Abstract
Research on the involvement of retroelements in developmental processes has been gaining momentum recently; however, most of the studies published so far have been focused on embryonic development. This commentary presents two recent papers, which document significant changes in transcriptional activity of retroelements in two different model systems, salamander limb regeneration and regeneration of radial organs in the sea cucumber Holothuria glaberrima. We hypothesize that transcriptional activity of the retrotransposons can be specifically controlled by the host and may play some hitherto unrecognized role in regeneration.
Keywords: LTR retrotransposons, differential gene expression, regeneration, echinoderms, holothurian, nervous system
Regeneration represents a unique biological phenomenon of re-initiation of developmental processes in animals in response to various kinds of traumatic injuries. The ability to regrow injured body parts is widely, but unevenly represented in the animal kingdom. Therefore, regenerative biology, as a field, has benefited from studies on animals, such as salamanders, echinoderms, planarians and coelenterates, that show amazing feats of regeneration not commonly found in classical animal models such as mice, Drosophila or C. elegans. Understanding gene regulatory mechanisms driving post-traumatic tissue re-growth in these regeneration-competent animals is important for two reasons. First, on a theoretical level, it contributes to our knowledge of fundamental principles of regeneration as a developmental phenomenon, its evolution and relationships with embryogenesis. A related, but more practical reason, is that the research on these model species can eventually inform biomedical studies seeking cures for diseases and disabilities.
Research in regenerative biology, as in developmental biology in general, has been mostly dominated by studies of protein-coding genes. This emphasis is completely justified given the fact that it is these genes that eventually determine the phenotype. However, there has been a shift in the paradigm in the recent years showing a growing appreciation of the developmental roles played by the genes, whose final products are not cellular proteins. Nowhere is this more evident than in the recent publications of the results of the ENCODE (The Encyclopedia of DNA Elements) project.1,2
Transposable elements occupy a significant portion of eukaryotic genomes and account for a large share of the output of sequencing projects.3 Most biologists generally consider them as “junk” sequences or genomic parasites. However, recent research has shown that activity of at least some of the transposable elements can be beneficial to their hosts.4-6 Of particular relevance to the field of developmental biology is the ability of transcriptionally active retrotransposons (the type of transposable elements producing RNA intermediates in their life cycle) to regulate expression of protein-coding genes of the host at both transcriptional and post-transcriptional levels.4,5,7 Retroelements have recently been reported to perform important functions in embryogenesis by, for instance, affecting pluripotency and cell fate decisions.7,8 In spite of the growing appreciation of the role of transposable elements in development, their involvement in animal regeneration has not been properly addressed until recently, when two papers9,10 aimed to tackle this issue have appeared earlier this year. The papers report transcriptional activity of retroelements in regeneration in two different animal model systems, the regenerating limb of the salamander Ambyostoma mexicanum and the regenerating radial organ complex of the echinoderm Holothuria glaberrima. The two groups of authors, although both showing differential expression of retroelements in regeneration, arrive at different interpretations of their results. This discrepancy stresses the need for further research on the role of transposable elements in post-traumatic recovery.
The first paper by Zhu et al.9 documents an effort to characterize transcriptomic profile at early stages of axolotl limb regeneration, which eventually led to discovery of drastic upregulation of the long interspersed nucleotide element-1 (LINE-1) in dedifferentiating tissues of the regenerate. Some preliminary evidence is also provided that other putative retrotransposons become transcriptionally active in the re-growing limb, but their expression profile may differ from that of LINE-1. The authors do not suggest any sort of function for LINE-1 or other elements in regeneration, but rather consider the transcriptional activity of retrotransposons as a non-specific consequence of general reduction of epigenetic silencing, which allows the dedifferentiating cells to partially return to the germline-like state.
In our paper,10 we describe a serendipitous finding of differential transcriptional activity of another group of retrolelements, long-terminal repeat (LTR) retrotansposons, in radial organ complex regeneration in the sea cucumber H. glaberrima (Fig. 1). Our interest in these animals is determined by the fact that echinoderms are among the best regenerating metazoan animals. As a basal phylum within the monophyletic group Deuterostomia, to which Chordata, our own phylum, also belongs, they stand out as attractive model organisms equally suitable both for evolutionary regenerative biology studies and for biomedicine-related research. We have been investing serious efforts in characterizing cellular processes and molecular events that underlie such an extraordinary regenerative potential in these animals (reviewed in refs. 11–13). One of our ongoing projects involves large-scale characterization of the transcriptome in various regenerating tissues. Analysis of gene expression profile in the regenerating radial organ complex in H. glaberrima showed that the majority of the differentially expressed protein-coding genes were up- or downregulated between two and 4-fold relative to their expression levels in the uninjured animals (Mashanov et al., in preparation). LTR retroelements initially attracted our attention because two of them, which were eventually designated as Gypsy-1_Hg and Gypsy-2_Hg, stood out as dramatically upregulated genes (~54-fold and ~26-fold, respectively). This prompted us to further explore the diversity of transcriptionally active retroelements and characterize their expression patterns in regeneration. Overall, we identified 36 LTR retrotransposons belonging to BEL and Gypsy clades, of which 20 (i.e., more than half) significantly changed their expression level in regeneration (11 were overexpressed, 8 showed downregulation and one element was initially upregulated in the early regenerate, but then showed significant downregulation at later stages). This suggested that differential expression of retroelements in regeneration could be a large-scale phenomenon. We then further focused on Gypsy-1_Hg expression at the tissue level and showed that it was abundantly expressed in both the radial glial cells and neurons of the regenerating central nervous system. Our original concern was that the increased transcriptional activity of the retroelement could eventually result in the death of the host cells due to, for example, deleterious impact on genomic integrity. However, multiple labeling experiments showed that the cells expressing Gypsy-1_Hg transcripts did not undergo cell death, but, instead, contributed to regeneration.
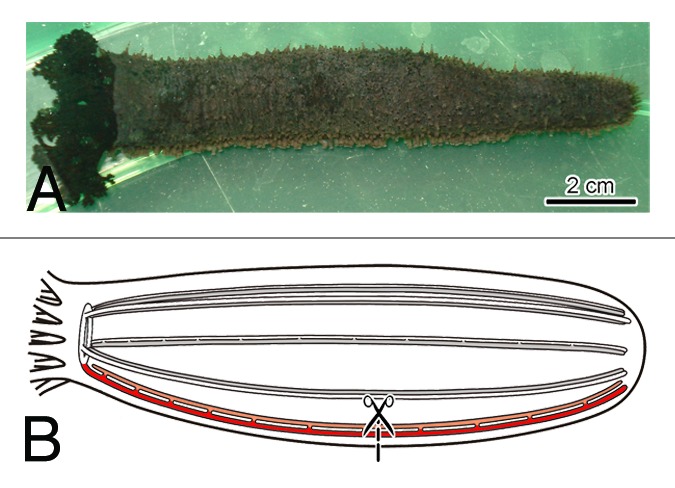
Figure 1. (A) The model organism, Holothuria glaberrima Selenka, 1867 (Echinodermata: Holothuroidea). (B) Injury paradigm. One of the five radial organ complexes (including the radial nerve, coelomic canal and muscle) was cut at about the mid-body level. For clarity, the diagram shows only the nervous system. The injured radial nerve is shaded in red.
Two crucial questions arise at this point: what causes the retroelements to change their expression levels in regenerating tissues, and what is the impact of this expression on the host organism? Unfortunately, we have not been able to provide definite answers due to the lack of the sequenced genome and the absence of established protocols for genetic manipulation for our model organism. Nevertheless, some preliminary insights can be derived from the available data. A traditional view would hold that transposons make use of the global injury-induced chromatin activation and transcriptional de-repression to amplify themselves and thereby increase their odds of surviving the lysis of their host cell to be taken up by other cells.9,14 However, as our data suggest, this might not be the case. First, different LTR retroelements show different kinds of responses during regeneration: besides upregulated retrotransposons, there are also elements, which do not show any change in their expression, and still others, which are less transcriptionally active after the injury than under normal conditions. This suggests that expression of retrotransposons is controlled in an element-specific way. Second, although transposon-derived transcripts are abundantly expressed in the vicinity of the injury, this expression is not ubiquitous, since there are always cells, where retroelements are not transcriptionally active. Therefore, expression of retroelements might be specifically controlled at a single-cell level. Third, our unpublished data suggest that some LTR retrotransposons behave differently in different regenerating organs of the same species, suggesting tissue-specific regulation of transcription. For example, there are significant differences in transcriptional activity of Gypsy-1_Hg and Gypsy-2_Hg between the regenerating digestive tube and the regenerating radial organ complex in the sea cucumber H. glaberrima (compare Fig. 2 of the present manuscript with Fig. 3B in Mashanov et al.10). Fourth, the cells, which extensively express retrotransposon-derived transcripts, do not undergo cell death and contribute to regeneration. Taken together, our findings indicate that the LTR retroelements do not merely exploit the transcriptional machinery of the host to propagate themselves, but rather are specifically controlled by the host, respond in a coordinated way to regeneration cues and thus may play some previously unrecognized roles in post-traumatic tissue re-growth.
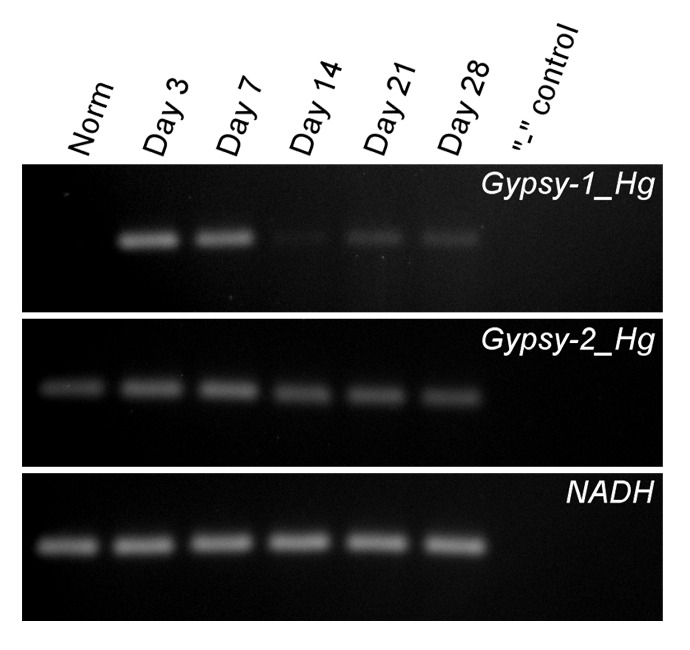
Figure 2. Reverse transcription polymerase chain reaction (RT-PCR) showing temporal expression of Gypsy-1_Hg and Gypsy-2_Hg at different time points of visceral regeneration (Day 3−Day 28) and in the normal (Norm) digestive tube of the sea cucumber H. glaberrima. RT-PCR was performed as described in reference 15. Primer sequences for Gypsy-1_Hg and Gypsy-2_Hg were from reference 10. NADH dehydrogenase subunit 5 was used as an internal control. The gel also includes a no-template control (“-” control) to test for contamination. Whereas Gypsy-1_Hg is not transcriptionally active in the normal gut but overexpressed in regeneration (in particular, at the early time points), Gypsy-2_Hg does not show any significant differences in expression level between the normal and regenerating digestive tube. This contrasts with expression of these genes in the radial organ complex regeneration in the same species (Fig. 3B in Mashanov et al.10), where both retroelements are barely detectable under the normal conditions, but show dramatic upregulation during regeneration.
Transcriptional activity of transposable elements is known to be able to affect expression of protein-coding genes in a variety of ways.5 The specific role of LTR retrotransposons in regeneration remains obscure and can be a promising subject of further research. One of the most important future directions will be developing a mechanistic model of LTR retrotransposon functioning in regeneration. We believe that our data on differential transcriptional activity of these elements in sea cucumber regeneration will attract other investigators working on model systems, where genetic manipulation techniques have been already established. In fact, informal discussions with several fellow researchers suggest that retrotransposon expression during regenerative events is a common phenomenon. That it has not been more widely reported is possibly due to a biased view that these “selfish transcripts” merely contaminate data. (Some experimental analyses even use filters to eliminate the “transposon noise” from the rest of the sequence data before any downstream annotation and/or quantification of the transcripts is performed.)
Thus, future experiments should focus on understanding the nature of the factors that trigger or suppress transcription of the retroelements and show if and how retroelements are integrated in gene regulatory networks. Such studies on a range of different organisms will improve our understanding of both the evolution of regenerative mechanisms in metazoans and on the evolution of relationships between transposable elements and their hosts.
Acknowledgments
This work was supported by NIH (grant numbers: 1SC1GM084770–01 and 1R03NS065275–01), NSF (grant number: IOS-0842870) and the University of Puerto Rico.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/22644
References
- 1.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monaghan JR, Epp LG, Putta S, Page RB, Walker JA, Beachy CK, et al. Microarray and cDNA sequence analysis of transcription during nerve-dependent limb regeneration. BMC Biol. 2009;7:1. doi: 10.1186/1741-7007-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muotri AR, Marchetto MCN, Coufal NG, Gage FH. The necessary junk: new functions for transposable elements. Hum Mol Genet. 2007;16 Spec No. 2:R159–67. doi: 10.1093/hmg/ddm196. [DOI] [PubMed] [Google Scholar]
- 5.Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 2008;9:397–405. doi: 10.1038/nrg2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faulkner GJ, Carninci P. Altruistic functions for selfish DNA. Cell Cycle. 2009;8:2895–900. doi: 10.4161/cc.8.18.9536. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muotri AR, Chu VT, Marchetto MCN, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435:903–10. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- 9.Zhu W, Kuo D, Nathanson J, Satoh A, Pao GM, Yeo GW, et al. Retrotransposon long interspersed nucleotide element-1 (LINE-1) is activated during salamander limb regeneration. Dev Growth Differ. 2012;54:673–85. doi: 10.1111/j.1440-169X.2012.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mashanov VS, Zueva OR, García-Arrarás JE. Posttraumatic regeneration involves differential expression of long terminal repeat (LTR) retrotransposons. Dev Dyn. 2012;241:1625–36. doi: 10.1002/dvdy.23844. [DOI] [PubMed] [Google Scholar]
- 11.Mashanov VS, Zueva OR, Heinzeller T. Regeneration of the radial nerve cord in a holothurian: a promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue Cell. 2008;40:351–72. doi: 10.1016/j.tice.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.García-Arrarás JE, Dolmatov IY. Echinoderms: potential model systems for studies on muscle regeneration. Curr Pharm Des. 2010;16:942–55. doi: 10.2174/138161210790883426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashanov VS, García-Arrarás JE. Gut regeneration in holothurians: a snapshot of recent developments. Biol Bull. 2011;221:93–109. doi: 10.1086/BBLv221n1p93. [DOI] [PubMed] [Google Scholar]
- 14.Wilkins AS. The enemy within: an epigenetic role of retrotransposons in cancer initiation. Bioessays. 2010;32:856–65. doi: 10.1002/bies.201000008. [DOI] [PubMed] [Google Scholar]
- 15.Mashanov VS, Zueva OR, Garcia-Arraras JE. Expression of Wnt9, TCTP, and Bmp1/Tll in sea cucumber visceral regeneration. Gene Expr Patterns. 2012;12:24–35. doi: 10.1016/j.gep.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


