Abstract
There is a fundamental gap in our understanding of how horizontal gene transfer contributes to the enormous range of genetic variations that are observed among bacteria. The objective of our study was to better understand how the acquisition of genetic material by natural transformation is regulated within a population of Vibrio cholerae cells. V. cholerae is an aquatic bacterium and a facultative human pathogen. It acquires natural competence for transformation in response to changing environmental signals, such as the presence of chitinous surfaces, the absence of monomeric sugars and quorum sensing-linked autoinducers. The latter play a distinctive role in V. cholerae as they fine-tune a switch from the degradation of extracellular DNA toward the uptake of intact DNA strands in competence-induced cells. The link between quorum sensing and natural competence for transformation will be discussed. Furthermore, we speculate on the overrepresentation of transformation-negative strains of V. cholerae in patient-derived culture collections, which might be the result of a biased sampling strategy as virulence and natural transformation are contrarily regulated by the quorum sensing network.
Keywords: horizontal gene transfer, chitin-induced natural competence, transformation, quorum sensing, V. cholerae
The Regulatory Network of Natural Competence and Transformation of Vibrio cholerae
Recombination between the bacterial chromosome and DNA fragments that enter the cell by horizontal gene transfer (HGT) can either replace damaged or mutated alleles by the original alleles, thereby repairing the gene, or transfer mutated alleles or even new genes to naïve strains. Thus, HGT plays a key role in the transfer of genetic information from one bacterium to another and in the balance between genome maintenance and evolution. Natural transformation is one of three modes of HGT in prokaryotes. Large pieces of DNA containing a series of genes can be transferred by natural transformation without any need for direct interaction with other microbes or the intercession of mobile genetic elements.
Many bacterial species are naturally competent for transformation, including the human pathogen Vibrio cholerae1 (for recent reviews on competence induction in Gram-positive and Gram-negative bacteria, see refs. 2, 3, 4). The physiological state of natural competence of V. cholerae is linked to its primary niche, the aquatic environment. Within this habitat V. cholerae is frequently found attached to the exoskeleton of zooplankton.5,6 The building block of such exoskeletons is the polymer chitin, which is the natural inducer of natural competence in V. cholerae.1 However, recent studies have demonstrated that even though chitin is essential for competence induction of V. cholerae, it is not sufficient.1,7-15 Additional environmental factors also contribute to the onset of natural competence in V. cholerae and the transformation process.4 These factors include the accumulation of intracellular cAMP, which concomitantly allows for activation of the cAMP receptor protein CRP. CRP-bound cAMP is essential in at least three steps of natural competence and transformation: chitin colonization, chitin degradation and competence gene expression.14 Finally, we and others have demonstrated that quorum sensing (QS) also plays a key role in natural competence and transformation of V. cholerae.1,7,12-15
In this context, we recently investigated the link between QS on one hand and competence induction and natural transformation on the other hand.15 In this study, we took advantage of a chitin-independent competence induction system, which allowed us to precisely investigate the correlation between QS and competence/transformation at both the transcriptional and protein level. As previously suggested,1 we confirmed that the main regulator of QS, HapR, is absolutely crucial for natural competence and transformation.15 However, more importantly, we showed an inverse correlation between the abundance of the HapR protein and the nuclease Dns using western blot analysis. Furthermore, we demonstrated that only a small number of genes involved in natural competence and transformation are regulated in a QS-dependent manner; such QS-regulated competence genes include comEA, which encodes a putative periplasmic DNA-binding protein,1 and comEC, which encodes a “DNA internalization-related competence protein.”16 Both of these proteins, ComEA and ComEC, show some degree of homology to their counterparts in Bacillus subtilis.12,17,18 We therefore concluded that a QS-mediated switch occurs in V. cholerae, which determines the fate of the surrounding DNA (Fig. 1). Specifically, at low cell density and low autoinducer concentrations, V. cholerae does not produce sufficient HapR protein.19 Thus, the extracellular nuclease-encoding gene dns is fully transcribed and translated, and the secreted enzyme degrades any free DNA that surrounds the bacteria and could potentially serve as transforming material (Fig. 1, left panel).7,15 This is counterproductive to natural transformation, which requires intact DNA strands to allow homologous recombination within the cell. Thus, natural transformation of V. cholerae does not occur at low cell density1,7 or within mutants mimicking a low cell density state, such as V. cholerae variants that are defective in autoinducer synthesis12,15 or devoid of the HapR protein.1,7,12,13,15 However, at high cell density (Fig. 1, right panel), the accumulated QS regulator HapR represses the dns gene,7 and the Dns protein level quickly diminishes15 (Fig. 1, right panel). Concomitantly, the expression of comEA and comEC is strictly dependent on positive regulation by HapR.15 These two proteins are predicted to be directly involved in DNA uptake. Thus, a switch from DNA degradation toward the uptake of intact DNA fragments is possible once the cells reach the high cell density state (Fig. 1, right panel).
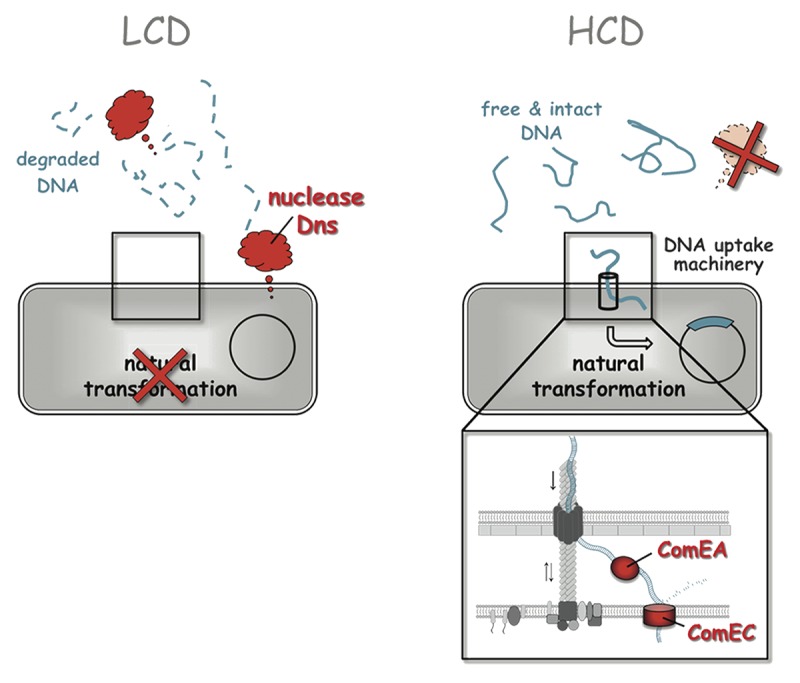
Figure 1. Quorum sensing regulates the expression of transformation-related genes, such as dns and comEA/comEC. The schematic demonstrates the quorum sensing-mediated switch between DNA degradation at low cell density (LCD, left panel) and DNA uptake by naturally competent V. cholerae cells at high cell density (HCD, right panel). For details, see the text.
The data described above were obtained using a uniform and chitin-independent competence-inducing system.15 However, in the aquatic environment and upon colonization of the chitinous exoskeleton of small zooplankton, the conditions are most likely more heterogeneous and fluctuate over time and space. Thus, it was not surprising that the induction of the competence genes, including those driven by QS, seemed rather heterogeneous under chitin surface-inducing conditions, with the majority of cells within this bacterial population being devoid of competence gene expression.15 We hypothesize that such heterogeneity is caused by the uneven distribution of autoinducers, chitin degradation products and N-acetyl-glucosamine monomers, the latter of which has a negative effect on intracellular cAMP levels.14,15 V. cholerae cells grown under chitin-dependent but surface-independent batch culture conditions behaved differently, thus supporting our hypothesis: in this scenario, the more homogenous competence gene expression pattern within the bacterial population mimicked the expression pattern of housekeeping genes.15
However, if QS plays a major role in natural transformation by serving as a switching mechanism from DNA degradation to DNA uptake, why are so many V. cholerae strains defective in QS and thus not naturally transformable1,20? Most notably, strain N16961, the first sequenced strain of V. cholerae,16 contains a frameshift mutation in hapR, which renders HapR non-functional.21 The main argument for this hapR frameshift mutation being the reason why N16961 was not naturally transformable was based on natural transformation being fully restorable in this strain solely by adding a functional copy of hapR.1 Furthermore, in a more recent study, Jun Zhu and coworkers evaluated hapR sequences from different V. cholerae isolates and found that many of these isolates had mutations in the hapR gene.22 These mutations either caused single amino acid exchanges or resulted in shifts of the reading frame, thereby changing and often shortening the encoded HapR protein.22 Not surprisingly, all representatives of such hapR mutant strains that we tested for chitin-induced competence were non-transformable (ref. 1 and Blokesch, unpublished). Based on all of these isolates having different mutations in hapR,22 the clonal expansion of one successful hapR mutant can be excluded. However, does that mean that natural competence and transformation are not important for V. cholerae in its natural environment? We hypothesize that this is not the case, as Staffan Kjelleberg and collaborators recently showed that hapR-defective V. cholerae strains have a fitness disadvantage against protozoan predation.23 Thus, a counter-selection against QS-defective isolates can be envisioned in the aquatic environment, which assures that QS is kept intact in the population. In accordance with this theory, V. cholerae strains in the environment will most likely have a functional link between QS and natural transformation. This hypothesis is consistent with the finding that environmental strains of V. cholerae isolated from the Californian coast are naturally transformable upon chitin induction.24
A Biased Sampling Strategy Might Result in an Overrepresentation Of Non-Transformable V. cholerae Strains in Patient-Derived Culture Collections
However, why are mutations within hapR so abundant in patient isolates? We hypothesize that this is the result of a biased patient sampling strategy. We base this hypothesis on the following facts. HapR only accumulates in the bacterium upon reaching a high cell density state; at this point, HapR leads to the downregulation of virulence genes [including those encoding the toxin-coregulated pilus (TCP) and cholera toxin (CT)] and repression of biofilm formation.21,25,26 However, such a downregulation would fail in hapR mutant strains that arise within the intestine of infected people. It has previously been speculated that “elimination of HapR […] may be an evolutionary step that improves V. cholerae adaptation to the human host” by “prolonging TCP and CT expression even at high cell density.”21 We suggest that this scenario might rather be the exception to the rule and that most patients recover from the disease upon the QS-dependent downregulation of virulence and onset of the mucosal escape response.27 However, patients carrying QS-defective V. cholerae strains will have worse symptoms due to the high bacterial load and the continuous production of cholera toxin and might therefore end up in a hospital environment for proper treatment. At the hospital, the chances of a patient being sampled for the V. cholerae strain associated with the disease is most likely significantly higher than for patients being treated in less well-equipped cholera treatment units/centers. This might, in the end, lead to most cholera patient-derived culture collections, at least from developing countries, containing an over-representative high proportion of QS-defective strains. In contrast, cholera patients are less frequently observed in developed countries, and when they are, the presence of V. cholerae is always confirmed, even in cases of only mild (or even no) symptoms. One prominent example for this is the common laboratory strain V. cholerae (92)A1552,28 which the Schoolnik laboratory initially obtained from the California Department of Health Services. The health authorities had isolated the strain and most likely other V. cholerae strains after several cholera cases appeared in the US in 1992. A link between those cholera cases in the US and a severe cholera outbreak in South America soon became obvious: the bacteria were ingested due to contaminated food served on an aircraft.29 More specifically, 336 passengers were on an airplane between Lima and Los Angeles while a major cholera epidemic took place in Peru.30 Out of the 336 passengers, 194 were identified in the US and tested for V. cholerae infection, and 100 passengers were carriers of V. cholerae O1 El Tor; 75 of those people were reported to have diarrhea. Fortunately, only 10 patients required hospitalization, of whom a 70-y old man died.29 The V. cholerae strain A1552 isolated from this outbreak is functional for QS and does not contain any mutation within the hapR gene. Notably, this was the strain in which chitin-induced natural transformation of V. cholerae was initially discovered.1 Thus, the negative correlation between the severity of cholera symptoms, often caused by a defective QS system of the pathogen, and the inability of those strains to quorum sense and thus undergo natural transformation might explain why natural transformation of V. cholerae remained undiscovered for many years. Future studies will shed more light on the complexity of the regulatory network of chitin-induced natural competence and transformation of the human pathogen and environmental bacterium V. cholerae.
Acknowledgments
This work and other studies related to the regulatory network of natural competence and transformation of V. cholerae in the Blokesch lab is supported by Swiss National Science Foundation (SNSF) grant 31003A_127029.
Glossary
Abbreviations:
- HGT
horizontal gene transfer
- QS
quorum sensing
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/22284
References
- 1.Meibom KL, Blokesch M, Dolganov NA, Wu C-Y, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–7. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 2.Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2:241–9. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 3.Claverys JP, Prudhomme M, Martin B. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu Rev Microbiol. 2006;60:451–75. doi: 10.1146/annurev.micro.60.080805.142139. [DOI] [PubMed] [Google Scholar]
- 4.Seitz P, Blokesch M. Cues and regulatory pathways involved in natural competence and transformation in pathogenic and environmental Gram-negative bacteria. FEMS Microbiol Rev. 2012 doi: 10.1111/j.1574-6976.2012.00353.x. In press. [DOI] [PubMed] [Google Scholar]
- 5.Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–31. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 6.Pruzzo C, Vezzulli L, Colwell RR. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol. 2008;10:1400–10. doi: 10.1111/j.1462-2920.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 7.Blokesch M, Schoolnik GK. The extracellular nuclease Dns and its role in natural transformation of Vibrio cholerae. J Bacteriol. 2008;190:7232–40. doi: 10.1128/JB.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marvig RL, Blokesch M. Natural transformation of Vibrio cholerae as a tool--optimizing the procedure. BMC Microbiol. 2010;10:155. doi: 10.1186/1471-2180-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Souza Silva O, Blokesch M. Genetic manipulation of Vibrio cholerae by combining natural transformation with FLP recombination. Plasmid. 2010;64:186–95. doi: 10.1016/j.plasmid.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto S, Morita M, Izumiya H, Watanabe H. Chitin disaccharide (GlcNAc)2 induces natural competence in Vibrio cholerae through transcriptional and translational activation of a positive regulatory gene tfoXVC. Gene. 2010;457:42–9. doi: 10.1016/j.gene.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Izumiya H, Mitobe J, Morita M, Arakawa E, Ohnishi M, et al. Identification of a chitin-induced small RNA that regulates translation of the tfoX gene, encoding a positive regulator of natural competence in Vibrio cholerae. J Bacteriol. 2011;193:1953–65. doi: 10.1128/JB.01340-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suckow G, Seitz P, Blokesch M. Quorum sensing contributes to natural transformation of Vibrio cholerae in a species-specific manner. J Bacteriol. 2011;193:4914–24. doi: 10.1128/JB.05396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonova ES, Hammer BK. Quorum-sensing autoinducer molecules produced by members of a multispecies biofilm promote horizontal gene transfer to Vibrio cholerae. FEMS Microbiol Lett. 2011;322:68–76. doi: 10.1111/j.1574-6968.2011.02328.x. [DOI] [PubMed] [Google Scholar]
- 14.Blokesch M. Chitin colonization, chitin degradation and chitin-induced natural competence of Vibrio cholerae are subject to catabolite repression. Environ Microbiol. 2012;14:1898–912. doi: 10.1111/j.1462-2920.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 15.Lo Scrudato M, Blokesch M. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet. 2012;8:e1002778. doi: 10.1371/journal.pgen.1002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–83. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inamine GS, Dubnau D. ComEA, a Bacillus subtilis integral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J Bacteriol. 1995;177:3045–51. doi: 10.1128/jb.177.11.3045-3051.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draskovic I, Dubnau D. Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol Microbiol. 2005;55:881–96. doi: 10.1111/j.1365-2958.2004.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blokesch M, Schoolnik GK. Serogroup conversion of Vibrio cholerae in aquatic reservoirs. PLoS Pathog. 2007;3:e81. doi: 10.1371/journal.ppat.0030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002;99:3129–34. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joelsson A, Liu Z, Zhu J. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect Immun. 2006;74:1141–7. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matz C, McDougald D, Moreno AM, Yung PY, Yildiz FH, Kjelleberg S. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc Natl Acad Sci U S A. 2005;102:16819–24. doi: 10.1073/pnas.0505350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MC, Keymer DP, Avelar A, Boehm AB, Schoolnik GK. Detection and transformation of genome segments that differ within a coastal population of Vibrio cholerae strains. Appl Environ Microbiol. 2007;73:3695–704. doi: 10.1128/AEM.02735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–56. doi: 10.1016/S1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 26.Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun. 2007;75:5542–9. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen AT, Dolganov NA, Otto G, Miller MC, Wu CY, Schoolnik GK. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2006;2:e109. doi: 10.1371/journal.ppat.0020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yildiz FH, Schoolnik GK. Role of rpoS in stress survival and virulence of Vibrio cholerae. J Bacteriol. 1998;180:773–84. doi: 10.1128/jb.180.4.773-784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eberhart-Phillips J, Besser RE, Tormey MP, Koo D, Feikin D, Araneta MR, et al. An outbreak of cholera from food served on an international aircraft. Epidemiol Infect. 1996;116:9–13. doi: 10.1017/S0950268800058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC From the Centers for Disease Control. Cholera--Peru, 1991. JAMA. 1991;265:1232. doi: 10.1001/jama.265.10.1232. [DOI] [PubMed] [Google Scholar]


