32. doi: 10.1101/gr.5826307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.