Abstract
Cataract is a key factor in the morbidity associated with diabetes. While the pathogenesis of diabetic cataract formation is poorly understood, previous research has identified aldose reductase (ALR2) as a key player. To elucidate a potential role for this enzyme in diabetic cataract formation, we created a series of transgenic mice designed for expression of human ALR2 (AKR1B1) in epithelial and outer cortical fiber cells of the lens. One of the founder lines, designated PAR39, developed an early onset cataract that involved formation of a plaque of cells at the anterior aspect of the lens. These cells appear to separate from the anterior epithelium and undergo a dramatic change that is reminiscent of the epithelial to mesenchymal transition (EMT). We characterized this phenotype in the PAR39 strain by examining rates of cell proliferation and by immunostaining for markers of EMT. Incorporation of the thymidine analog bromodeoxyuridine (BrdU) was used to estimate cell proliferation in two functional areas of the lens epithelium: the mitotically active germinative zone (GZ) and the less proliferative center zone (CZ). Staining cell nuclei with diamido 4',6-diamidino-2-phenylindole (DAPI) was used to establish a total cell count in the demarcated areas. Lens epithelium in PAR39 transgenic mice demonstrated a decrease in the percentage of BrdU/DAPI staining within the GZ as compared to nontransgenic littermate controls (8.1% vs. 10.9%). A similar decrease in BrdU/DAPI was observed in the CZ (0.6% compared to 3.3%). However, cell density was greater within the GZ of PAR39 mice as compared with nontransgenic controls, while it was not significantly different in the CZ among the two groups. Furthermore, cells associated with the epithelial plaque did not stain positive for BrdU, but were strongly positive for alpha-smooth muscle actin, a classical marker for EMT. These findings suggest that ALR2 over-expression is associated with an alteration in the balance between proliferation and apoptosis of epithelial cells in the mouse lens, and that cells associated with epithelial plaques in the PAR39 lens have features in common with cells undergoing EMT.
Keywords: cataract, EMT, epithelial-to-mesenchymal transition, aldo-keto reductase, diabetes
1. INTRODUCTION
Diabetes mellitus is one of the leading causes of blindness worldwide. Chronic diabetes is associated with changes in the retina, retinal vasculature, lens, cornea, iris and the sensory, motor, and autonomic nerves serving these structures. Given the increase in recent years in the incidence of this disease, diabetic eye disease is expected to place an increasingly large burden on the productivity and quality of life of society. Although studies have shown that normalization of circulating blood sugar is the most effective means to prevent the onset and/or progression of diabetic complications such as retinopathy, the personal challenges required to achieve sustained euglycemia are practically impossible to overcome [24]. Thus, therapeutic strategies are needed to deal with the long-term consequences of hyperglycemia.
Many theories have been advanced to explain the pathogenesis of diabetic eye disease. One of the most prominent areas of research has centered on aldose reductase (ALR2)1 and its role in the conversion of glucose to sorbitol. ALR2-mediated polyol accumulation has been shown to lead to osmotic imbalances and oxidative stress that result in fiber cell swelling, liquefaction, and eventually development of cataracts [7, 10]. Animal species that are deficient in ALR2 gene expression in lens, such as the mouse, are relatively resistant to the onset of diabetic cataracts [25]. In contrast, genetic polymorphisms linked to the human AKR1B1 gene have been associated with higher tissue levels of ALR2 and development of more advanced diabetic retinopathy [20, 31].
Srivastava and coworkers have demonstrated over the past several years that ALR2 plays a critical role in regulating intracellular signaling mediated by a variety of protein kinases [18]. While the mechanisms linking ALR2 function and protein kinase regulation are not well understood, it now appears that there is a broad spectrum of cellular phenotypes controlled by ALR2, including regulation of the inflammatory response [14, 17, 19, 23, 29] and cell proliferation [3, 15, 16]. Among the latter phenotypes, it was demonstrated that inhibitors of ALR2 were effective in suppressing proliferation of lens epithelial cells and their transition to mesenchymal cells in a process referred to as epithelial-to-mesenchymal transition [EMT, see ref. 28]. The EMT process is thought to underlie development of posterior capsule opacification (PCO), a relatively common post-surgical complication of cataract surgery [27].
In the course of our studies on mechanisms of diabetic eye disease, we produced a series of transgenic mice designed for expression of human ALR2 (AKR1B1) and human aldose reductase-like protein 1 (AKR1B10) selectively in the lens. While lenses from AKR1B10 transgenic mice were not significantly different from nontransgenic control mice in most respects [6], one of the strains of AKR1B1 transgenic mice, designated PAR39, was observed to develop a severe lens phenotype. In the current study, we present evidence that the cellular basis for this lens phenotype has many features that are reminiscent of cells involved with development of PCO reported from a porcine model [28]. These results are consistent with a role for AKR1B1 in the etiology of PCO in human cataract patients.
2. MATERIALS AND METHODS
2.1 Transgenic Mice
Transgenic mice were produced in strain C57BL6 by standard procedures. The transgene construct was prepared essentially as described previously for AKR1B10 transgenic mice, with the exception that coding sequences for recombinant protein were taken from the AKR1B1 cDNA [6, 13]. PCR-based genotyping was used to identify founder mice, which were then backcrossed to C57BL6 (Jackson Laboratories, Bar Harbor, ME) to maintain transgene sequences in a hemizygous state. The genetic stability of our strains was confirmed when we observed transmission of transgene sequences in the expected Mendelian pattern following multiple backcrosses to wild type breeding stock. Mice were housed in a germ-free environment and were maintained on standard laboratory chow. The presence and/or extent of lens changes were monitored in animals without the use of a general anesthetic. Eyes were dilated with topical administration of a mydriatic cocktail containing tropicamide and phenylephrine, and were then examined using a slit lamp ophthalmoscope (Topcon); images were captured by in-line digital camera. Eye globes for morphological and histological assessment were obtained from donor animals immediately after euthanasia. Lenses were carefully dissected free from other tissues while the eye was immersed in sterile PBS. Lens transparency and the presence of structural defects were documented by bright field microscopy using a Nikon SMZ800 microscope fitted to a Nikon DS-Fi1 digital camera.
2.2 Histology and Immunofluorescence
Eyes for histological analysis were treated with buffered formalin and then embedded in paraffin by standard procedures. Tissue sections for immunostaining were deparaffinized and rehydrated through a series of graded alcohols. Nonspecific antibody binding was minimized by treating tissues with 5% goat serum for 1 hour at room temperature, and then treated with primary antibodies diluted according to source. Alexa-488 labeled goat anti-rabbit antibody (Invitrogen, Carlsbad, CA) was used to detect immune complexes. Tissue sections were treated with 4',6-diamidino-2-phenylindole (DAPI) to visualize cell nuclei.
Antibodies to αSMA were diluted 500-fold into blocking buffer. Antibodies to γ-crystallin were diluted 1:1000 [5]. Analysis of tissue by immunofluorescence was carried out using a Nikon Eclipse 80i light microscope fitted to a Nikon DS Qi1Mc camera. In some cases, eyes were embedded in plastic and thin sections were stained by the toluidine blue method.
2.3 BrdU Labeling of Lens Epithelial Cells
Transgenic and nontransgenic P14 mice were injected intraperitoneally with a mixture containing 50 micrograms per gram of body weight of 5′-bromodeoxyuridine (BrdU; Invitrogen). One hour after injection the mice were euthanized and lenses dissected from the eye globe. Using fine forceps, the lens capsule-epithelium was carefully removed from the lens mass and flat mounted onto glass slides. The tissue was then fixed in 10% neutral buffered formalin in 1× PBS at room temperature for 10 min. After rinsing, tissues were treated with an Alexa-488 conjugated monoclonal antibody to BrdU (1:500; Invitrogen) and the fluorescent nucleic acid stain DAPI. Digital images were collected with a Nikon DS Qi1Mc camera fitted to the microscope, and cell counts performed visually by overlaying the image with a digital grid consisting of boxes measuring 50×50 pixels for comparison of cell density. Cells were counted in each box by their DAPI signal. Similarly, BrdU labeled cells were also counted. By this method, the average number of DAPI and BrdU positive cells per square were then calculated for each group of epithelium. The BrdU index was assessed by the ratio of BrdU to DAPI cells per box.
Two zones are functionally defined within the lens epithelium, namely the mitotically active germinative zone (GZ) and the less proliferative central region, defined here as the center zone (CZ) [2, 30]. The GZ is positioned equatorially on the lens and as a peripheral ring of cells on the flat mounts. The CZ covers the anterior surface of the lens and occupies the central portion of the flat mounts, bounded by the GZ. The areas were demarcated with a digital overlay based upon position and counted separately.
2.4 Statistical Analysis
Differences between samples were evaluated using Student's t-test. Statistical significance was determined when p< 0.05.
3. RESULTS
3.1 Production of AKR1B1 transgenic mice
To produce transgenic mice designed for over-expression of AKR1B1 in the ocular lens, we assembled a transgene construct composed of the hybrid α/δ-crystallin promoter fused to the complete cDNA sequence encoding the enzyme [21]. A full description of the characteristics of the 5 resulting independent founder lines will be published elsewhere. In brief, the transgenic lines differed primarily in the abundance of AKR1B1 transgene product expressed in the lens. Two of the lines, designated PAR37 and PAR39, respectively, contained the highest level of AKR1B1 as judged by a standard enzyme activity assay involving the NADPH-dependent reduction of DL-glyceraldehyde. There was a strong correspondence between apparent AKR1B1 activity and the abundance of AKR1B1 in lens homogenates assessed by Western blotting. The level of AKR1B1 activity was measured to be approximately 34±11 mU/mg protein in 21 day old PAR39 transgenic mice; this is about 3-times higher than the apparent aldo-keto reductase activity we detected in lens homogenates prepared from 3 month old rats. Aldo-keto reductase activity in nontransgenic littermates was essentially negligible. Thus, our transgenic approach allowed us to assess the effect of human aldose reductase (AKR1B1) expression in the lens of an animal model that has very low levels of endogenous aldose reductase.
3.2 Lens abnormalities associated with over-expression of AKR1B1
At the time of weaning at P21, lenses from PAR37 and PAR39 transgenic animals had developed cataracts and were noticeably opaque even to the unaided eye of the observer (for simplicity, only images for PAR39 are shown; Fig 1A). Dissection of the intact lens from the eye revealed the development of refractive abnormalities in the outer cortical layers of the lens, as evidenced by the intense areas of light refraction observed under bright field microscopy. In contrast, lenses from nontransgenic littermate controls were uniformly transparent and free from refractive discontinuities (Figs. 1B, 1C). Plastic thin sections produced from these lenses showed that the PAR39 lens was extensively disorganized in the anterior cortical region. Based on histological staining with toludine blue, the anterior cortex appears to have developed an extensive array of vacuoles that stain only weakly for intracellular materials; nontransgenic control lenses produced uniform staining when analyzed under the same conditions (Fig. 1D, 1E).
Figure 1. Structural abnormalities induced by over-expression of AKR1B1 in the mouse lens.
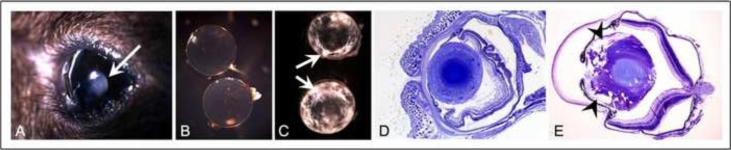
(A) Frank lens opacity (arrow) in P21 lens of AKR1B1 transgenic mice, strain PAR39; (B) Transparency of nontransgenic lens; (C) Refractive abnormalities in PAR30 transgenic lenses (arrows); (D) Toluidine blue staining of thin plastic section taken from nontransgenic control lens. Uniform staining is observed throughout the outer cortical region; (E) Extensive vacuole formation in outer cortex of PAR39 transgenic lens (arrowheads).
By the age of P21, most lenses from the PAR39 strain contained an unusual plaque of cells at the anterior surface. Unlike the surrounding lens cortical fiber cells, which have an elongated fiber-like morphology, the cells associated with the plaque body appeared more compact in shape. The lens plaque typically appeared to be physically detached from the surrounding lens fiber cells and the lens capsule (Fig. 2A,B)
Figure 2. Lens plaque formation in AKR1B1 transgenic lens.
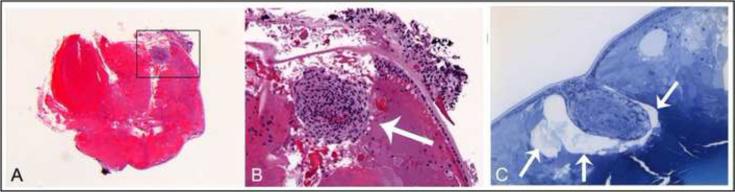
Animals were euthanized at P35 and paraffin sections were produced for H&E staining. (A) A dense cellular plaque was observed at the anterior aspect of lenses from transgenic strain PAR39. (B) Higher magnification of area demarcated in panel A. The dense cellular plaque is indicated by the arrow. (C) Some lenses were embedded in plastic and used to produce thin sections for toluidine blue staining. The lens plaque appears to be separated from surrounding cortical fiber cells and surrounded by large vacuoles (arrows).
3.3 Markers of epithelial-to-mesenchymal transition (EMT) in AKR1B1 transgenic mice
Following mechanical injury, lens epithelial cells are known to undergo a process of epithelialto-mesenchymal transition (EMT) characterized by upregulation of a number of markers such as α-smooth muscle actin (αSMA). In humans, the EMT process in lens can be stimulated by eye trauma or surgery such as phacoemulsification.
We carried out immunofluorescence studies to characterize cells associated with the lens plaque in PAR39 animals. When tissue sections were immunostained for γ-crystallin, a well-known cytoplasmic marker of lens fiber cell differentiation, cells surrounding the lens plaque were strongly positive, as expected. However, cells that comprise the plaque itself were not positive for this marker, indicating that they had not committed to terminal differentiation and develop into lens fiber cells (Fig. 3A,B). In contrast, cells associated with the plaque stained positive for αSMA, a well-known marker of EMT (Fig. 3C).
Figure 3. Markers for EMT in AKR1B1 transgenic lens.
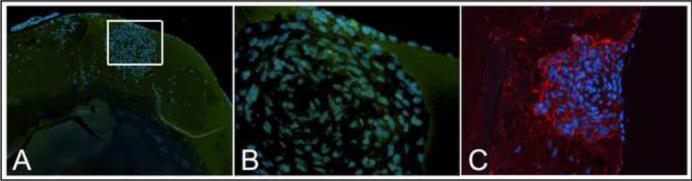
(A) Sections were stained with an antibody to γ-crystallin, a marker for lens fiber cell differentiation (green). Sections were also stained with DAPI to identify cell nuclei (blue). Lack of γ-crystallin staining in the cytoplasm of cells associated with the lens plaque, in contrast to uniform staining of surrounding cortical fiber cells, demonstrates that cells in the plaque have deviated from the normal programmed differentiation of epithelial cells into fiber cells. (C) Sections were also stained with an antibody to αSMA (red), a marker expressed by cells that are undergoing epithelial-to-mesenchymal transition (EMT). Cells associated with the lens plaque stain positive for αSMA, in contrast to surrounding lens fiber cells. Cell nuclei are identified by DAPI staining (blue).
3.4 Markers of epithelial cell proliferation in lens epithelium of AKR1B1 transgenic mice
AKR1B1 has been reported to be associated with induction of cell proliferation in disease models such as colon cancer [23]. To determine if over-expression of AKR1B1 induced abnormal proliferation of lens epithelial cells in our transgenic mouse model, we compared the BrdU labeling index in the germinative and central zones of the lens from transgenic and nontransgenic age-matched control animals.
3.4.1 Germinative Zone
The lens epithelium of nontransgenic animals demonstrated an average of 14.7±0.2 cells per square with 1.6±0.1 of these being BrdU positive within the germinative zone. This gives a BrdU labeling index of 10.9%±0.8%. This value is consistent with previous studies of the replicative index of the germinative zone of 2 week old mice [30]. In contrast, the PAR39 transgenic lens epithelia had an average of 18.4±0.3 cells per square with 1.5±0.1 BrdU positive. This gives a BrdU labeling index of 8.1%±0.7%. The difference of cell density in the germinative zone was significantly different between the transgenic and nontransgenic samples (P<0.0001). However, the difference of BrdU-labeled cells in the germinative zone was not significant (P>0.05).
3.4.2 Center Zone
For nontransgenic animals, a cell count of 15.3±0.3 and BrdU of 0.5±0.1 gives a BrdU index of 3.3%±0.7. Transgenic animals exhibited a cell count of 15.4±0.2, a BrdU count of 0.1±0.03 and a BrdU index of 0.6%±0.2%. With respect to the center zone, the cell densities were not significantly different (P>0.05). However, the AKR1B1 transgenic mice demonstrated a decreased BrdU-labeling index throughout the lens (p<0.0001), suggesting a decreased proliferative state of lens epithelial cells. With regard to cell density, the lens epithelial cells in AKR1B1 transgenic mice showed increased density in the germinative zone but similar density in the central zone as compared to nontransgenic.
4. Discussion
Relatively elevated levels of ALR2 are found within the lens, retina, schwann cells, liver, kidney, placenta, red blood cells, ovaries, sperm and seminal vesicles. This constellation of tissues is one of the reasons why ALR2 has been investigated as a possible therapeutic target for diabetic complications. Specifically, its localization in the lens and retina has made it a prime suspect in the pathogenesis of diabetic cataract and diabetic retinopathy, respectively [11, 12]. Past theories of the mechanism by which increased ALR2 activity mediates its pathologic effects in the lens have focused on osmotic imbalances due to buildup of polyols [7]. However, this model does not explain the other disturbances increased ALR2 activity can have on tissues. Of particular interest are the correlations to proliferation of cells in mouse colon cancer models [23] and in posterior capsular opacity development in the pig eye [28]. The proliferative state of lens epithelial cells is of paramount importance as this layer of cells is the only type of replicating cell of the lens mass and it is the regulated generation of new cells that result in the normal maturation of the lens. Epithelial cells form a monolayer on the anterior surface of the lens, which is continuously replenished by new cells arising from stem cells within the germinative zone near the equatorial region. A majority of the newly produced LECs move posteriorly and differentiate into lens fiber cells in the bow area [1].
The health of the LEC and parameters related to cell proliferation have been of interest in past studies [4, 26]. It has been long questioned if the cell density of the LEC has a bearing on the development of cataracts. However, the relationship between cell proliferation and cataracts is difficult to establish, particularly in a causal context.
Struck et al sought to investigate this issue in their study of lens epithelial cell densities in type-II diabetics vs. non-diabetics undergoing cataract surgery [22]. They collected capsulotomy specimens from 59 human subjects and analyzed the cell density of the LEC with respect to diabetic status and cataract type. The mean cell density in type-II diabetics was significantly lower than in the non-diabetic group. In addition, among cataract types the nuclear cataract had the highest cell density, while the lowest mean cell density was associated with posterior subcapsular cataracts (PSC.) PSC is a cataract form classically associated with diabetic disease and has a higher prevalence in this population than in non-diabetics [8].
Furthermore, Kumamoto et al investigated if there was a correlation between levels of erythrocyte ALR2 and LEC density in diabetics versus non-diabetic patients. They too used capsulotomy specimens from patients undergoing cataract surgery. In this study, higher levels of erythrocyte ALR2 correlated significantly with not only decreased LEC density, but also diabetic retinopathy and higher HbA1c [9]. These results led them to conclude that the polyol pathway via ALR2 may be associated with the reduction of epithelial cell density in the eyes of patients with DM.
Of note, the capsulotomy specimens of both of these studies only contained the central zone of the LEC, therefore, a statement of the density of the germinative zone could not be made. In addition, no analysis of the replicative state was taken either.
Hence, our finding of a decreased BrdU index would suggest that the decreased LEC density observed by Kumamoto and Struck may be secondary to decreased cell proliferation. The region sampled in the capsulotomy specimens would correlate to the CZ region of our analysis. While the cell density of the CZ was not decreased in transgenic as compared with nontransgenic controls, this could be due to the young age of our animals. The decreased density may develop as the animal ages when the low proliferation rate fails to populate the expanding CZ as the lens increases in size and old cells undergo apoptosis. It could also be due to defects in cell migration, as the cells typically originate in the GZ, with some cells mobilizing to the CZ.
Interestingly, our investigation of the germinative zone demonstrated increased cell density. However, when examining the proliferative state of this region, the BrdU index was decreased. This would suggest that a hyper proliferative state is not the reason for the increased cell density, as we would have expected from prior studies [23, 28]. Again, the explanation of disturbed LEC migration could explain this as cells would fail to mobilize to other parts of the lens. This issue of cell migration is certainly an area to be pursued in understanding the pathogenesis of diabetic cataract.
Our finding of increased density of the germinative zone thus prompts the question if this too is a finding that would be observed in human subjects. As the anatomic location of the GZ is in proximity of the zonules and ciliary body, it is unlikely that this area could be safely sampled during cataract surgery. Thus, cadaver studies could supply the information needed.
5. Conclusions
We propose that the PAR39 transgenic mouse model, which demonstrates characteristics of human diabetic eyes, will be a useful tool in the investigation of ALR2 and its causative role in diabetic complications of the vision system. ALR2-mediated induction of cellular changes similar to EMT in our transgenic mice suggests that ALR2 may play a key role in posterior capsular opacification that can develop in human patients following cataract surgery.
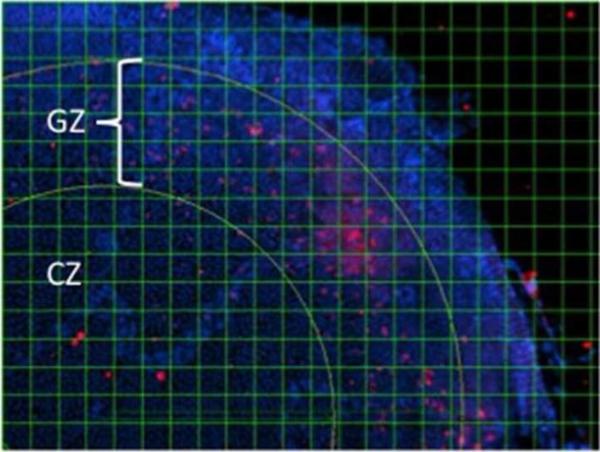
Figure 4. BrdU-labeling to quantify cell proliferation in the lens.
Lens capsule-epithelium flatmounts were prepared from BrdU-treated PAR39 transgenic and nontransgenic littermate control mice as described in the Materials and Methods section. Tissues were immunostained with an Alexa-conjugated antibody to BrdU and visualized by fluorescence microscopy (red). Cell nuclei were visualized by staining with DAPI (blue). BrdU-positive cells were concentrated in the germinative zone (GZ), a radial band of cells located roughly near the outer portion of the flatmounted tissue, demarcated by the yellow radial lines. The central zone (CZ) is the region located at the central region of the anterior surface of the lens. The labeling index was calculated by expressing the number of BrdU-positive cells relative to the total cell count revealed by DAPI staining in each 50 × 50 pixel area from the digital grid pattern.
6. Acknowledgements
Funding for this project was provided by grant EY005856 from the National Eye Institute (to JMP) and by a Boyscast Fellowship (to S. Palla) from the Ministry of Science and Technology of India. The authors gratefully acknowledge the assistance of Mike Casey, Theresa Harter, Belinda McMahan, and Sue Penrose in various aspects of the project. Dr. Lixing Reneker (University of Missouri, Columbia) generously provided the α/δ promoter transgene construct used in these studies. The authors wish to dedicate this paper to the memory of Professor Henry Weiner, whose tireless efforts to organize a biennial research conference devoted to carbonyl metabolism helped to launch the careers of countless scientists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Conflict of Interest Statement The authors declare that there are no conflicts of interest.
In cases referring to aldose reductase in a generic sense, the abbreviation ALR2 will be used. In cases referring to aldose reductase of a defined species origin, we will use the standard AKR nomenclature, such as AKR1B1 for human aldose reductase.
8. References Cited
- [1].Bassnett S. Three-dimensional reconstruction of cells in the living lens: the relationship between cell length and volume. Experimental Eye Research. 2005;81:716–723. doi: 10.1016/j.exer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [2].Bhat SP. The ocular lens epithelium. Bioscience Reports. 2001;21:537–563. doi: 10.1023/a:1017952128502. [DOI] [PubMed] [Google Scholar]
- [3].Chandra D, Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Role of aldose reductase in TNF-alpha-induced apoptosis of vascular endothelial cells. Chem.Biol.Interact. 2003;143–144:605–612. doi: 10.1016/s0009-2797(02)00191-6. [DOI] [PubMed] [Google Scholar]
- [4].Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci. 1998;39:2696–2706. [PubMed] [Google Scholar]
- [5].Hay RE, Andley UP, Petrash JM. Expression of recombinant bovine gamma B-, gamma C- and gamma D-crystallins and correlation with native proteins. Exp.Eye Res. 1994;58:573–584. doi: 10.1006/exer.1994.1052. [DOI] [PubMed] [Google Scholar]
- [6].Huang SP, Palla S, Ruzycki P, Varma A, Harter T, Reddy GB, Petrash JM. Aldo-keto reductases in the eye. Journal of Ophthalmology. 2010 doi: 10.1155/2010/521204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kinoshita JH, Nishimura C. The involvement of aldose reductase in diabetic complications. Diabetes-Metabolism Reviews. 1988;4:323–337. doi: 10.1002/dmr.5610040403. [DOI] [PubMed] [Google Scholar]
- [8].Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49–55. doi: 10.3109/09286589509071451. [DOI] [PubMed] [Google Scholar]
- [9].Kumamoto Y, Takamura Y, Kubo E, Tsuzuki S, Akagi Y. Epithelial cell density in cataractous lenses of patients with diabetes: association with erythrocyte aldose reductase. Exp.Eye Res. 2007;85:393–399. doi: 10.1016/j.exer.2007.06.007. [DOI] [PubMed] [Google Scholar]
- [10].Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. Faseb Journal. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- [11].Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp.Diabetes Res. 2007;2007:61038–61047. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: mechanisms and management. Diabetes/Metabolism Research and Reviews. 2010;26:172–180. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- [13].Petrash JM, Harter TM, Devine CS, Olins PO, Bhatnagar A, Liu SQ, Srivastava SK. Involvement of cysteine residues in catalysis and inhibition of human aldose reductase - site-directed mutagenesis of cys-80, cys-298, and cys-303. Journal Of Biological Chemistry. 1992;267:24833–24840. [PubMed] [Google Scholar]
- [14].Pladzyk A, Reddy AB, Yadav UC, Tammali R, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol.Vis.Sci. 2006;47:5395–5403. doi: 10.1167/iovs.06-0469. [DOI] [PubMed] [Google Scholar]
- [15].Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. Journal Of Biological Chemistry. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- [16].Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Srivastava SK. Aldose reductase mediates the mitogenic signals of cytokines. Chem.Biol.Interact. 2003;143–144:587–596. doi: 10.1016/s0009-2797(02)00194-1. [DOI] [PubMed] [Google Scholar]
- [17].Ramana KV, Fadl AA, Tammali R, Reddy ABM, Chopra AK, Srivastava SK. Aldose Reductase Mediates the Lipopolysaccharide-induced Release of Inflammatory Mediators in RAW264.7 Murine Macrophages. Journal Of Biological Chemistry. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- [18].Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- [19].Ramana KV, Tammali R, Reddy AB, Bhatnagar A, Srivastava SK. Aldose reductase-regulated tumor necrosis factor-alpha production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology. 2007;148:4371–4384. doi: 10.1210/en.2007-0512. [DOI] [PubMed] [Google Scholar]
- [20].Reddy GB, Satyanarayana A, Balakrishna N, Ayyagari R, Padma M, Viswanath K, Petrash JM. Erythrocyte aldose reductase activity and sorbitol levels in diabetic retinopathy. Mol.Vis. 2008;14:593–601. [PMC free article] [PubMed] [Google Scholar]
- [21].Reneker LW, Chen Q, Bloch A, Xie L, Schuster G, Overbeek PA. Chick delta1-crystallin enhancer influences mouse alphaA-crystallin promoter activity in transgenic mice. Invest Ophthalmol Vis.Sci. 2004;45:4083–4090. doi: 10.1167/iovs.03-1270. [DOI] [PubMed] [Google Scholar]
- [22].Struck HG, Heider C, Lautenschlager C. Changes in the lens epithelium of diabetic and non-diabetic patients with various forms of opacities in senile cataract. Klin Monbl Augenheilkd. 2000;216:204–209. doi: 10.1055/s-2000-10545. [DOI] [PubMed] [Google Scholar]
- [23].Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].C.The Diabetes. G. Complications Trial Research The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial. Archives Of Ophthalmology. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- [25].Varma SD, Kinoshita JH. The absence of cataracts in mice with congential hyperglycemia. Experimental Eye Research. 1974;19:577–582. doi: 10.1016/0014-4835(74)90095-5. [DOI] [PubMed] [Google Scholar]
- [26].Vasavada AR, Cherian M, Yadav S, Rawal UM. Lens epithelial cell density and histomorphological study in cataractous lenses. J Cataract Refract Surg. 1991;17:798–804. doi: 10.1016/s0886-3350(13)80415-4. [DOI] [PubMed] [Google Scholar]
- [27].Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009;88:257–269. doi: 10.1016/j.exer.2008.10.016. [DOI] [PubMed] [Google Scholar]
- [28].Yadav UC, Ighani-Hosseinabad F, van Kuijk FJ, Srivastava SK, Ramana KV. Prevention of posterior capsular opacification through aldose reductase inhibition. Invest Ophthalmol Vis.Sci. 2009;50:752–759. doi: 10.1167/iovs.08-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yadav UCS, Srivastava SK, Ramana KV. Aldose Reductase Inhibition Prevents Endotoxin-Induced Uveitis in Rats. Investigative Ophthalmology Visual Science. 2007;48:4634–4642. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yamamoto N, Majima K, Marunouchi T. A study of the proliferating activity in lens epithelium and the identification of tissue-type stem cells. Med Mol Morphol. 2008;41:83–91. doi: 10.1007/s00795-008-0395-x. [DOI] [PubMed] [Google Scholar]
- [31].Yang B, Millward A, Demaine A. Functional differences between the susceptibility Z−2/C-106 and protective Z+2/T-106 promoter region polymorphisms of the aldose reductase gene may account for the association with diabetic microvascular complications. Biochimica et Biophysica Acta. 2003;1639:1–7. doi: 10.1016/s0925-4439(03)00095-4. [DOI] [PubMed] [Google Scholar]