Abstract
Insect root herbivores can alter plant community structure by affecting the competitive ability of single plants. However, their effects can be modified by the soil environment. Root herbivory itself may induce changes in the soil biota community, and it has recently been shown that these changes can affect plant growth in a subsequent season or plant generation. However, so far it is not known whether these root herbivore history effects (i) are detectable at the plant community level and/or (ii) also determine plant species and plant community responses to new root herbivore attack. The present greenhouse study determined root herbivore history effects of click beetle larvae (Elateridae, Coleoptera, genus Agriotes) in a model grassland plant community consisting of six common species (Achillea millefolium, Plantago lanceolata, Taraxacum officinale, Holcus lanatus, Poa pratensis, Trifolium repens). Root herbivore history effects were generated in a first phase of the experiment by growing the plant community in soil with or without Agriotes larvae, and investigated in a second phase by growing it again in the soils that were either Agriotes trained or not. The root herbivore history of the soil affected plant community productivity (but not composition), with communities growing in root herbivore trained soil producing more biomass than those growing in untrained soil. Additionally, it influenced the response of certain plant species to new root herbivore attack. Effects may partly be explained by herbivore-induced shifts in the community of arbuscular mycorrhizal fungi. The root herbivore history of the soil proved to be a stronger driver of plant growth on the community level than an actual root herbivore attack which did not affect plant community parameters. History effects have to be taken into account when predicting the impact of root herbivores on grasslands.
Introduction
Insect root herbivores can alter plant community structure by affecting the competitive ability of single plants [1]. However, their effects can be modified by the soil environment [2]–[3]. Root herbivory itself may induce changes in the soil biota community [4]–[6]. It has recently been shown that these changes can affect plant growth in a subsequent season or plant generation [7]. However, it is not known whether these root herbivore history effects (i) are detectable at the plant community level and/or (ii) also determine plant species and plant community responses to new root herbivore attack.
Several studies documented that root symbiotic arbuscular mycorrhizal fungi (AMF) and root herbivores influence each other's performance and effect on the host plant [8]–[11]. One study [12] even found consequences of these interactive effects on the plant community level. AMF provide mineral nutrients to the plant in exchange for photosynthates [13]. Their effect on plant growth proved to be plant as well as AMF species specific [14]. As root herbivores can alter AMF community structure [15] their history effects may potentially be generated through changes in this important soil biota group.
Click beetle larvae (Elateridae, Coleoptera) of the genus Agriotes are dominant generalist root herbivores in European grasslands [16]–[18] and also pests in different economically important crops. While their effects on cropping systems are well studied, their impact on grassland plant communities is almost unknown. Studies that included measurements on background soil biota have so far only been done in a single plant system (Plantago lanceolata), and found no effects of Agriotes larvae on the microbial carbon source utilization in the rhizosphere [19] and root colonization by arbuscular mycorrhizal fungi [20]–[21].
The presented greenhouse study aimed at determining root herbivore history effects of Agriotes spp. larvae on a grassland plant community. The root herbivore history of the soil was generated by growing a model plant community without or with Agriotes larvae in soil biota communities from two different grassland sites in a first phase of the experiment. Root herbivore history effects on plant growth and response to a new Agriotes attack were determined by growing the model plant community without or with Agriotes larvae in the either herbivore untrained (absence of Agriotes larvae in phase 1) or trained (presence of Agriotes larvae in phase 1) soil, in a second phase of the experiment. We further investigated effects of present or past Agriotes herbivory on AMF as one important group within the soil biota community. Measurements included AMF community parameters in soil as well as community parameters and colonization levels in the roots of the model plant P. lanceolata. We hypothesized that (i) the root herbivore history of the soil influences plant growth and response to a new root herbivore attack, (ii) this has consequences for plant community structure, and (iii) root herbivore history effects can be explained by herbivore induced shifts in the AMF community.
Materials and Methods
The experiment was conducted within the frame of the Biodiversity Exploratories project [22]. Background soil and inocula were collected from grassland sites in the Schorfheide exploratory, 100 km north of Berlin, Germany. A general field work permit was issued by the Landesumweltamt Brandenburg. The collection did not involve endangered or protected species.
Background soil
The background soil was an alfisol that was collected from the upper 5–30 cm of a mown pasture. Stones and coarse roots were sieved out (1 cm mesh size) and the soil was steamed for 4 h at 90°C prior to usage to kill root herbivores.
Establishment of plant community and Agriotes treatment
A total of 120 round 2 L plastic pots (Albert Treppens & Co Samen GmbH, Berlin, Germany) were prepared by sealing the drainage holes with water permeable non-woven material (Plantex®, DuPont, Germany) to prevent escape of Agriotes larvae in Agriotes treatments. Pots were filled with background soil, and soil biota other than soil macrofauna was reintroduced by means of inocula that were specific for the two phases of the experiment (see detailed experimental set up of phase 1 and 2 below). A plant community consisting of six grassland plant species (Achillea millefolium L., P. lanceolata L., Taraxacum officinale Wiggers, Holcus lanatus L., Poa pratensis L., Trifolium repens L.) was established in each pot, with one individual per species. The plant species chosen were common on the two grassland sites from where the soil biota inocula for phase 1 (see below) were collected. The proportion of plant functional types (two grasses, three herbs and one legume) resembled those at the sites. Pots in each treatment were allocated to three different sowing schemes to account for neighboring effects, and plant species were sown at evenly distanced positions in each pot, with four seeds (Appels Wilde Samen GmbH, Germany) per position, according to the sowing schemes. Each pot was covered with a perforated plastic bag (15×66 cm, EDNA International GmbH, Germany) to prevent invasion of unwanted herbivores. Seedlings were thinned to one per position approximately one week after sowing. Pots were randomized once a week and watered from the bottom as needed during the course of the experiment. To establish the Agriotes treatment, Agriotes spp. larvae, collected from a fallow grassland app. 10 km south of Berlin, were added to half of the pots in each soil biota/training treatment (see below) four weeks after sowing, with three larvae per pot. Five randomly chosen larvae from that fallow grassland were identified as A. obscurus [23]. The composition of instars reflected those at the site but was not assessed in detail. Larvae were randomly allocated to the pots. Larvae were rinsed with tap water prior to addition. The rinsing water was collected and evenly allocated to Agriotes-free pots to correct for microorganisms that were introduced with the larvae.
Experimental set up
Phase 1
40 pots were each filled with 1790+/−1 g of background soil. The upper 790 g of background soil in each pot were mixed with 174+/−1 g of one of two soil biota inocula, with 20 pots per inoculum. Each inoculum consisted of un-steamed soil that had been collected from the upper 10 cm of one of two alfisol grassland sites (SEG 33 and SEG 37). Sites for inocula collection were chosen (i) to be of the same soil type as the background soil to ensure establishment of the soil biota in the pots and (ii) to differ in management (SEG 33: fertilized mown pasture, SEG 37: unfertilized pasture) to maximize differences between the two soil biota communities in general and AMF communities [24] in particular. Macrofauna and coarse material was sieved out (4 mm mesh size) from the inocula, roots were cut to 1 cm pieces and added back, and the inocula were air dried prior to usage to kill remaining root herbivore eggs. The plant community and Agriotes treatment were established as described above, resulting in four treatments (two soil biota communities (SEG 33, SEG 37), either without or with Agriotes larvae), with 10 replicates each. Plants were grown for two months in a climate chamber at 20/18°C day/night temperature, 69% air humidity and 16 h day length.
Phase 2
80 pots were each filled with 1140+/−1 g of background soil. The upper 540 g of background soil in each pot were mixed with 825+/−1 g of a soil biota inoculum. Soil biota inocula consisted of root free soil from phase 1 pots. The comparably high amount of inoculum was chosen to transfer not only the soil biota themselves, but also the respective abiotic conditions that potentially resulted from the treatments in phase 1. After the harvest of phase 1, Agriotes larvae were removed from the respective treatments and the soil of each phase 1 pot was well mixed and air dried for storage until the set up of phase 2. The soil from one phase 1 pot then served as inoculum for two pots in phase 2. The plant community and Agriotes treatment were established as described above, with each pair of pots being split between control and Agriotes treatment. This resulted in eight treatments (two soil biota communities (SEG 33, SEG 37), either untrained or Agriotes trained during phase 1 (together with respective abiotic soil conditions), each without or with new Agriotes larvae in phase 2), with 10 replicates each. Plants were grown for two months in June/July in a greenhouse at 20/19°C minimal day/night temperature with 16 h additional light per day.
Harvests and measurements
Each phase was harvested by cutting the shoots at soil surface level. Shoot biomass for each plant was determined gravimetrically after drying at 56°C for 72 h as gram dry weight (gDW). Shoot biomass data (means (se)) for all plant species grown in different treatments are reported as supporting information (Supporting Tables S1). Shannon's diversity index was calculated per pot as H′ = ∑pi*ln pi, where pi is the share of shoot biomass represented by species i and is calculated as shoot biomass i/shoot biomass total. Roots were sieved (1 mm mesh size) from the soil, and P. lanceolata roots were separated as far as possible. As roots were strongly entangled it was not possible to further separate plant species. Thus, total roots per pot were washed, and root biomass (including P. lanceolata) per pot was determined gravimetrically after drying at 56°C for 72 as gDW.
Soil carbon (C) and nitrogen (N) content was determined from air dried soil of the original soil biota inocula and of phase 1 pots at harvest by means of complete combustion and chromatographical detection (analyzer EuroEA, HEKATech GmbH, Germany). Soil CN ratio was calculated as surrogate for nutrient mineralization [25]–[27].
Mycorrhizal structures in P. lanceolata roots from all pots in phase 1 and half of the pots in each treatment in phase 2 were stained with the ink-vinegar method [28]. The percentage of root length colonized [%RLC] by arbuscules and mycorrhizal structures in total (total AMF, including arbuscules, vesicles and intraradical hyphae) was determined at 200 fold magnification using the gridline intersect method [29]. The length of extraradical AMF hyphae in soil (LEH [m/g soil]) was determined for phase 1 and 2 from half of the pots in each treatment applying the methods of [30] and [31]. The AMF community was characterized in phase 1 and 2 from fresh substrate (stored at −80°C until analysis) and dried P. lanceolata roots by means of terminal restriction fragment length polymorphism (TRFLP) using the database TRFLP approach as outlined in [32]. Briefly, soil DNA was extracted from root and soil samples of each pot and amplified using AM fungal specific primers for the ribosomal small subunit gene (SSU). PCR amplicons were pooled for root and soil samples separately, cloned and 129 clones were sequenced to obtain a database of AM fungal operational taxonomic units (OTUs) present in the samples. This database was used to calibrate the T-RFLP approach, which was then applied on the PCR products obtained from each sample separately. Obtained TRFLP peaks were compared with the database to identify present OTUs using the TRAMPR package in R [33]. A detailed description of the methods is available as supporting information (Protocol S1). AMF data (means (se)) in different treatments are reported as supporting information (Supporting Tables S1).
Statistical analyses
All statistical analyses were performed using the software program ‘R’, version 2.12.0 [34]. Data were analyzed separately for phase 1 and 2 of the experiment. Effects of sowing scheme, soil biota- and Agriotes treatments on plant biomass parameters, P. lanceolata root length colonized by AMF, length of extraradical AMF hyphae (LEH) and soil CN ratio were analyzed with generalized least square models (GLS) for phase 1 and linear mixed effects models (lme) for phase 2. Identities of phase 1 pots were included as random factor in the models to analyze effects in phase 2. Inhomogeneity of variances among data of different treatments was accounted for by applying the varIdent command [35]. The factor sowing scheme did not affect any of the parameters and was therefore excluded from the models. Effects of soil biota- and Agriotes treatments on AMF community composition were analyzed with permutation tests based on Jaccard distance matrices using the adonis command [36]. AMF species extracted from P. lanceolata roots in phase 1 could not be analyzed due to very low species detection. To visualize AM fungal communities in the pots of phase 2, we calculated a non-metric analysis and plotted mean community composition and standard error for the four treatment combinations of soil biota and Agriotes training.
Results
The two phases of the experiment differed in plant biomass and community composition. In phase 1, total plant biomass per pot was on average 3.5 times higher than in phase 2 (Table 1). T. repens clearly dominated the plant community at the harvest of phase 1, while in phase 2 the plant community was only slightly dominated by P. lanceolata.
Table 1. Plant and AMF parameters and Soil CN ratio (mean (se)) in different soil treatments (soil biota communities SGE 33 and SEG 37, Agriotes untrained (−AP1) or trained (+AP1) soil substrate) in phase 1 and 2.
| Phase 1 | Phase 2 | |||||
| SEG 33 | SEG 37 | SEG 33 | SEG 33 | SEG 37 | SEG 37 | |
| (−AP1) | (+AP1) | (−AP1) | (+AP1) | |||
| Shannon's H | 1.29 (0.04) | 1.27 (0.03) | 1.53 (0.03) | 1.51 (0.03) | 1.39 (0.03) | 1.40 (0.04) |
| Plant biomass total (gDW) | 20.73 (0.83) | 22.18 (0.92) | 5.70 (0.33) | 6.32 (0.31) | 6.13 (0.30) | 6.54 (0.18) |
| Root biomass total (gDW) | 7.72 (0.43) | 9.23 (0.42) | 2.01 (0.17) | 2.33 (0.15) | 2.38 (0.22) | 2.56 (0.16) |
| Shoot biomass total (gDW) | 13.00 (0.59) | 12.95 (0.75) | 3.70 (0.17) | 3.99 (0.17) | 3.74 (0.15) | 3.99 (0.14) |
| Shoot biomass (gDW) | ||||||
| A. millefolium | 1.21 (0.16) | 0.66 (0.14) | 0.42 (0.06) | 0.46 (0.05) | 0.67 (0.12) | 0.47 (0.05) |
| P. lanceolata | 1.82 (0.21) | 1.91 (0.27) | 0.97 (0.10) | 1.10 (0.08) | 1.25 (0.14) | 1.16 (0.11) |
| T. officinale | 0.25 (0.04) | 0.35 (0.07) | 0.75 (0.11) | 0.81 (0.12) | 0.76 (0.14) | 1.19 (0.14) |
| H. lanatus | 3.01 (0.24) | 3.85 (0.29) | 0.91 (0.15) | 1.10 (0.18) | 0.63 (0.15) | 0.93 (0.16) |
| P. pratensis | 0.23 (0.03) | 0.25 (0.04) | 0.07 (0.02) | 0.09 (0.01) | 0.02 (0.01) | 0.02 (0.01) |
| T. repens | 6.49 (0.74) | 5.94 (0.71) | 0.57 (0.10) | 0.46 (0.08) | 0.42 (0.11) | 0.22 (0.05) |
| LEH [m/gDW soil] | 0.47 (0.07) | 1.24 (0.21) | 2.07 (0.47) | 1.16 (0.29) | 1.40 (0.25) | 1.87 (0.42) |
| No of AMF species in soil | 3.75 (0.61) | 5.15 (0.23) | 2.40 (0.26) | 3.33 (0.52) | 3.50 (0.49) | 2.61 (0.52) |
| No of AMF species in roots | 3.00 (0.48) | 2.90 (0.43) | 4.25 (0.45) | 3.83 (0.46) | ||
| Total AMF [% P. lanceolata RLC] | 18.95 (2.37) | 24.05 (2.50) | 71.50 (3.00) | 66.80 (3.81) | 59.80 (3.53) | 46.50 (3.72) |
| Arbuscules [% P. lanceolata RLC] | 8.20 (1.81) | 5.70 (0.95) | 16.50 (2.66) | 17.10 (2.99) | 12.50 (1.60) | 10.75 (2.12) |
| Soil CN ratio | 12.09 (0.15) | 11.98 (0.12) | ||||
gDW: gram dry weight, LEH: length of extraradical AMF hyphae, AMF: arbuscular mycorrhizal fungi, RLC: root length colonized.
Phase 1
Soil biota community effects
Root biomass of the plant community was 20% higher when grown with SEG 37 than with SEG 33, while total shoot and plant biomass as well as plant diversity, measured as Shannon's H′ on the basis of shoot biomass was not affected by the soil biota community (Tables 1 and 2). However, the soil biota community affected shoot biomass of three out of six plant species, with A. millefolium growing 83% larger with soil biota community SEG 33 than with SEG 37, while T. officinale and H. lanatus grew 40% and 28% larger with SEG 37 than with SEG 33.
Table 2. Effects of soil biota community (SB, SEG 33 and SEG 37) and Agriotes presence (AP1, without and with) on plant and AMF parameters and soil CN in phase 1.
| SB | AP1 | SB x AP1 | ||||
| df:1 | df:1 | df:1 | ||||
| F | p | F | p | F | p | |
| Shannon's H | 0.28 | ns | 0.75 | ns | 0.02 | ns |
| Plant biomass total | 3.10 | ns | 0.01 | ns | 0.00 | ns |
| Root biomass total | 13.00 | *** | 1.98 | ns | 1.46 | ns |
| Shoot biomass total | 0.01 | ns | 0.61 | ns | 0.55 | ns |
| Shoot biomass | ||||||
| A. millefolium | 30.65 | *** | 9.54 | ** | 7.11 | * |
| P. lanceolata | 0.16 | ns | 9.94 | ** | 2.57 | ns |
| T. officinale | 4.21 | * | 0.16 | ns | 0.82 | ns |
| H. lanatus | 9.66 | ** | 0.57 | ns | 0.18 | ns |
| P. pratensis | 0.35 | ns | 1.50 | ns | 2.52 | ns |
| T. repens | 0.62 | ns | 3.10 | ns | 1.02 | ns |
| LEH | 22.65 | *** | 1.68 | ns | 0.62 | ns |
| AMF community in soil | 14.36 | ** | 0.16 | ns | 2.42 | ns |
| No of AMF species in soil | 5.77 | * | 0.47 | ns | 0.74 | ns |
| Total AMF (P. lanceolata) | 4.15 | * | 0.01 | ns | 0.10 | ns |
| Arbuscules (P. lanceolata) | 2.84 | ns | 0.00 | ns | 0.11 | ns |
| Soil CN ratio | 0.62 | ns | 0.01 | ns | 0.73 | ns |
significance level:
p<0.05,
p<0.01,
p<0.001,
ns = not significant, LEH: length of extraradical AMF hyphae, AMF: arbuscular mycorrhizal fungi.
The two soil biota communities differed in their AMF community composition in soil, with 1.4 AMF species more present and extraradical hyphae being 2.6 times longer in soil of SEG 37 than of SEG 33. Total AMF structures of SEG 37 colonized 27% more of P. lanceolata roots length than AMF of SEG 33. Soil CN ratio did not differ for the two soil biota communities.
Root herbivory effects
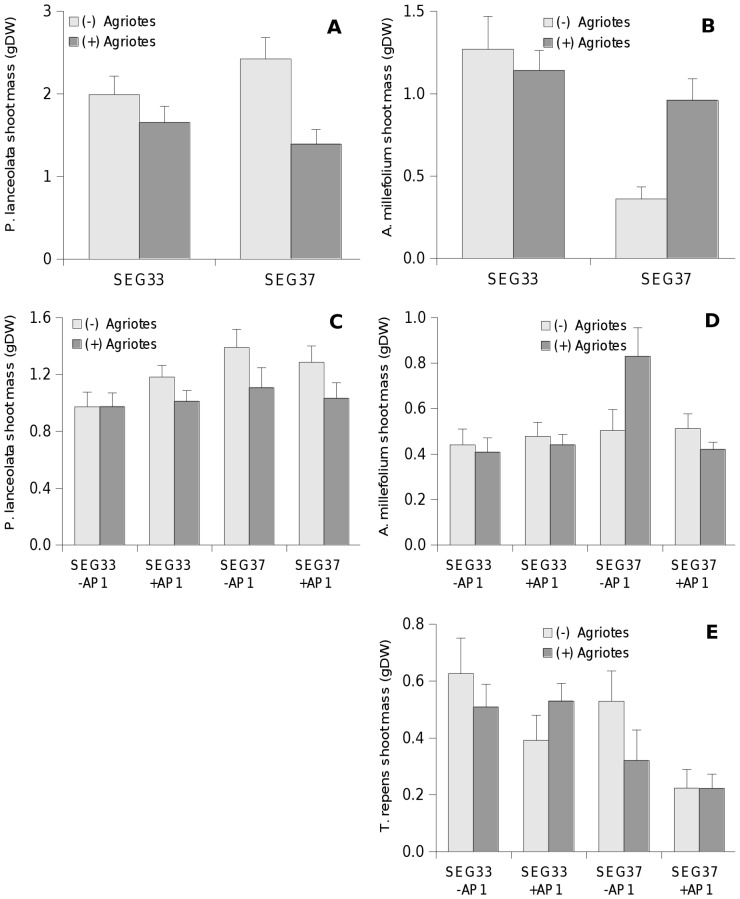
Plant community parameters did not respond to the presence of Agriotes larvae. On the level of single plants, Agriotes larvae reduced shoot biomass of P. lanceolata (Figure 1A) by 31% on average and the effect did not differ significantly for the two soil biota communities. On the contrary, shoot biomass of A. millefolium (Figure 1B) was 2.7 times higher in the presence than in the absence of Agriotes larvae, but only when grown in soil biota community SEG 37. AMF parameters as well as the soil CN ratio were not affected by the presence of Agriotes larvae.
Figure 1. Shoot biomass (mean + se) of plants affected by the presence of Agriotes larvae.
Phase 1 (A) P. lanceolata (B) A. millefolium; phase 2 (C) P. lanceolata (D) A. millefolium, (E) T repens; SEG33, SEG37: soil biota from respective grassland sites; −AP1, +AP1: untrained and Agriotes trained soil from phase 1, respectively.
Phase 2
Soil biota community effects
Soil biota community effects on plant community biomasses were similar to phase 1, with root biomass of the plant community being 14% higher when grown with SEG 37 than with SEG 33, while total shoot and plant biomass was not affected (Tables 1 and 3). The plant community was 9% more diverse when grown with SEG 33 than with SEG 37. The effect of the soil biota community on single plant species differed from that in phase 1, with P. pratensis and T. repens now being affected and growing 400% and 61%, larger with soil biota community SEG 33 than SEG 37, respectively, while responsive plant species in phase 1 (A. millefolium, T. officinale, H. lanatus) were not affected anymore.
Table 3. Effects of soil biota community (SB, SEG 33 and SEG 37), Agriotes training (AP1, Agriotes presence in phase 1, untrained and trained) and Agriotes presence (AP2, without and with) on plant and AMF parameters in phase 2.
| SB | AP1 | AP2 | SB X AP1 | SB x AP2 | AP1 x AP2 | SB x AP1 x AP2 | ||||||||
| df:1 | df:1 | df:1 | df:1 | df:1 | df:1 | df:1 | ||||||||
| F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| Shannon's H | 22.17 | *** | 0.04 | ns | 2.30 | ns | 0.44 | ns | 0.04 | ns | 0.44 | ns | 0.02 | ns |
| Plant biomass total | 1.90 | ns | 5.26 | * | 0.31 | ns | 0.20 | ns | 1.03 | ns | 2.11 | ns | 0.59 | ns |
| Root biomass total | 4.42 | * | 3.14 | ns | 0.01 | ns | 0.34 | ns | 0.46 | ns | 2.58 | ns | 1.06 | ns |
| Shoot biomass total | 0.02 | ns | 3.98 | 0.05 | 0.84 | ns | 0.02 | ns | 0.45 | ns | 0.04 | ns | 0.02 | ns |
| Shoot biomass | ||||||||||||||
| A. millefolium | 2.31 | ns | 2.26 | ns | 0.02 | ns | 4.76 | * | 0.42 | ns | 3.77 | 0.06 | 3.66 | 0.06 |
| P. lanceolata | 3.64 | ns | 0.05 | ns | 7.81 | ** | 1.40 | ns | 2.14 | ns | 0.35 | ns | 0.64 | ns |
| T. officinale | 3.15 | ns | 5.32 | * | 0.92 | ns | 3.39 | ns | 0.12 | ns | 1.01 | ns | 0.04 | ns |
| H. lanatus | 2.90 | ns | 3.24 | ns | 3.62 | ns | 0.25 | ns | 1.17 | ns | 0.16 | ns | 0.54 | ns |
| P. pratensis | 28.51 | *** | 0.48 | ns | 1.74 | ns | 0.13 | ns | 0.01 | ns | 0.00 | ns | 0.13 | ns |
| T. repens | 8.14 | ** | 4.51 | * | 0.10 | ns | 0.43 | ns | 2.21 | ns | 4.98 | * | 0.06 | ns |
| LEH | 0.00 | ns | 0.97 | ns | 0.02 | ns | 7.12 | * | 4.89 | * | 0.67 | ns | 0.00 | ns |
| AMF community in soil | 7.93 | ** | 0.99 | ns | −0.39 | ns | 4.99 | ** | 3.44 | * | 2.57 | ns | 0.37 | ns |
| No of AMF species in soil | 0.41 | ns | 0.00 | ns | 0.04 | ns | 6.11 | * | 3.57 | ns | 0.44 | ns | 0.03 | ns |
| AMF community in roots | 9.30 | *** | 0.06 | ns | −0.01 | ns | 0.26 | ns | 1.93 | ns | 0.51 | ns | 0.11 | ns |
| No of AMF species in roots | 9.10 | ** | 0.49 | ns | 0.74 | ns | 0.19 | ns | 0.03 | ns | 0.47 | ns | 0.42 | ns |
| Total AMF (P. lanceolata) | 15.21 | ** | 4.98 | * | 0.22 | ns | 1.20 | ns | 0.09 | ns | 6.95 | * | 4.65 | * |
| Arbuscules (P. lanceolata) | 4.91 | * | 0.05 | ns | 0.03 | ns | 0.26 | ns | 5.72 | * | 6.40 | * | 0.17 | ns |
significance level:
p<0.05,
p<0.01,
p<0.001,
ns = not significant, LEH: length of extraradical AMF hyphae, AMF: arbuscular mycorrhizal fungi.
The two soil biota communities differed in their AMF community composition in soil and P. lanceolata roots, with 1.09 AMF species more present in P. lanceolata roots grown in SEG 37 than in SEG 33. The length of extraradical hyphae did not differ for AMF from the two soil biota communities. Contrary to phase 1, AMF from SEG 33 colonized a higher percentage of P. lanceolata roots length than AMF from SEG 37. This was true for mycorrhizal structures in total as well as for arbuscules (30% and 45%, higher colonization with SEG 33 than SEG 37, respectively).
Root herbivore history effects
Total plant biomass was higher (9%; Table 1) when grown in Agriotes trained soil (presence of Agriotes larvae in phase 1) compared to untrained soil. The same tendency was observed for total shoot biomass (7%; lme: p = 0.05). Root biomass and plant diversity were not affected by the herbivore history of the soil. On the level of single plants, Agriotes trained soil facilitated shoot growth of T. officinale by 32% but reduced shoot growth of T. repens by 31% compared to untrained soil.
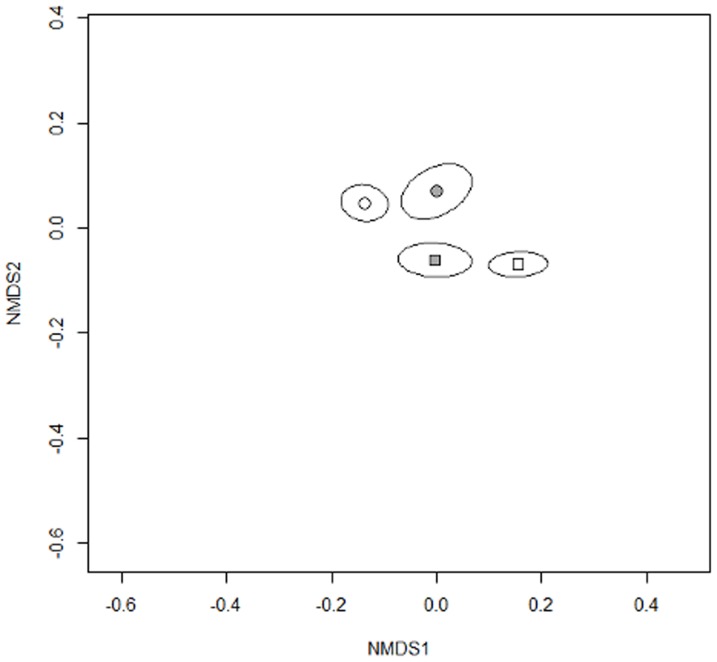
The effect of the Agriotes training on AMF community composition differed for the two soil biota communities (Figure 2). The community of Agriotes trained AMF consisted of 0.93 more species and 44% shorter extraradical hyphae for SEG 33 but of 0.42 fewer species and 34% longer hyphae for SEG 37 compared to the respective untrained AMF communities. Total structures of Agriotes trained AMF colonized 14% less of P. lanceolata roots length than structures of untrained AMF. Other AMF parameters were not affected by the Agriotes training.
Figure 2. Non-metric multidimensional scaling plot of AMF communities in soils after phase 2.
Circles and squares give centroids (means) of AMF communities for SEG33 and SEG37, respectively, open symbols show those of untrained and filled symbols those of trained AMF communities. Ellipsoids give standard error. The two soil biota origins are clearly differentiated along the second axis while the Agriotes training leads to opposing community shifts along the first axis.
Root herbivory effects
As in phase 1, plant diversity as well as plant community biomasses did not respond to the presence of Agriotes larvae. Also in accordance with phase 1, Agriotes larvae reduced shoot biomass of P. lanceolata (Figure 1C) by 15% on average and statistically independent of the soil biota community and Agriotes training, while shoot biomass of A. millefolium (Figure 1D) was not affected by the presence of Agriotes larvae when grown in soil biota community SEG 33, but enhanced by 65% when grown in untrained SEG 37. As indicated by a marginally non-significant three way interaction (lme: p = 0.06) of original soil biota community (SB), Agriotes training (AP1) and Agriotes presence (AP2), the positive effect of the presence of Agriotes larvae with soil biota community SEG 37 was lost when the soil was already Agriotes trained. Shoot biomass of T. repens (Figure 1E) was reduced by 28% by Agriotes larvae in phase 2, but only with untrained soil.
Contrarily to phase 1, AMF parameters were affected by the presence of Agriotes larvae. For the community composition in soil, the length of extraradical hyphae (LEH) and the percentage of P. lanceolata root length colonized by arbusclues the effect depended on the original soil biota community. LEH from SEG 33 was reduced by 27% (from 1.9 (se = 0.3) to 1.4 (se = 0.4) m/g DW soil) by Agriotes larvae while LEH from SEG 37 was increased by 49% (from 1.3 (se = 0.3) to 2.0 (se = 0.3) m/g DW soil). For AMF arbuscules an additional interaction of the factors Agriotes training and presence of Agriotes larvae resulted in colonization by Agriotes trained AMF being less negatively affected in SEG 33 and positively affected in SEG 37 by the presence of Agriotes larvae compared to arbuscules colonization by untrained AMF (50% reduction (from 22.0 (se = 2.7) to 11.0 (se = 1.0) %RLC) in untrained SEG 33 compared to no effect in trained SEG 33 (mean 17.1 (se = 4.4) %RLC), and 210% increase (from 5.3 (se = 0.5) to 16.3 (se = 1.5) %RLC) in trained SEG 37 compared to no effect in untrained SEG 37 (mean 12.5 (se = 2.4) %RLC)). On the level of total AMF structures, the same two way interaction of the presence of Agriotes larvae and Agriotes training as well as a three way interaction including original soil biota led to a similar effect for total AMF structures from SEG 37 (38% increase (from 39.0 (se = 3.4) to 54.0 (se = 2.4) %RLC) in trained SEG 37), whereas total AMF structures from SEG 33 were not affected (mean 69.2 (se = 4.9) %RLC).
Discussion
The study aimed at determining root herbivore history effects of Agriotes spp. larvae on a grassland plant community. As hypothesized, the root herbivore history of the soil influenced plant growth as well as plant growth response to new root herbivore attack. It affected plant community productivity (but not composition), showing for the first time that root herbivore induced changes in soil conditions can impact plant communities in a subsequent season. The root herbivore history of the soil proved to be a stronger driver of plant growth on the community level than an actual root herbivore attack which did not affect plant community parameters.
The two phases of the experiment differed in plant growth and community composition due to different growth conditions. Pots in phase 2 supported less plant biomass than pots in phase 1, presumably because they contained a smaller proportion of steamed, nutrient rich background soil.
Soil biota community effects
The original soil biota community affected the root biomass of the plant community and the shoot growth of several plant species in both phases of the experiment. The significant effects of the soil biota treatment confirm that (i) the two soil biota communities differed in their abundance/composition and (ii) the structure of soil biota communities can have pronounced impact on plant community structure [37].
Differences in community composition were also directly confirmed for AMF. Similar CN ratios of soil biota inocula (SEG 33 = 11.0 , SEG 37 = 11.8) suggest that factors other than nutrient provision by the decomposer community, like plant species specific interactions with beneficial or detrimental soil organisms were involved in creating the soil biota effect. The identity of the plant species that were affected by the soil biota community differed between the two phases of the experiment. Additionally, plant diversity was only affected by the soil biota community in phase 2. Differences in soil biota effects between phase 1 and 2 may be ascribed to differences in (i) soil biota/AMF community composition due to plant species specific accumulation of associated organisms (plant-soil feedback effects [38]) during phase 1 and due to the dry dormancy between phases and/or (ii) nutrient content of the growth substrate, with effects of soil organisms being more pronounced under the nutrient poor conditions [39] in phase 2.
Root herbivore history effects
The Agriotes training of the soil enhanced plant community productivity (total shoot and plant growth). Effects of the Agriotes training indicate changes in plant growth conditions due to the root herbivore that persisted even when the root herbivore was no longer present [7]. Our results show that these root herbivore history effects are even detectable at the plant community level. On the level of single plants Agriotes training enhanced shoot growth of T. officinale but reduced shoot growth of T. repens.
Effects on plant community biomasses and the N indicator species T. officinale [40] were positive, pointing to enhanced nutrient supply. Similar CN ratios of untrained and trained soil inocula suggest that nutrient provision by the decomposer community did again not cause the training effect. Instead, AMF communities differed between trained and untrained soils. The difference was not preceded by an actual root herbivore effect on AMF communities in the first phase of the experiment, indicating that it may have been generated through an influence on viability of AMF spores that changed community structure only after the dry dormancy between the two phases of the experiment. Though AMF community shifts due to root herbivore training differed depending on the original soil biota community, they may still have been towards higher abundance of species beneficial for those plants that contributed to enhanced plant community productivity (e.g. T. officinale) in both soil biota communities. The reduction in T. repens shoot biomass points to reduced competitive advantage through its symbiotic N fixation at improved nutrient acquisition by AMF for other plants.
Root herbivory effects
Plant community parameters did not respond to the presence of the root herbivore. For single plant species our results show that actual root herbivory effects are influenced by the root herbivore history of the soil, indicating that root herbivore induced changes in plant growth conditions also determine plant responses to new root herbivore attack.
Shoot growth of A. millefolium was facilitated by Agriotes larvae when grown in untrained soil biota community SEG 37, however, this effect was lost when the soil was already Agriotes trained. Shoot biomass of T. repens was reduced by Agriotes larvae, but only with the two untrained soils in phase 2. AMF communities in phase 2 were affected by the herbivore presence (in interaction with the original soil biota community). However, the actual root herbivore effect on AMF was less strong than the herbivore history effect. When grown individually in SEG 37 soil [21] shoot biomass of A. millefolium and T. repens did not respond to herbivory by Agriotes larvae. Thus, in the present study, responses of A. millefolium and T. repens to root herbivory may be best explained by (i) herbivore history induced differences in AMF species composition and (ii) shifts in the plant community composition. In untrained SEG 37 soil, roots of A. millefolium may have replaced those of other members of the community that were consumed by the root herbivore. Low root losses that did not result in changes in shoot biomass may have been sufficient, as A. millefolium responds strongly to release from below ground competition [41]. The shift was not detected in Agriotes trained SEG 37 where plants were potentially better nourished by AMF (see Root herbivore history effects) and thus able to compensate the herbivore damage [2], and in SEG 33 where plant community root biomass was lower and release from below ground competition thus less pronounced. Trifolium repens did not respond to Agriotes presence in phase 1 when it was the strongest competitor in the plant community but in phase 2 where it was less strong. In phase 2 the shoot mass reduction only occurred with untrained, potentially less well nourished plants where T. repens presumably benefited more from its N fixing symbiosis, thus, suggesting a root herbivore effect on the symbiosis [42] that became apparent under these conditions. In contrast, shoot biomass of P. lanceolata was reduced by Agriotes larvae in both phases of the experiment and the effect did not differ significantly for the two original soil biota communities and the training treatments, suggesting independence of soil conditions for the root herbivore effect on this plant species. This was despite the fact that the AMF community in P. lanceolata roots differed significantly between the two original soil biota treatments in phase 2 and quantitative root colonization parameters were additionally affected by the herbivore history and herbivore presence. In accordance, this plant species showed similar growth response to several AMF species and no connection of growth response to the degree of root colonization in other studies ([43] and [44], resp.). Substantial root biomass loss in the presence of Agriotes larvae in two experiments where P. lanceolata was grown individually ([19], [21]) point to a strong direct grazing effect that may have overridden indirect root herbivore effects for this plant species.
In conclusion, our study found evidence for root herbivore history effects on grassland plant communities. The effects may partly be explained by herbivore induced shifts in the AMF community. Interestingly, the root herbivore history of the soil proved to be a stronger driver of plant growth on the community level than an actual herbivore attack. History effects have to be taken into account when predicting the impact of root herbivores in grasslands.
Supporting Information
Includes the tables S1 and S2. Plant biomass data and AMF parameters in different soil treatments in phase1 and phase2.
(DOCX)
Detailed protocol for characterization of AMF community.
(DOC)
Acknowledgments
We thank the managers of the three exploratories, Swen Renner, Sonja Gockel, Andreas Hemp and Martin Gorke and Simone Pfeiffer for their work in maintaining the project infrastructure, and Markus Fischer, Elisabeth Kalko, Eduard Linsenmair, Dominik Hessenmöller, Jens Nieschulze, Daniel Prati, Ingo Schöning, François Buscot, Ernst-Detlef Schulze and Wolfgang W. Weisser for their role in setting up the Biodiversity Exploratories project. We thank Uta Schumacher for providing information about the management of Schorfheide sites and Bernhard Warzecha for his work on part of the TRFLP samples. We are very grateful to Uta Schumacher and Andreas Hemp for hands-on help with collecting soil.
Funding Statement
The work has been funded by the Deutsche Forschungsgemeinschaft (http://www.dfg.de/index.jsp) Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” (DFG-WU 603/3-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown VK, Gange AC (1990) Insect herbivory below ground. Advances in Ecological Research 20: 1–58. [Google Scholar]
- 2. Dosdall LM, Clayton GW, Harker KN, O'Donovan JT, Stevenson FC (2004) The effects of soil fertility and other agronomic factors on infestations of root maggots in canola. Agronomy Journal 96: 1306–1313. [DOI] [PubMed] [Google Scholar]
- 3. Kalb DW, Bergstrom GC, Shields EJ (1994) Prevalence, severity, and association of fungal crown and root rots with injury by the clover root curculio in New York alfalfa. Plant Disease 78: 491–495. [Google Scholar]
- 4. Yeates GW, Saggar S, Denton CS, Mercer CF (1998) Impact of clover cyst nematode (Heterodera trifolii) infection on soil microbial activity in the rhizosphere of white clover (Trifolium repens) - a pulse-labelling experiment. Nematologica 44: 81–90. [Google Scholar]
- 5. Bardgett RD, Denton CS, Cook R (1999) Below-ground herbivory promotes soil nutrient transfer and root growth in grassland. Ecology Letters 2: 357–360. [Google Scholar]
- 6. Treonis AM, Grayston SJ, Murray PJ, Dawson LA (2005) Effects of root feeding cranefly larvae on soil microorganisms and the composition of rhizosphere solutions collected from grassland plants. Applied Soil Ecology 28: 203–215. [Google Scholar]
- 7. Kostenko O, van de Voorde TFJ, Mulder PPJ, van der Putten WH, Bezemer TM (2012) Legacy effects of aboveground–belowground interactions. Ecology Letters 15: 813–821. [DOI] [PubMed] [Google Scholar]
- 8. Gange AC, Brown VK, Sinclair GS (1994) Reduction of black vine weevil larval growth by vesicular-arbuscular mycorrhizal infection. Entomologia Experimentalis et Applicata 70: 115–119. [Google Scholar]
- 9. Gange AC (2001) Species-specific responses of a root- and shoot-feeding insect to arbuscular mycorrhizal colonization of its host plant. New Phytologist 150: 611–618. [Google Scholar]
- 10. Currie AF, Murray PJ, Gange AC (2006) Root herbivory by Tipula paludosa larvae increases colonization of Agrostis capillaris by arbuscular mycorrhizal fungi. Soil Biology & Biochemistry 38: 1994–1997. [Google Scholar]
- 11. Currie AF, Murray PJ, Gange AC (2011) Is a specialist root-feeding insect affected by arbuscular mycorrhizal fungi? Applied Soil Ecology 47: 77–83. [Google Scholar]
- 12. Gange AC, Brown VK (2002) Soil food web components affect plant community structure during early succession. Ecological Research 17: 217–227. [Google Scholar]
- 13.Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. London: Elsevier Science Ltd.
- 14. Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417: 67–70. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez-Echeverría S, de la Peňa E, Moens M, Freitas H, van der Putten WH (2009) Can root-feeders alter the composition of AMF communities? Experimental evidence from the dunegrass Ammophila arenaria. Basic and Applied Ecology 10: 131–140. [Google Scholar]
- 16. Parker WE, Seeney FM (1997) An investigation into the use of multiple site characteristics to predict the presence and infestation level of wireworms (Agriotes spp., Coleoptera: Elateridae) in individual grass fields. Annuals of Applied Biology 130: 409–425. [Google Scholar]
- 17. Hemerik L, Gerrit G, Brussaard L (2003) Food preference of wireworms analyzed with multinomial logit models. Journal of Insect Behavior 16: 647–665. [Google Scholar]
- 18. Jedlicka P, Frouz J (2007) Population dynamics of wireworms (Coleoptera, Elateridae) in arable land after abandonment. Biologia 62: 103–111. [Google Scholar]
- 19. Wurst S, van der Putten WH (2007) Root herbivore identity matters in plant-mediated interactions between root and shoot herbivores. Basic and Applied Ecology 8: 491–499. [Google Scholar]
- 20. Wurst S, van Dam NM, Monroy F, Biere A, van der Putten WH (2008) Intraspecific variation in plant defense alters effects of root herbivores on leaf chemistry and aboveground herbivore damage. Journal of Chemical Ecology 34: 1360–1367. [DOI] [PubMed] [Google Scholar]
- 21. Sonnemann I, Baumhaker H, Wurst S (2012) Species specific responses of common grassland plants to a generalist root herbivore (Agriotes spp. larva). Basic and Applied Ecology 13: 579–586. [Google Scholar]
- 22. Fischer M, Bossdorf O, Gockel S, Hänsel F, Hemp A, et al. (2010) Implementing largescale and longterm functional biodiversity research: The Biodiversity Exploratories. Basic and Applied Ecology 11: 473–485. [Google Scholar]
- 23.Klausnitzer B (1994) Die Larven der Kaefer Mitteleuropas. Krefeld: Goecke & Evers Verlag. 325 p.
- 24. Börstler B, Renker C, Kahmen A, Buscot F (2006) Species composition of arbuscular mycorrhizal fungi in two mountain meadows with differing management types and levels of plant biodiversity. Biol Fertil Soils 42: 286–298. [Google Scholar]
- 25. Schoenholtz SH, Van Miegroet H, Burger JA (2000) A review of chemical and physical properties as indicators offorest soil quality: challenges and opportunities. Forest Ecology and Management 138: 335–356. [Google Scholar]
- 26. Watt MS, Coker G, Clinton PW, Davis MR, Parfitt R, et al. (2005) Defining sustainability of plantation forests through identification of site quality indicators influencing productivity—A national view for New Zealand. Forest Ecology and Management 216: 51–63. [Google Scholar]
- 27. Watt MS, Davis MR, Clinton PW, Coker G, Ross C, et al. (2008) Identification of key soil indicators influencing plantation productivity and sustainability across a national trial series in New Zealand. Forest Ecology and Management 256: 180–190. [Google Scholar]
- 28. Vierheilig H, Coughlan A, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Mircobiology 64: 5004–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist 115: 495–501. [DOI] [PubMed] [Google Scholar]
- 30. Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytologist 120: 371–380. [Google Scholar]
- 31. Tennant D (1975) A test of a modified line intersect method of estimating root length. Journal of Ecology 63: 995–1001. [Google Scholar]
- 32. Dickie IA, FitzJohn RG (2007) Using terminal restriction fragment length polymorphism (T-RFLP) to identify mycorrhizal fungi: a methods review. Mycorrhiza 17: 259–270. [DOI] [PubMed] [Google Scholar]
- 33. FitzJohn RG, Dickie IA (2007) TRAMPR: An R package for analysis and matching of terminal-restriction fragment length polymorphism (TRFLP) profiles. Molecular Ecology Notes 7: 583–587. [Google Scholar]
- 34.R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
- 35.Zuur AF, Ieno EN, Walker NJ, Saveliew AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. New York: Springer.
- 36. McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 82: 290–297. [Google Scholar]
- 37. Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, et al. (2004) Ecological linkages between aboveground and belowground biota. Science 304: 1629–1633. [DOI] [PubMed] [Google Scholar]
- 38. Bever JD, Westover KM, Antonovics J (1997) Incorporating the soil community into plant population dynamics: the utility of the feedback approach. Journal of Ecology 85: 561–573. [Google Scholar]
- 39. Araujo ASF, Leite LFC, Iwata BD, Lira MD, Xavier GR, et al. (2012) Microbiological process in agroforestry systems. A review. Agronomy for Sustainable Development 32: 215–226. [Google Scholar]
- 40.Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen. Stuttgart: Ulmer Verlag.
- 41. Kosola KR, Gross KL (1999) Resource competition and suppression of plants colonizing early successional old fields. Oecologia 118: 69–75. [DOI] [PubMed] [Google Scholar]
- 42. Dawson LA, Grayston SJ, Murray PJ, Pratt SM (2002) Root feeding behavior of Tipula paludosa (Meig.) (Diptera: Tipulidae) on Lolium perenne (L.) and Trifolium repens (L.). Soil Biology & Biochemistry 34: 609–615. [Google Scholar]
- 43. Zaller JG, Thomas F, Drapela T (2011) Soil sand content can alter effects of different taxa of mycorrhizal fungion plant biomass production of grassland species. European Journal of Soil Biology 47: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin SL, Mooney SJ, Dickinson MJ, West HM (2012) The effects of simultaneous root colonisation by three Glomus species on soil pore characteristics. Soil Biology & Biochemistry 49: 167–173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes the tables S1 and S2. Plant biomass data and AMF parameters in different soil treatments in phase1 and phase2.
(DOCX)
Detailed protocol for characterization of AMF community.
(DOC)