Abstract
Spermidine is a naturally occurring polyamine involved in multiple biological processes, including DNA metabolism, autophagy and aging. Like other polyamines, spermidine is also indispensable for successful reproduction at several stages. However, a direct influence on the actual fertilization process, i.e., the fusion of an oocyte with a spermatocyte, remains uncertain. To explore this possibility, we established the mating process in the yeast Saccharomyces cerevisiae as a model for fertilization in higher eukaryotes. During human fertilization, the sperm capacitates and the acrosome reaction is necessary for penetration of the oocyte. Similarly, sexually active yeasts form a protrusion called “shmoo” as a prerequisite for mating. In this study, we demonstrate that pheromone-induced shmoo formation requires spermidine. In addition, we show that spermidine is essential for mating in yeast as well as for egg fertilization in the nematode Caenorhabditis elegans. In both cases, this occurs independently from autophagy. In synthesis, we identify spermidine as an important mating component in unicellular and multicellular model organisms, supporting an unprecedented evolutionary conservation of the mechanisms governing fertilization-related cellular fusion.
Keywords: Caenorhabditis elegans, spermidine, mating, fertilization, Saccharomyces cerevisiae, shmoo, autophagy, sexual reproduction
Introduction
The alarming decrease in fertility rates across industrialized countries is often interpreted to be rooted in a consciously decided decline of the desired number of children. This, in turn, is often thought to be caused by a change in the social and behavioral lifestyle. However, it seems premature to rely only on a sociopsychological explanation for this phenomenon, because prominent lifestyle changes as well as exposure to environmental hazards might have led to endocrine disruption as well.1 Therefore, thorough understanding of the basic mechanisms and molecular players involved in mammalian reproduction is pivotal to tackle the ever-increasing problems related to human infertility.
Polyamines are known to be indispensable for successful mammalian reproduction at several stages, including spermatogenesis, oogenesis, embryogenesis, implantation or placentation.2 Interestingly, some correlative data indicate a possible involvement in the actual fertilization process. For instance, the seminal plasma of infertile men contains markedly lower levels of the polyamines spermine and spermidine compared to normospermic controls.3,4 Also, the fertilization efficiency gradually declines with advanced paternal age, paralleling the decrease in intracellular polyamine concentrations.5,6 Declining fertilization efficiency may be associated with reduced semen volume, sperm concentration as well as polyamine-dependent sperm motility and morphology.5 However, it remains to be elucidated if polyamines also determine the fusion efficiency of oocytes and spermatozoa, which would be crucial for the understanding and improvement of both in vivo and in vitro fertilization.
The budding yeast Saccharomyces cerevisiae has blossomed into a major, genetically tractable model organism for the analysis of basic molecular processes like the cell cycle, autophagy and protein-protein interactions.7,8 In addition, the last two decades have established yeast as a model for the study of cancer,9,10 aging11-13 and neurodegenerative disorders.14-16
During the yeast mating process, two haploid cells of opposed mating types (“MATa” and “MATα”) combine to form a single diploid cell. This event is reminiscent of the human fertilization process, in which the fusion of two haploid cells, the spermatocyte and the oocyte, results in a diploid zygote. Here, we establish the budding yeast S. cerevisiae as a new model organism for the analysis of fertilization mechanisms in higher eukaryotes. Moreover, we unveil a conserved and specific role of spermidine in the positive regulation of this process.
Results
Disruption of spermidine production entails a severe mating defect in yeast
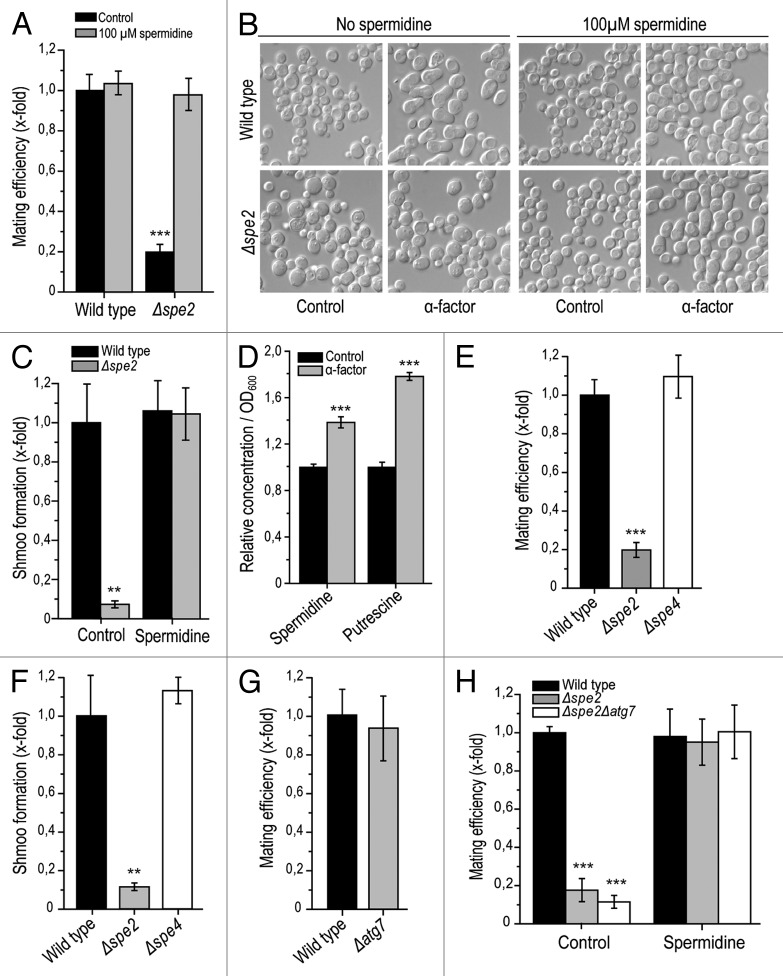
Polyamines are known to be indispensable for successful mammalian reproduction and have been implicated in both prenatal and postnatal processes, such as embryogenesis, placentation and lactation.2 Interestingly, polyamines have been suggested to be involved in the modulation of membrane fusion events.17 However, their impact on the actual fertilization process remains undetermined, perhaps because of the technical difficulty of mechanistically exploring the fusion of spermatocytes and oocytes.18 To develop a method to model this process, we investigated sexual reproduction in the baker’s yeast S. cerevisiae, during which two haploid cells of opposed gender or mating types (“MATa” and “MATα”) combine to generate one diploid yeast cell. To specifically explore the contribution of the polyamine spermidine, yeast mating was monitored between “MATα” wild-type cells and “MATa” deletion mutants lacking SPE2 (Δspe2), the gene coding for S-adenosyl-methionine decarboxylase, which is essential for spermidine biosynthesis. This disruption of spermidine production in one of the mating partners drastically reduced the overall mating efficiency as compared to the control mating scenario, involving “MATa” and “MATα” wild-type cells (Fig. 1A). Preceeding addition of exogenous spermidine to co-cultures of “MATa” Δspe2 cells restored the mating efficiency to control levels (Fig. 1A). In conclusion, spermidine is essential for efficient mating in S. cerevisiae.
Figure 1. Yeast mating efficiency and shmoo formation depends on the availability of spermidine but is autophagy-independent. (A) Mating efficiency of wild-type and Δspe2 cells with or without external administration of 100 µM spermidine as determined via clonogenic mating assay. Mating efficiency of wild-type control was set at 1. Data represent mean ± S.E.M. (n = 3; ***p < 0.001). (B) Representative sample pictures of wild-type and Δspe2 cells (MATa) after treatment with 1 µM α -pheromone for 3 h with or without external administration of 100 µM spermidine. (C) Microscopic quantification of cells treated as described in (B) and displaying a shmoo. Only viable cells were considered, and at least 3,000 cells per strain and experiment were manually counted. The portion of cells displaying a shmoo was normalized to that of the untreated wild type control, which was set at 1. Data represent means ± S.E.M (n = 4; **p < 0.01). (D) Relative intracellular concentration of spermidine and its precursor putrescine per OD600 of wild-type cells (mating type a) either treated with 1 µM α-pheromone or left untreated (control), as determined using LC/MS/MS and normalized to the intracellular concentrations of the untreated control. Data represent means ±S.E.M. (n = 5; ***p < 0.001). (E) Mating efficiency of wild-type, Δspe2 and Δspe4 strains. Data were determined via clonogenic mating assay and represent means ± S.E.M. (n = 3; ***p < 0.001). (F) Shmoo-formation in wild-type, Δspe2 and Δspe4 cells (mating type a) after treatment with 1 µM α-pheromone for 3 h (compare to Fig. 1B). Data represent means ± S.E.M. (n = 4; **p < 0.01). (G) Mating efficiency of wild-type and Δatg7 cells. Data were determined via clonogenic mating assay and represent means ± S.E.M. (n = 3; ***p < 0.001). Mating efficiency of wild-type control was set at 1. (H) Mating efficiency of wild-type, Δspe2 and Δspe2Δatg7 strains with or without external administration of 100 µM spermidine. Data was determined via clonogenic mating assays and represent means ± S.E.M. (n = 3; ***p < 0.001). Mating efficiency of wild-type control was set at 1.
Pheromone-induced shmoo formation depends on spermidine
An indispensable step during human fertilization is spermatocyte capacitation and the acrosome reaction, which is necessary for penetration of the egg by the spermatocyte.19-21 Similarly, sexually reproducing yeast cells must form a nodule called “shmoo” for mating.22,23 The shmoo is produced upon sensing the mating pheromone of the opposite sex, which is released by and signalizes the availability of suitable mating partners.23,24 Thus, to further characterize the impact of spermidine on yeast mating, we quantified shmoo formation upon external administration of 1 µM α-pheromone to wild-type and Δspe2 “MATa” cells. As expected, α-pheromone induced pronounced shmoo formation in viable wild-type cells as quantified by microscopic analysis (Fig. 1B and C). In contrast, phermone-treated viable Δspe2 cells only occasionally developed shmoos (Fig. 1B and C). Again, external supplementation of 100 µM spermidine reverted the deficient shmoo formation in spe2 disruptants to wild-type control levels (Fig. 1B and C). Thus, spermidine is indispensable for shmoo formation, which, in turn, is required for successful mating in yeast. Of note, intracellular levels of spermidine as well as those of its precursor putrescine are significantly elevated upon treatment with mating pheromone (Fig. 1D).
Polyamine-mediated mating control in yeast is spermidine-specific
To evaluate the specificity of spermidine in accounting for the observed positive effect on yeast sexual reproduction, we performed mating experiments with cells lacking spermine synthase (Spe4p). This enzyme catalyzes the generation of the polyamine spermine from spermidine in the polyamine biosynthetic pathway. Intriguingly, deletion of SPE4 did not compromise mating efficiency, which showed similar levels as the wild-type control (Fig. 1E). Accordingly, Δspe4 cells displayed no defect in shmoo formation (Fig. 1F). Altogether, these results point towards a specific role for spermidine (rather than spermine) in the promotion of yeast mating.
Spermidine determines yeast mating in an autophagy-independent manner
We next asked whether the significance of spermidine during mating might involve autophagy, a process of cellular self-digestion that is potently induced by spermidine.25-30 Deletion of the autophagy-related gene ATG7, which is essential for autophagic execution in S. cerevisiae, did not influence mating efficiency (Fig. 1G). Furthermore, the mating efficiency of Δatg7 yeast cells additionally deleted in SPE2 could still be restored to wild-type levels via external spermidine supply (Fig. 1H). These data suggest that spermidine-controlled mating in yeast does not depend on autophagy.
Spermidine modulates fertilization in the nematode Caenorhabditis elegans
In order to establish whether our results might be relevant to fertilization in higher eukaryotes, we explored the impact of spermidine on fertilization in C. elegans. Disruption of spermidine biosynthesis through deletion of spermidine synthase (SPDS-1) led to a significant reduction of the total number of fertilized eggs laid by the worm (Fig. 2A). As in yeast, this deficiency could be largely prevented by external supply of spermidine to the culture medium (Fig. 2A). This spermidine-mediated fertilization control also seems to be autophagy-independent in worms, since RNAi-driven knockdown of bec-1, a gene encoding a cardinal autophagic regulator in C. elegans, did not influence egg fertilization rates (Fig. 2B). Thus, spermidine modulates fertilization in worms in a manner akin to yeast, suggesting that yeast mating constitutes a bona fide model for the fertilization process of oocytes by spermatocytes.
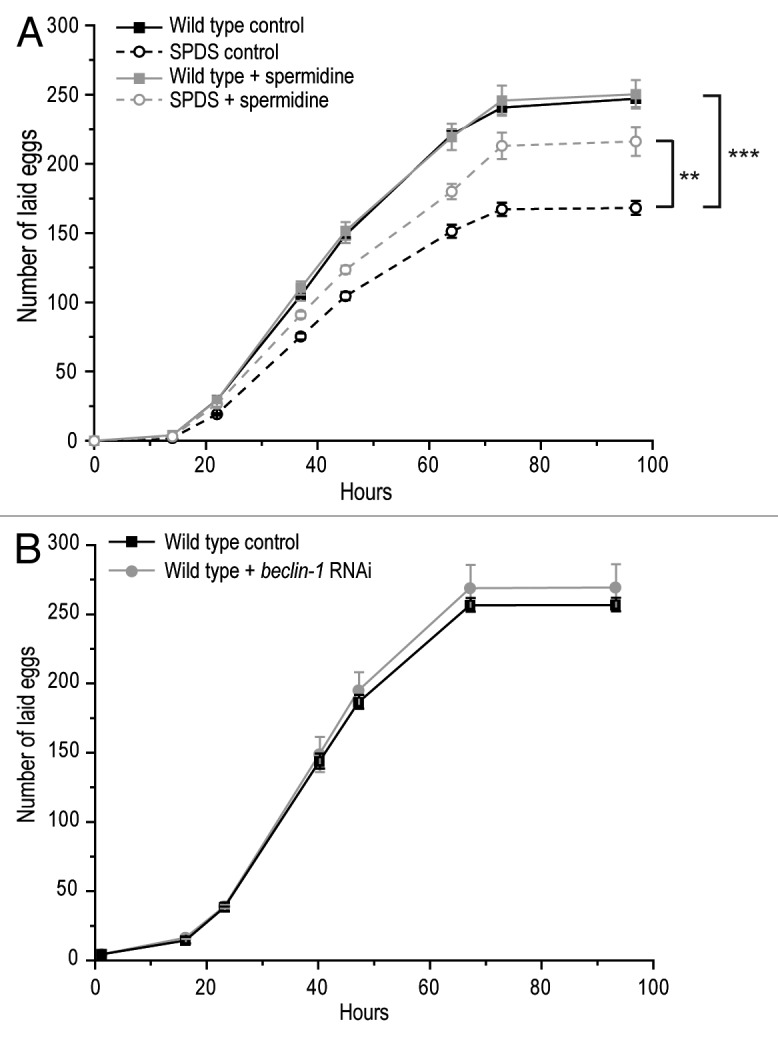
Figure 2. Fertilization in C. elegans is modulated by spermidine. (A) Total number of fertilized eggs laid by wild-type and spermidine synthase (SPDS-1)-deficient worms, fed with or without 50 µM spermidine-supplemented food. Data represent mean ± S.E.M. (n = 5; **p < 0.01, ***p < 0.001). (B) Total number of fertilized eggs laid by wild-type and bec-1-deficient worms. Data represent mean ±S.E.M. (n = 5).
Discussion
Here, we demonstrate that sexually reproducing yeast may constitute a valuable model for studying basic mechanisms that govern the fertilization process in higher eukaryotes. Specifically, we identified the polyamine spermidine to be indispensable for successful mating in S. cerevisiae. Moreover, we observed that spermidine is equally crucial for effective fertilization in the nematode C. elegans. In both cases, the mechanisms underlying this pro-fertilizing effect are autophagy-independent. Altogether, we propose that—as it occurs in the sexual reproductive variant of baker’s yeast—the fusion of two haploid yeast cells forming a diploid cell mirrors the fertilization process of higher eukaryotes, where two haploid gametes conjugate to generate a diploid zygote.
Our hypothesis arguing for a conserved regulation of such haploid fusion events during fertilization is supported by recent evidence suggesting that mating-associated yeast cell fusion may represent a useful model system for studying the mechanisms of cytogamy and karyogamy.31-33 Intriguingly, a recent report has established structural similarities between proteins involved in sperm-egg recognition in mammals and fusion of haploid yeast cells.34 Our results with yeast and nematodes now reinforce the concept that the mechanisms accounting for the fusion among gametes are much more conserved than anticipated.
This study determines spermidine as a crucial regulator of mating/fertilization. Interestingly, spermidine levels have been shown to continuously decrease during yeast chronological aging11,30 and with increasing human age.5,6 This is, in turn, accompanied by decreased fertilization efficiency.5 We show that the intracellular concentrations of spermidine and its precursor putrescine are significantly elevated upon mating induction via treatment with the corresponding mating pheromone. However, such increase could also originate in other effects elicited through treatment with mating pheromone, which, e.g., also induces cell cycle arrest.23 In fact, high spermidine concentrations have been previously linked to repression of cell division.35-37 Thus, a causal relationship between the herein identified elevation of intracellular spermidine levels and the induction of mating will need to be further elucidated in future studies.
Spermidine is required for shmooing, which is a prerequisite for successful mating and represents the polarized growth of the yeast cell towards the mating partner. Shmoo formation in yeast on the one hand and capacitation as well as the acrosome reaction in spermatocytes of higher eukaryotes on the other hand exhibit strong similarities: both manifest prior to the fusion of two haploid cells; both are indispensable for fusion efficiency, and both are morphologically similar to the extent that they involve protruding areas of the mating cells. In our hands, shmooing depends specifically on spermidine but does not seem to involve the generation of the closely related polyamine spermine. In contrast, recent in vitro data do not suggest such specificity for the acrosome reaction. In the ram spermatozoid, externally applied spermine does localize to the acrosome,38 and low concentrations of spermine can stimulate the acrosome of bovine spermatozoa.39 However, these in vitro studies did not compare spermine to spermidine and did not consider the possibility that spermine oxidase can convert spermine into spermidine. As a result, it is conceivable that such supposedly spermine-related effects might in fact be connected to the generation of effective spermidine levels. This will need clarification in future studies. Our conclusion that spermidine is specific for shmooing and mating control, however, is based on the observation that deletion of spermine synthase does not affect mating efficiency or shmooing. This does not exclude the possibility that spermine generation may be alternatively achieved through yet to be identified biosynthetic polyamine routes.40
Spermidine potently induces autophagy in a range of model organisms including mice, worms, flies and yeast.30 This had raised the question whether the positive effects on the sexual reproduction of yeast and nematodes might rely on its autophagy-promoting potential. Our data demonstrate that mating/fertilization is normal in yeast cells and nematodes lacking proteins that are essential for autophagy, like Atg7p in S. cerevisiae or beclin-1 in C. elegans. Moreover, external administration of spermidine restored the mating efficiency of spermidine biosynthesis-deficient yeast cells to wild-type levels, even in the context of deficient autophagy. Thus, autophagy is not involved in the positive effects of spermidine on mating/fertilization. Consequently, alternative pathways must be responsible for the herein observed effects and involve a different molecular target of spermidine. Possible candidates include calcium channels, which are regulated by spermidine41 and required for effective yeast mating as well as for capacitation and the acrosome reaction in humans.42,43 It is thus conceivable that a distortion of spermidine-tuned calcium flux regulation deriving from unbalanced spermidine levels might result in adverse mating/fertilization effects. Whether spermidine affects calcium channels that are crucial for haploid cell fusion remains to be explored.
Altogether, this study provides first in vivo evidence that the mechanisms underlying mating-related cell fusion of haploid yeast cells might parallel fertilization of higher eukaryotes. It is tempting to speculate that yeast cell fusion might also model somatic fusion events as they occur in mammals during the formation of the syncytiotrophoblast, skeleton muscle fibers and osteoclasts.44-47 In fact, polyamines have been previously linked to the modulation of membrane fusion and aggregation.17 Yeast’s enormous technical advantages, such as its inexpensiveness, convenient handling, rapid growth and uncomplicated DNA modification, have defined its value as a model for many areas of molecular biology. Thus, sexual reproduction and specifically fertilization might join as a further area to be explored in S. cerevisiae and thereby even more intimately link yeast and human.
Materials and Methods
Reagents
Spermidine (Sigma) was dissolved in sterilized water to a stock solution concentration of 100 mM and stored as single use aliquots at -20°C. Propidium iodide (PI, Sigma) was stored at -20°C as a stock water solution of 100 µg/ml.
Yeast strains and growth conditions
Yeast experiments were carried out in Saccharomyces cerevisiae BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and the respective null mutants Δspe2 and Δspe4 as well as in BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0), which were all obtained from Euroscarf. All strains were grown on liquid SC medium containing 0.17% yeast nitrogen base (BD Diagnostics), 0.5% (NH4)2SO4, 30mg/l of all amino acids (except 80mg/l histidine, 60 mg/l lysine and 200 mg/l leucine), 30mg/l adenine, 320mg/l uracil and 2% glucose. For experiments, yeast strains from 25% glycerol stocks at -80°C were streaked on YPD plates and stored at 4°C for up to 10 d, from which overnight cultures were inoculated. Incubation of all liquid cultures was performed with 145 rpm at 28°C. Cell densities were determined using an automated cell counter (CASY1, Roche Innovatis).
Yeast-mating efficency
To determine mating efficiency, BY4741 cultures were first inoculated into 100 ml quadruple-indented flasks at 10 ml cell culture each from fresh overnight cultures to a cell density of 1 × 106 cells/ml and, depending on the experiment, supplemented or not with 100 µM spermidine. After 20‒24 h of growth, 2 × 106 cells of each flask were transferred to a new non-indented flask containing 10 ml SCD medium and mixed with 1 × 107 cells BY4742 of a freshly made overnight culture. Of note, comparable viability of all cultures was verified by clonogenic survival plating on YPD as described before.12,13 Three hours after mixing, an aliquot was plated on two different agar plates, respectively: (1) 2,000 cells were plated on agar plates containing 0.17% yeast nitrogen base, 0.5% (NH4)2SO4, 320 mg/l uracil, 200 mg/l leucine, 80 mg/l histidine and 30 mg/l methionine to determine the amount of both viable BY4741 (addition of methionine, see genotype) and diploid cells (arisen from successful mating of one BY4741 and one BY4742 cell) and (2) 2,000 cells were plated on agar plates containing 0.17% yeast nitrogen, 0.5% (NH4)2SO4, 320 mg/l uracil, 200 mg/l leucine and 80 mg/l histidine to assess the number of viable diploid cells only. All plates were incubated at 28°C for 2 d, and resulting colony-forming units (CFU) were counted. The mating efficiency was determined as the number of diploid colonies per number of colonies corresponding to BY4741 cells, i.e. CFU of plate 2/CFU of plate 1.
Yeast shmooing efficency
For shmooing efficiency experiments, cultures were inoculated from fresh overnight cultures to a density with an absorbance of 0.1 as determined using a photometer; this absorbance correlated to approximately 1 × 106 cells/ml. Depending on the experiment, the culture was supplemented or not with 100 µM spermidine and let grow for 20‒24 h. Thereafter, the cultures were inoculated to a density with an absorbance of 0.3 in new non-indented flasks containing 10 ml fresh spermidine-free SCD medium that included 50 µM Na-succinate and was adjusted to pH 4.3 to retard pheromone proteolysis during subsequent pheromone treatment.48 Cultures were then treated with 1 μM α-pheromone. After 3 h, cultures were centrifuged (3 min., 3,000 rpm) and diluted in PBS buffer containing propidium iodide (PI) at a concentration of 0.1 µg/ml. PI as an intercalating, fluorescent agent was used to evaluate cell viability and exclude inviable cells from subsequent microscopic quantification. Cells were incubated for 10 min and used for microscopy.
Microscopy
Microscopy was performed with a Zeiss Axioskop microscope using a Zeiss Plan-Neofluar objective lense with 40 × magnification and 1.0 numerical aperture in oil (using Zeiss Immersol) at room temperature. Imaging medium was PBS. Fluorochrome was propidium iodide (PI). Fluorescence microscopic sample images were taken with a Diagnostic Instruments camera (Model: SPOT 9.0 Monochrome-6), acquired using the Metamorph software (version 6.2r4, Molecular Devices) and processed with IrfanView (version 3.97, Wiener Neustadt) software. Specifically, picture processing involved coloring of PI pictures, creation of corresponding DIC/PI overlays (used to determine inviable cells to exclude from quantification) with Metamorph and cropping of representative areas with IrfanView.
C. elegans strains and growth conditions
Experiments were carried out using the Caenorhabditis elegans N2 wild-type strain. The following mutant strain was used: RB2481 spds-1(ok3421) II (referred to in the text as SPDS-1). We followed standard procedures for C. elegans strain maintenance.49 Nematode rearing temperature was kept at 20°C.
C. elegans fertility determination
Preparation of Escherichia coli (OP50) plates for worm feeding was performed as previously described.49 Briefly, bacteria on seeded 7 ml NGM plates were killed by exposure to UV irradiation for 10 min (0.5 J/cm2) using a UV crosslinker (BIO-LINK‒BLX-E365, Vilber Lourmat). Spermidine was prepared by dilutions in 50 μl sterilized water and applied to the top of the OP50 lawn on the agar medium (7 ml NGM plates). Identical solutions of drug-free water were used for the control plates. Plates were then allowed to dry overnight. N2 and SPDS-1 worms previously grown to L4 stage were transferred to fresh plates with or without spermidine. After 2 d, the progeny was transferred to corresponding fresh plates. Upon reaching L4 stage, single worms were transferred to plates without spermidine. One worm was transferred per plate. The day of this transfer was defined as t = 0. After t = 0, worms were transferred to fresh plates without spermidine every day in order to facilitate egg counting for each day. The number of eggs laid by each single worm was monitored until egg laying ceased.
For fertility experiments involving RNAi-suppression of bec-1, a previously described50,51 plasmid, which directs the synthesis of a dsRNA corresponding to the bec-1 gene in E. coli, was used. HT115(DE3) bacteria were transformed with either this bec-1 RNAi plasmid or the pL4440 control vector. Before seeding, 2 mM IPTG were added to the bacterial culture and incubated for 15 min. The plates seeded with RNAi or pL4440 vector-containing bacteria were subsequently used as described above for the OP50-seeded plates in the fertility assay experiments. Each fertility assay was conducted at least twice, and figures represent typical assays.
Extraction and measurements of polyamines
Extraction and measurements of polyamines were essentially performed as described in Eisenberg et al.30 For acid extraction of polyamines from yeast cells, culture equivalents of 2 OD600 were washed with ddH2O, resuspended in 1,000μl ice-cold 5% TCA with isotopically labelled internal standards for spermidine (13C4-spermidine) and putrescine (2H8-putrescine) and incubated on ice for 1h, with vortexing every 15min. [Calibration standards were prepared by spiking extraction buffer with specific concentrations of spermidine and putrescine and internal standards. Polyamines were derivatized to carbamyl-derivatives by adding 125 µl of 1 M carbonate buffer (pH 9), 800 µl water and 20 µl of isobutyl chloroformate to 100 µl of sample or calibration standard containing internal standards.] Supernatants were neutralized with 100μl of 2M K2HPO4 and stored at -80°C until polyamine measurements.
Polyamines were determined using LC/MS/MS. All analyses were carried out on an Ultimate 3000 System (Dionex, LCPackings) coupled to a Quantum TSQ Ultra AM (Thermo Scientific) using an electrospray ion source. The system was controlled by Xcalibur Software 2.0. The stationary phase was a Kinetex 2.6 µm C18 100A 50 mm × 2.1 mm column (Phenomenex). Two hundred and fifty µl of the derivatized samples were loaded on to an online-SPE column (Strata X, Phenomenex) using eluent A (flow rate 1.5 ml min). After 2 min, online SPE was switched to the analytical column and the polyamines were eluted and separated on the analytical column within 4 min using isocratic conditions (80% eluent B, flow rate 250 µl/min). Polyamines were detected in MRM mode using following transitions: Spermidine (m/z 446 -> 298V), putrescine (m/z 289 -> 115), 13C4-spermidine (m/z 450 -> 302), 2H8-putrescine (m/z 297 -> 123).
Statistical analyses
Error bars (±S.E.M.) are shown for independent experiments. In cases when experiments were performed in parallel, a common ONC for each strain was used and (1) fresh medium in separate flasks was inoculated directly from the corresponding ONCs (knockout studies). The number of independent data points (n) is indicated in the figure legends of the corresponding graphs. Statistical analyses were carried out using the Microsoft Office 2003 Excel software package (Microsoft Corporation). Mean values were compared using unpaired t-tests.
Acknowledgments
We thank Ulrike Potocnik and Angela Pasparaki for assistance. We are grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B12 and P24381-B20. N.T. acknowledges support by grants from the European Research Council (ERC) and the European Commission 7th Framework Programme. This work is supported by grants to G.K. from the Ligue Nationale contre le Cancer (Equipe labellisée), Agence Nationale pour la Recherche (ANR), AXA Chair for Longevity Research, European Commission (ArtForce, ChemoRes), Fondation pour la Recherche Médicale (FRM), Institut National du Cancer (INCa), Cancéropôle Ile-de-France, Fondation Bettencourt-Schueller, the LabEx Onco-Immunology and the Paris Alliance of Cancer Research Institutes. The authors declare no conflict of interest.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23199
References
- 1.Phillips KP, Tanphaichitr N. Human exposure to endocrine disrupters and semen quality. J Toxicol Environ Health B Crit Rev. 2008;11:188–220. doi: 10.1080/10937400701873472. [DOI] [PubMed] [Google Scholar]
- 2.Lefèvre PL, Palin MF, Murphy BD. Polyamines on the reproductive landscape. Endocr Rev. 2011;32:694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- 3.Calandra RS, Rulli SB, Frungieri MB, Suescun MO, González-Calvar SI. Polyamines in the male reproductive system. Acta Physiol Pharmacol Ther Latinoam. 1996;46:209–22. [PubMed] [Google Scholar]
- 4.Shohat B, Maayan R, Singer R, Sagiv M, Kaufman H, Zukerman Z. Immunosuppressive activity and polyamine levels of seminal plasma in azo-ospermic, oligospermic, and normospermic men. Arch Androl. 1990;24:41–50. doi: 10.3109/01485019008986857. [DOI] [PubMed] [Google Scholar]
- 5.Stewart AF, Kim ED. Fertility concerns for the aging male. Urology. 2011;78:496–9. doi: 10.1016/j.urology.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Scalabrino G, Ferioli ME. Polyamines in mammalian ageing: an oncological problem, too? A review. Mech Ageing Dev. 1984;26:149–64. doi: 10.1016/0047-6374(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 7.Pertschy B, Saveanu C, Zisser G, Lebreton A, Tengg M, Jacquier A, et al. Cytoplasmic recycling of 60S preribosomal factors depends on the AAA protein Drg1. Mol Cell Biol. 2007;27:6581–92. doi: 10.1128/MCB.00668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hohmann S. The Yeast Systems Biology Network: mating communities. Curr Opin Biotechnol. 2005;16:356–60. doi: 10.1016/j.copbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Carmona-Gutierrez D, Eisenberg T, Büttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17:763–73. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- 10.Ruckenstuhl C, Büttner S, Carmona-Gutierrez D, Eisenberg T, Kroemer G, Sigrist SJ, et al. The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS One. 2009;4:e4592. doi: 10.1371/journal.pone.0004592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmona-Gutiérrez D, Bauer MA, Ring J, Knauer H, Eisenberg T, Büttner S, et al. The propeptide of yeast cathepsin D inhibits programmed necrosis. Cell Death Dis. 2011;2:e161. doi: 10.1038/cddis.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–7. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madeo F, Herker E, Maldener C, Wissing S, Lächelt S, Herlan M, et al. A caspase-related protease regulates apoptosis in yeast. Mol Cell. 2002;9:911–7. doi: 10.1016/S1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 14.Krobitsch S, Lindquist S. Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc Natl Acad Sci USA. 2000;97:1589–94. doi: 10.1073/pnas.97.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Büttner S, Bitto A, Ring J, Augsten M, Zabrocki P, Eisenberg T, et al. Functional mitochondria are required for alpha-synuclein toxicity in aging yeast. J Biol Chem. 2008;283:7554–60. doi: 10.1074/jbc.M708477200. [DOI] [PubMed] [Google Scholar]
- 16.Braun RJ, Büttner S, Ring J, Kroemer G, Madeo F. Nervous yeast: modeling neurotoxic cell death. Trends Biochem Sci. 2010;35:135–44. doi: 10.1016/j.tibs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Schuber F, Hong K, Düzgünes N, Papahadjopoulos D. Polyamines as modulators of membrane fusion: aggregation and fusion of liposomes. Biochemistry. 1983;22:6134–40. doi: 10.1021/bi00295a015. [DOI] [PubMed] [Google Scholar]
- 18.Leahy T, Gadella BM. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction. 2011;142:759–78. doi: 10.1530/REP-11-0310. [DOI] [PubMed] [Google Scholar]
- 19.Yanagimachi R. In: Neill EKJ, ed. The Physiology of Reproduction. New York: Raven Press, 1988:135-85. [Google Scholar]
- 20.Yanagimachi R. In: Biggers LMJD, ed. Fertilization and embryonic development in vitro. New York: Plenum Press, 1981:82-182. [Google Scholar]
- 21.Russell L, Peterson R, Freund M. Direct evidence for formation of hybrid vesicles by fusion of plasma and outer acrosomal membranes during the acrosome reaction in boar spermatozoa. J Exp Zool. 1979;208:41–56. doi: 10.1002/jez.1402080106. [DOI] [PubMed] [Google Scholar]
- 22.Madden K, Snyder M. Cell polarity and morphogenesis in budding yeast. Annu Rev Microbiol. 1998;52:687–744. doi: 10.1146/annurev.micro.52.1.687. [DOI] [PubMed] [Google Scholar]
- 23.Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2004;25:1465–76. doi: 10.1016/j.peptides.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Cross F, Hartwell LH, Jackson C, Konopka JB. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–57. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- 25.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–6. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 26.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 2011;3:716–32. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–29. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, et al. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012;3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G. Spermidine: a novel autophagy inducer and longevity elixir. Autophagy. 2010;6:160–2. doi: 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 31.Ydenberg CA, Rose MD. Yeast mating: a model system for studying cell and nuclear fusion. Methods Mol Biol. 2008;475:3–20. doi: 10.1007/978-1-59745-250-2_1. [DOI] [PubMed] [Google Scholar]
- 32.Ydenberg CA, Stein RA, Rose MD. Cdc42p and Fus2p act together late in yeast cell fusion. Mol Biol Cell. 2012;23:1208–18. doi: 10.1091/mbc.E11-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sens KL, Zhang S, Jin P, Duan R, Zhang G, Luo F, et al. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J Cell Biol. 2010;191:1013–27. doi: 10.1083/jcb.201006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson WJ, Aagaard JE, Vacquier VD, Monné M, Sadat Al Hosseini H, Jovine L. The molecular basis of sex: linking yeast to human. Mol Biol Evol. 2011;28:1963–6. doi: 10.1093/molbev/msr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeb F, van der Weele CM, Wolniak SM. Spermidine is a morphogenetic determinant for cell fate specification in the male gametophyte of the water fern Marsilea vestita. Plant Cell. 2010;22:3678–91. doi: 10.1105/tpc.109.073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gömürgen AN, Mutlu F, Bozcuk S. Effects of polyamines (putrescine, spermidine and spermine) on root tip mitosis and chromosomes in Allium cepa L. Cytologia (Tokyo) 2005;70:217–24. doi: 10.1508/cytologia.70.217. [DOI] [Google Scholar]
- 37.Osborne HB, Cormier P, Lorillon O, Maniey D, Bellé R. An appraisal of the developmental importance of polyamine changes in early Xenopus embryos. Int J Dev Biol. 1993;37:615–8. [PubMed] [Google Scholar]
- 38.Rubinstein S, Breitbart H. Cellular localization of polyamines: cytochemical and ultrastructural methods providing new clues to polyamine function in ram spermatozoa. Biol Cell. 1994;81:177–83. doi: 10.1016/S0248-4900(94)80008-1. [DOI] [PubMed] [Google Scholar]
- 39.Rubinstein S, Lax Y, Shalev Y, Breitbart H. Dual effect of spermine on acrosomal exocytosis in capacitated bovine spermatozoa. Biochim Biophys Acta. 1995;1266:196–200. doi: 10.1016/0167-4889(95)00007-F. [DOI] [PubMed] [Google Scholar]
- 40.Michael AJ. Exploring polyamine biosynthetic diversity through comparative and functional genomics. Methods Mol Biol. 2011;720:39–50. doi: 10.1007/978-1-61779-034-8_2. [DOI] [PubMed] [Google Scholar]
- 41.Gomez M, Hellstrand P. Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflugers Arch. 1999;438:445–51. doi: 10.1007/s004240051060. [DOI] [PubMed] [Google Scholar]
- 42.Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol Cell Biol. 1994;14:8259–71. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orta G, Ferreira G, José O, Treviño CL, Beltrán C, Darszon A. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol. 2012;590:2659–75. doi: 10.1113/jphysiol.2011.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vignery A. Osteoclasts and giant cells: macrophage-macrophage fusion mechanism. Int J Exp Pathol. 2000;81:291–304. doi: 10.1046/j.1365-2613.2000.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pötgens AJ, Schmitz U, Bose P, Versmold A, Kaufmann P, Frank HG. Mechanisms of syncytial fusion: a review. Placenta. 2002;23(Suppl A):S107–13. doi: 10.1053/plac.2002.0772. [DOI] [PubMed] [Google Scholar]
- 46.Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 47.Chen EH, Olson EN. Unveiling the mechanisms of cell-cell fusion. Science. 2005;308:369–73. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- 48.Ciejek E, Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979;18:623–35. doi: 10.1016/0092-8674(79)90117-X. [DOI] [PubMed] [Google Scholar]
- 49.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samara C, Syntichaki P, Tavernarakis N. Autophagy is required for necrotic cell death in Caenorhabditis elegans. Cell Death Differ. 2008;15:105–12. doi: 10.1038/sj.cdd.4402231. [DOI] [PubMed] [Google Scholar]
- 51.Tavernarakis N, Pasparaki A, Tasdemir E, Maiuri MC, Kroemer G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy. 2008;4:870–3. doi: 10.4161/auto.6730. [DOI] [PubMed] [Google Scholar]