Abstract
BACKGROUND
The role of renin-angiotensin inhibition in older patients with diastolic heart failure and chronic kidney disease remains unclear.
METHODS
Of the 1340 patients (age ≥65 years), with diastolic heart failure (ejection fraction ≥45%) and chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1.73 m2), 717 received angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Propensity scores for the use of these drugs, estimated for each of the 1340 patients, were used to assemble a cohort of 421 pairs of patients, receiving and not receiving these drugs, who were balanced on 56 baseline characteristics.
RESULTS
During more than 8 years of follow-up, all-cause mortality occurred in 63% and 69% of matched patients with chronic kidney disease receiving and not receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, respectively (hazard ratio {HR}, 0.82; 95% confidence interval {CI}, 0.70–0.97; p=0.021). There was no association with heart failure hospitalization (HR, 0.98; 95% CI, 0.82–1.18; p=0.816). Similar mortality reduction (HR, 0.81; 95% CI, 0.66–0.995; p=0.045) occurred in a subgroup of matched patients with an estimated glomerular filtration rate <45 ml/min/1.73 m2. Among 207 pairs of propensity-matched patients without chronic kidney disease, the use of these drugs was not associated with mortality (HR, 1.03; 95% CI, 0.80–1.33; p=0.826) or heart failure hospitalization (HR, 0.99; 95% CI, 0.76–1.30; p=0.946).
CONCLUSIONS
A discharge prescription for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was associated with a significant reduction in all-cause mortality in older patients with diastolic heart failure and chronic kidney disease including those with more advanced chronic kidney disease.
Keywords: Angiotensin-converting enzyme inhibitors, Angiotensin receptor blockers, Chronic kidney disease, Diastolic heart failure
Chronic kidney disease is common in patients with heart failure and is associated with poor outcomes.1, 2 Angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers may improve clinical outcomes in older adults with systolic heart failure and chronic kidney disease, although this benefit appeared more marked in those without chronic kidney disease.3 Heart failure in older adults is often associated with preserved ejection fraction, also known as diastolic heart failure, which is more common among older women often with a history of hypertension.4, 5 Although heart failure symptoms do not vary by ejection fraction, 4, 5 diastolic heart failure patients generally have better outcomes.6, 7 Yet, compared to those without heart failure, these patients are at an increased risk of death.8 However, inhibitors of renin-angiotensin system have not been shown to improve outcomes in clinical trials enrolling chronic stable outpatients with diastolic heart failure.9–11 Because treatment effect is often more pronounced in subgroups with poorer prognosis, 12 and the intrinsic effect of chronic kidney disease on mortality may be more pronounced in diastolic than in systolic heart failure, 2 we hypothesized that rennin-angiotensin inhibitors would improve outcomes in diastolic heart failure patients with chronic kidney disease. Therefore, the objective of the current study was to examine the clinical effectiveness of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in hospitalized older patients with diastolic heart failure and chronic kidney disease.
MATERIALS AND METHODS
Data Source and Study Patients
The current study is based on the Alabama Heart Failure Project, the details of which have been described previously.3, 13 Briefly, 9649 charts of fee-for-service Medicare beneficiaries hospitalized with heart failure during 1998–2001 in 106 Alabama hospitals were abstracted. A primary discharge diagnosis of heart failure was ascertained using the International Classification of Diseases, 9th Revision, Clinical Modification codes for heart failure. These hospitalizations occurred in 8555 unique heart failure patients, of whom 7058 patients age 65 years or older were discharged alive, of whom 2166 had diastolic heart failure or left ventricular ejection fraction ≥45%. Of the 2166 diastolic heart failure patients, data on baseline serum creatinine was available on 2137 patients, of whom 1340 had chronic kidney disease, defined as an estimated glomerular filtration rate <60 ml/min/1.73 m2. Data on baseline demographics, clinical history including admission medications, hospital course and discharge medications were collected.
Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use
Of the 1340 patients with diastolic heart failure and chronic kidney disease, 717 (54%) received discharge prescriptions for angiotensin-converting enzyme inhibitors (n=558), angiotensin receptor blockers (n=147) or both (n=12). We used guideline recommended doses for systolic heart failure to categorize patients into those receiving below-target and target (at or above) doses of these drugs.3
Mortality and Hospitalization
The primary outcome was all-cause mortality over 8 years of follow-up through April 2, 2007. Secondary outcomes included all-cause and heart failure hospitalizations. All outcomes data were obtained from the Centers for Medicare and Medicaid Services Medicare fee-for-service claims files.3, 13
Assembly of a Balanced Cohort
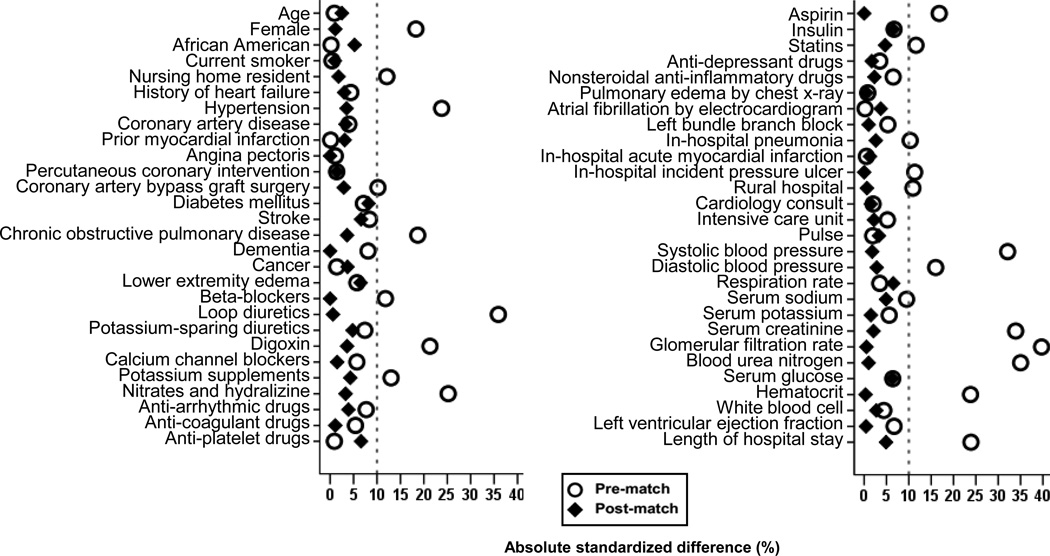
Because of the imbalances in baseline characteristics between patients receiving and not receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (Table 1 and Figure 1), we assembled a cohort of propensity-matched patients in which the two treatment groups would be well-balanced on all measured baseline covariates.3, 14–16 We used a nonparsimonious multivariable logistic regression model adjusting for 56 baseline characteristics (Figure 1) to estimate propensity scores for the receipt of these drugs for each of the 1340 patients.17, 18 Using a greedy matching protocol, we were able to match 421 pairs of patients receiving and not receiving these drugs who had similar propensity scores.19–21 Covariate balance before and after matching was assessed by estimating absolute standardized differences and presented as Love plots.22–24 An absolute standardized difference of 0% indicates no residual bias and differences <10% are considered inconsequential.
Table 1.
Baseline Patient Characteristics of Older Diastolic Heart Failure Patients with Chronic Kidney Disease by Discharge Prescriptions for Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers, Before and After Propensity Score Matching
| n (%) or mean (±SD) | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| Use of Angiotensin- Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
P Value | Use of Angiotensin- Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
P Value | |||
| No (n=623) | Yes (n=717) | No (n=421) | Yes (n=421) | |||
| Age (years) | 79 (±8) | 79 (±8) | 0.867 | 79 (±8) | 79 (±8) | 0.715 |
| Female | 415 (67) | 537 (75) | 0.001 | 300 (71) | 302 (72) | 0.937 |
| African American | 103 (17) | 119 (17) | 0.975 | 64 (15) | 72 (17) | 0.509 |
| Nursing home residents | 53 (9) | 39 (5) | 0.027 | 29 (7) | 31 (7) | 0.894 |
| Current smoker | 42 (7) | 49 (7) | 0.947 | 23 (5) | 24 (6) | 1.000 |
| Prior ACEI intolerance | 11 (1.8) | 10 (1.4) | 0.586 | 5 (1.2) | 5 (1.2) | 1.000 |
| Left ventricular ejection fraction (%) | 56 (±8) | 56 (±8) | 0.222 | 56 (±8) | 56 (±8) | 0.955 |
| Past medical history | ||||||
| Prior heart failure | 405 (65) | 481 (67) | 0.423 | 276 (66) | 282 (67) | 0.708 |
| Hypertension | 453 (73) | 592 (83) | <0.001 | 332 (79) | 338 (80) | 0.664 |
| Coronary artery disease | 328 (53) | 363 (51) | 0.460 | 213 (51) | 220 (52) | 0.684 |
| Myocardial infarction | 115 (19) | 132 (18) | 0.982 | 72 (17) | 77 (18) | 0.716 |
| Angina pectoris | 112 (18) | 126 (18) | 0.847 | 78 (19) | 78 (19) | 1.000 |
| Percutaneous coronary intervention | 83 (13) | 99 (14) | 0.796 | 58 (14) | 56 (13) | 0.920 |
| Coronary artery bypass graft | 145 (23) | 137 (19) | 0.062 | 87 (21) | 92 (22) | 0.735 |
| Left bundle branch block | 37 (6) | 52 (7) | 0.336 | 27 (6) | 26 (6) | 1.000 |
| Diabetes mellitus | 263 (42) | 328 (46) | 0.194 | 167 (40) | 184 (44) | 0.257 |
| Atrial fibrillation | 183 (29) | 210 (29) | 0.973 | 126 (30) | 119 (28) | 0.650 |
| Stroke | 144 (23) | 141 (20) | 0.124 | 77 (18) | 88 (21) | 0.396 |
| Chronic obstructive pulmonary disease | 230 (37) | 202 (28) | 0.001 | 131 (31) | 138 (33) | 0.643 |
| Dementia | 62 (10) | 55 (8) | 0.140 | 39 (9) | 39 (9) | 1.000 |
| Cancer | 11 (2) | 14 (2) | 0.801 | 8 (2) | 6 (1) | 0.774 |
| Clinical findings | ||||||
| Pulse (beats per minute) | 85 (±21) | 85 (±22) | 0.734 | 84 (±23) | 84 (±21) | 0.635 |
| Systolic blood pressure (mmHg) | 149 (±33) | 160 (±34) | <0.001 | 153 (±31) | 154 (±33) | 0.781 |
| Systolic blood pressure <80 (mmHg) | 3 (0.2) | 0 (0) | 0.063 | 1 (0.1) | 0 (0) | 0.317 |
| Diastolic blood pressure (mmHg) | 77 (±19) | 80 (±21) | 0.004 | 77 (±20) | 78 (±18) | 0.674 |
| Respiration (breaths per minute) | 23 (±6) | 23 (±6) | 0.523 | 23 (±5) | 23 (±6) | 0.341 |
| Peripheral edema | 458 (74) | 545 (76) | 0.294 | 307 (73) | 319 (76) | 0.382 |
| Pulmonary edema by chest x-ray | 440 (71) | 509 (71) | 0.884 | 292 (69) | 293 (70) | 1.000 |
| Tests and procedures | ||||||
| Serum sodium (mEq/L) | 138 (±5) | 139 (±5) | 0.082 | 139 (±4) | 138 (±5) | 0.479 |
| Serum potassium (mEq/L) | 4.3 (±0.7) | 4.3 (±0.7) | 0.302 | 4.3 (±0.7) | 4.3 (±0.7) | 0.836 |
| Serum potassium ≥5.5 (mEq/L) | 33 (5) | 32 (5) | 0.479 | 24 (6) | 25 (6) | 0.883 |
| Serum creatinine (mEq/L) | 2.0 (±1.3) | 1.6 (±0.9) | <0.001 | 1.8 (±1.2) | 1.7 (±0.9) | 0.758 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 38 (±14) | 43 (±12) | <0.001 | 40 (±13) | 40 (±13) | 0.941 |
| Estimated glomerular filtration rate < 15 (ml/min/1.73m2) | 48 (8) | 22 (3) | <0.001 | 19 (5) | 22 (5) | 0.631 |
| Blood urea nitrogen (mg/dL) | 35 (±20) | 29 (±14) | <0.001 | 31 (±16) | 32 (±17) | 0.883 |
| Serum glucose (mg/dL) | 147 (±64) | 151 (±68) | 0.244 | 144 (±65) | 148 (±63) | 0.361 |
| Hematocrit (%) | 34 (±6) | 36 (±6) | <0.001 | 35 (±6) | 35 (±6) | 0.966 |
| White blood cell (103/µL) | 9 (±5) | 9 (±6) | 0.421 | 9 (±4) | 9 (±5) | 0.650 |
| Hospital and care characteristics | ||||||
| Pneumonia | 185 (30) | 180 (25) | 0.060 | 116 (28) | 121 (29) | 0.751 |
| Acute myocardial infarction | 22 (4) | 26 (4) | 0.926 | 14 (3) | 13 (3) | 1.000 |
| Pressure ulcer | 61 (10) | 48 (7) | 0.039 | 34 (8) | 34 (8) | 1.000 |
| Rural hospital | 128 (21) | 180 (25) | 0.048 | 98 (23) | 97 (23) | 1.000 |
| Cardiology consult | 398 (64) | 451 (63) | 0.709 | 268 (64) | 265 (63) | 0.888 |
| Intensive care unit | 30 (5) | 27 (4) | 0.342 | 19 (5) | 21 (5) | 0.871 |
| Length of stay (days) | 8 (±6) | 7 (±5) | <0.001 | 7 (±6) | 7 (±5) | 0.464 |
| Discharge medications | ||||||
| Beta-blockers (heart failure) | 112 (18) | 163 (23) | 0.032 | 84 (20) | 84 (20) | 1.000 |
| Loop diuretics | 434 (70) | 606 (85) | <0.001 | 328 (78) | 329 (78) | 1.000 |
| Potassium-sparing diuretics | 58 (9) | 83 (12) | 0.178 | 44 (10) | 38 (9) | 0.567 |
| Digoxin | 159 (26) | 253 (35) | <0.001 | 133 (32) | 126 (30) | 0.659 |
| Calcium channel blockers | 228 (37) | 243 (34) | 0.301 | 156 (37) | 153 (36) | 0.884 |
| Potassium supplements | 239 (38) | 321 (45) | 0.018 | 190 (45) | 181 (43) | 0.571 |
| Nitrates and hydralazine | 40 (6) | 11 (2) | <0.001 | 8 (2) | 10 (2) | 0.815 |
| Anti-arrhythmic drugs | 63 (10) | 90 (13) | 0.161 | 47 (11) | 42 (10) | 0.644 |
| Anti-coagulants | 142 (23) | 180 (25) | 0.323 | 96 (23) | 94 (22) | 0.937 |
| Anti-platelet drugs | 66 (11) | 78 (11) | 0.867 | 35 (8) | 43 (10) | 0.410 |
| Aspirin | 210 (34) | 300 (42) | 0.002 | 158 (38) | 158 (38) | 1.000 |
| Insulin | 104 (17) | 138 (19) | 0.226 | 61 (14) | 71 (17) | 0.387 |
| Statins | 85 (14) | 128 (18) | 0.036 | 58 (14) | 65 (15) | 0.551 |
| Anti-depressants | 131 (21) | 161 (22) | 0.528 | 93 (22) | 90 (21) | 0.864 |
| Non-steroidal anti-inflammatory drugs | 62 (10) | 86 (12) | 0.234 | 45 (11) | 48 (11) | 0.815 |
Figure 1.
Love plots displaying absolute standardized differences for 56 baseline characteristics between older diastolic heart failure patients with chronic kidney disease receiving versus not receiving discharge prescriptions for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, before and after propensity score matching.
Of the 2137 diastolic heart failure patients with data on baseline serum creatinine, 797 patients were without chronic kidney disease, defined as estimated glomerular filtration rate ≥60 ml/min/1.73 m2. Using this cohort of 797 patients and employing the above propensity-matching approach, we assembled a second balanced cohort of 207 pairs of diastolic heart failure patients without chronic kidney disease receiving and not receiving these drugs.
Statistical Analysis
Baseline characteristics between the two treatment groups were compared using Pearson’s Chi square and Wilcoxon rank-sum tests for the pre-match data, and McNemar’s test and paired sample t-test for post-match comparisons, as appropriate. Our primary outcome of interest was all-cause mortality during 8 years of follow-up.3 When a cohort of older heart failure patients is followed for a long duration, mortality is expected to be 100% regardless of treatment or intervention. Therefore, instead of comparing proportions of events, we compared times to events using Kaplan-Meier and Cox regression analyses. For hospitalization outcomes, to adjust for competing risk of death, we also examined associations with time to composite endpoints of allcause mortality or heart failure hospitalization and all-cause mortality or all-cause hospitalization. Formal sensitivity analyses were conducted to quantify the degree of a hidden bias that would be required to explain away a significant association among matched patients. Subgroup analyses were conducted to determine the homogeneity of association. We then examined the associations of below-target and target doses of these drugs with outcomes using patients not receiving these drugs as reference.25 Finally, we examined the associations of these drugs with outcomes in those with chronic kidney disease Stage ≥3B (estimated glomerular filtration rate <45 ml/min/1.73 m2) not receiving renal replacement therapy. All statistical tests were two-tailed with a p-value <0.05 considered significant. Statistical analyses were performed using SPSS-18 for Windows (SPSS, Inc., 2009, Chicago, IL).
RESULTS
Baseline Characteristics
Matched patients (n=842) had a mean age (±SD) of 79 (±8) years, 71% were women, and 16% were African American. Pre-match imbalances in the distribution of various baseline characteristics between the two treatment groups were well balanced after matching (Table 1 and Figure 1). Post-match absolute standardized differences for all measured covariates were <10% (most <5%) suggesting substantial bias reduction. Matched diastolic heart failure patients without chronic kidney disease (n=414) had a mean age (±SD) of 79 (±8) years, 66% were women, and 23% were African American, who were also well balanced after matching (Table 2).
Table 2.
Baseline Patient Characteristics of Older Diastolic Heart Failure Patients without Chronic Kidney Disease by Discharge Prescriptions for Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers, Before and After Propensity Score Matching
| n (%) or mean (±SD) | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| Use of Angiotensin- Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
P Value | Use of Angiotensin- Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
P Value | |||
| No (n=330) | Yes (n=467) | No (n=207) | Yes (n=207) | |||
| Age (years) | 79 (±8) | 78 (±8) | 0.094 | 79 (±8) | 79 (±8) | 0.603 |
| Female | 220 (67) | 295 (63) | 0.309 | 135 (65) | 138 (67) | 0.836 |
| African American | 68 (21) | 136 (29) | 0.007 | 51 (25) | 46 (22) | 0.640 |
| Nursing home residents | 30 (9) | 20 (4) | 0.006 | 17 (8) | 14 (7) | 0.711 |
| Current smoker | 35 (11) | 42 (9) | 0.448 | 22 (11) | 22 (11) | 1.000 |
| Prior ACEI intolerance | 2 (0.6) | 6 (1.3) | 0.344 | 0 (0) | 4 (1.9) | 0.044 |
| Left ventricular ejection fraction (%) | 58 (±8) | 56 (±8) | 0.0001 | 57 (±8) | 58 (±9) | 0.486 |
| Past medical history | ||||||
| Prior heart failure | 183 (56) | 274 (59) | 0.366 | 117 (57) | 119 (58) | 0.922 |
| Hypertension | 200 (61) | 361 (77) | <0.001 | 150 (73) | 140 (68) | 0.332 |
| Coronary artery disease | 140 (42) | 199 (43) | 0.958 | 85 (41) | 80 (39) | 0.691 |
| Myocardial infarction | 36 (11) | 85 (18) | 0.005 | 31 (15) | 31 (15) | 1.000 |
| Angina pectoris | 57 (17) | 82 (18) | 0.916 | 32 (16) | 35 (17) | 0.795 |
| Percutaneous coronary intervention | 33 (10) | 53 (11) | 0.545 | 16 (8) | 18 (9) | 0.856 |
| Coronary artery bypass graft | 43 (13) | 76 (16) | 0.206 | 27 (13) | 25 (12) | 0.878 |
| Left bundle branch block | 13 (4) | 35 (8) | 0.038 | 11 (5) | 11 (5) | 1.000 |
| Diabetes mellitus | 98 (30) | 195 (42) | 0.001 | 73 (35) | 73 (35) | 1.000 |
| Atrial fibrillation | 110 (33) | 130 (28) | 0.096 | 59 (29) | 64 (31) | 0.661 |
| Stroke | 58 (18) | 84 (18) | 0.881 | 38 (18) | 41 (20) | |
| Chronic obstructive pulmonary disease | 123 (37) | 175 (38) | 0.954 | 85 (41) | 77 (37) | 0.505 |
| Dementia | 49 (15) | 37 (8) | 0.002 | 24 (12) | 21 (10) | 0.755 |
| Cancer | 12 (4) | 3 (1) | 0.002 | 4 (2) | 3 (1) | 1.000 |
| Clinical findings | ||||||
| Pulse (beats per minute) | 92 (±24) | 85 (±22) | <0.001 | 88 (±21) | 90 (±22) | 0.552 |
| Systolic blood pressure (mmHg) | 152 (±31) | 164 (±31) | <0.001 | 158 (±32) | 159 (±30) | 0.670 |
| Systolic blood pressure <80 (mmHg | 1 (0.3) | 0 (0) | 0.234 | 0 (0) | 0 (0) | 0.000 |
| Diastolic blood pressure (mmHg) | 80 (±16) | 83 (±19) | 0.026 | 81 (±16) | 82 (±19) | 0.760 |
| Respiration (breaths per minute) | 23 (±6) | 23 (±5) | 0.044 | 23 (±6) | 23 (±6) | 0.753 |
| Peripheral edema | 233 (71) | 345 (74) | 0.308 | 143 (69) | 150 (73) | 0.505 |
| Pulmonary edema by chest x-ray | 209 (63) | 288 (62) | 0.633 | 129 (62) | 133 (64) | 0.755 |
| Tests and procedures | ||||||
| Serum sodium (mEq/L) | 138 (±5) | 139 (±5) | 0.137 | 138 (±5) | 138 (±5) | 0.872 |
| Serum potassium (mEq/L) | 4.1 (±0.5) | 4.1 (±0.5) | 0.269 | 4.1 (±0.5) | 4.1 (±0.5) | 0.521 |
| Serum potassium >5.5 (mEq/L) | 5 (2) | 6 (1) | 0.784 | 3 (1) | 2 (1) | 1.000 |
| Serum creatinine (mEq/L) | 0.9 (±0.19) | 0.9 (±0.18) | <0.001 | 0.9 (±0.18) | 0.9 (±0.18) | 0.833 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 82 (±23) | 78 (±18) | 0.004 | 80 (±20) | 79 (±18) | 0.744 |
| Blood urea nitrogen (mg/dL) | 17 (±8) | 17 (±6) | 0.455 | 16 (±7) | 17 (±6) | 0.414 |
| Serum glucose (mg/dL) | 138 (±54) | 143 (±60) | 0.284 | 139 (±54) | 142 (±57) | 0.489 |
| Hematocrit (%) | 37 (±6) | 38 (±6) | 0.441 | 37 (±6) | 38 (±6) | 0.506 |
| White blood cell (103/µL) | 9 (±4) | 9 (±4) | 0.095 | 9 (±4) | 9 (±4) | 0.613 |
| Hospital and care characteristics | ||||||
| Pneumonia | 80 (24) | 108 (23) | 0.715 | 50 (24) | 53 (26) | 0.815 |
| Acute myocardial infarction | 19 (6) | 16 (3) | 0.114 | 11 (5) | 9 (4) | 0.824 |
| Pressure ulcer | 28 (9) | 27 (6) | 0.138 | 14 (7) | 12 (6) | 0.845 |
| Rural hospital | 79 (24) | 113 (24) | 0.933 | 52 (25) | 50 (24) | 0.908 |
| Cardiology consult | 193 (59) | 303 (65) | 0.067 | 127 (61) | 126 (61) | 1.000 |
| Intensive care unit | 17 (5) | 16 (3) | 0.228 | 9 (4) | 8 (4) | 1.000 |
| Length of stay (days) | 7 (±5) | 6 (±4) | 0.010 | 6 (±4) | 7 (±4) | 0.592 |
| Discharge medications | ||||||
| Beta-blockers (heart failure) | 38 (12) | 72 (15) | 0.116 | 25 (12) | 31 (15) | 0.488 |
| Loop diuretics | 233 (71) | 396 (85) | <0.001 0.001 | 165 (80) | 166 (80) | 1.000 |
| Potassium-sparing diuretics | 18 (6) | 59 (13) | 0.001 | 14 (7) | 16 (8) | 0.845 |
| Digoxin | 85 (26) | 160 (34) | 0.010 | 55 (27) | 57 (28) | 0.911 |
| Calcium channel blockers | 117 (36) | 118 (25) | 0.002 | 68 (33) | 67 (32) | 1.000 |
| Potassium supplements | 150 (46) | 246 (53) | 0.045 | 104 (50) | 97 (47) | 0.547 |
| Nitrates and hydralazine | 3 (0.9) | 2 (0.4) | 0.397 | 2 (1) | 2 (1) | 1.000 |
| Anti-arrhythmic drugs | 38 (12) | 43 (9) | 0.288 | 22 (11) | 18 (9) | 0.608 |
| Anti-coagulant drugs | 73 (22) | 106 (23) | 0.848 | 46 (22) | 41 (20) | 0.615 |
| Anti-platelet drugs | 27 (8) | 56 (12) | 0.083 | 21 (10) | 22 (11) | 1.000 |
| Aspirin | 98 (30) | 184 (39) | 0.005 | 66 (32) | 69 (33) | 0.824 |
| Insulin | 33 (10) | 80 (17) | 0.004 | 23 (11) | 25 (12) | 0.878 |
| Statins | 21 (6) | 71 (15) | <0.001 | 20 (10) | 19 (9) | 1.000 |
| Anti-depressant drugs | 64 (19) | 83 (18) | 0.561 | 39 (19) | 35 (17) | 0.689 |
| Non-steroidal anti-inflammatory drugs | 31 (9) | 64 (14) | 0.064 | 20 (10) | 19 (9) | 1.000 |
All-Cause Mortality in Diastolic Heart Failure and Chronic Kidney Disease
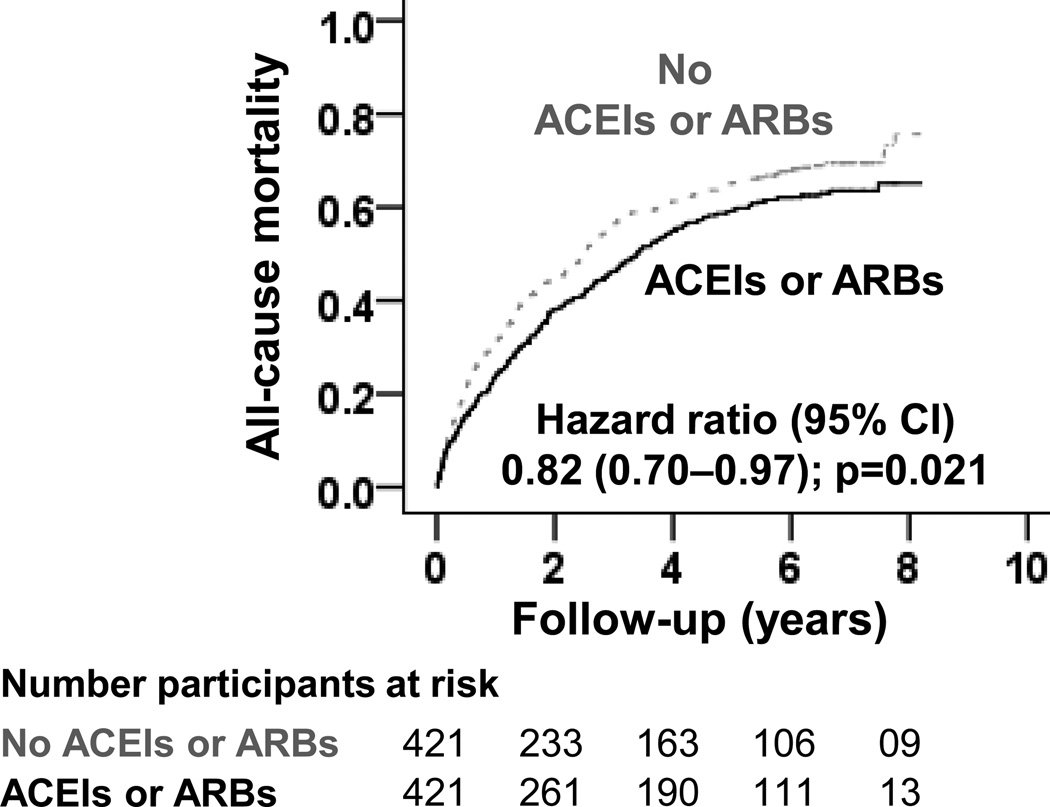
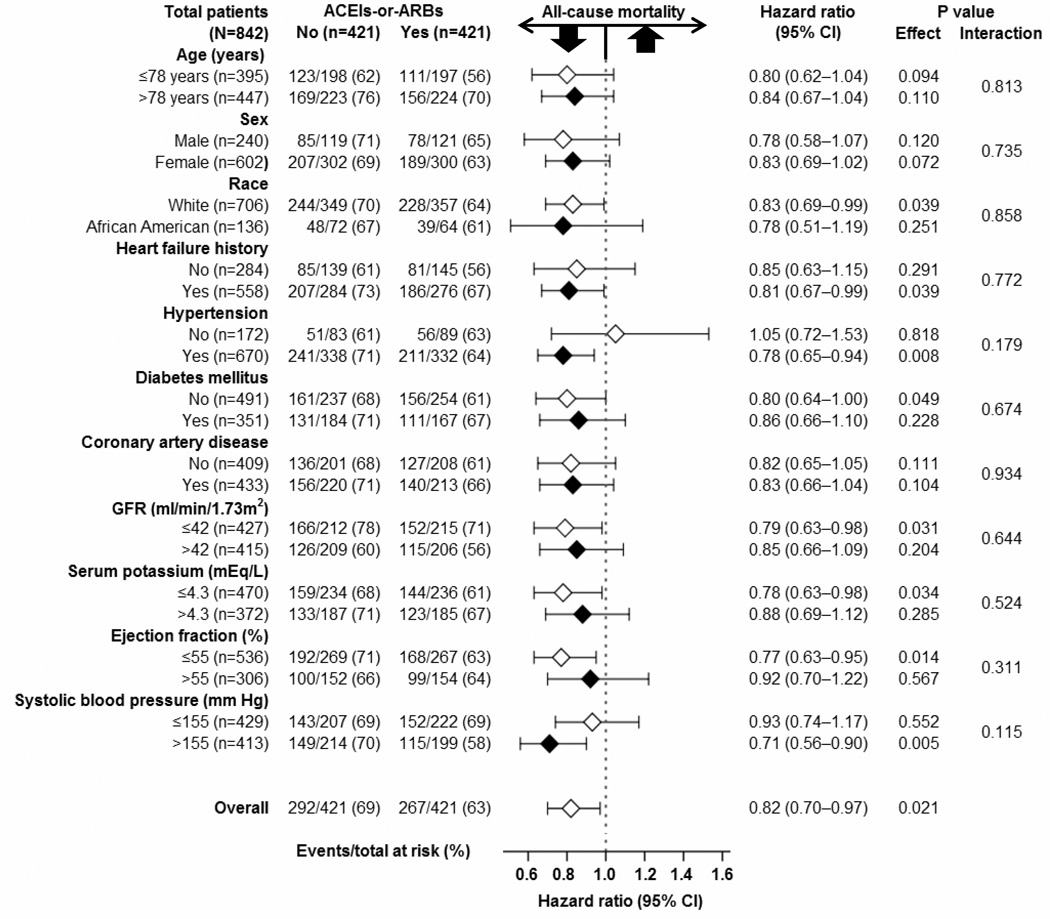
Among matched patients with diastolic heart failure and chronic kidney disease a discharge prescription for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was associated with a 11-month longer (41 versus 30 months for those not receiving those drugs) median survival, corresponding with a 18% relative risk reduction (hazard ratio {HR}, 0.82; 95% confidence interval {CI}, 0.70–0.97; p=0.021; Table 3 and Figure 2). A hidden covariate that is a near-perfect predictor of mortality may potentially explain away this association if it would increase the odds of discharge prescription for these drugs by about 1%. This association was homogeneous across various subgroups of patients (Figure 3). Similar risk-adjusted associations were observed in 1340 pre-match patients with chronic kidney disease (Table 3).
Table 3.
Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use with All-Cause Mortality in Older Diastolic Heart Failure Patients with and without Chronic Kidney Disease, Before and After Propensity Score Matching
| % (Total Events/Total Patients); Median Time to Event (95% CI) in Months |
||||
|---|---|---|---|---|
| All-Cause Mortality | Hazard Ratio* (95% CI) |
P Value | ||
| Use of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
||||
| No | Yes | |||
| Chronic kidney disease | ||||
| Pre-match, unadjusted | 73% (456/623); 27 (22–31) |
63% (450/717); 47 (41–53) |
0.70 (0.61–0.80) | <0.001 |
| Pre-match, multivariable-adjusted | --- | --- | 0.81 (0.70–0.93) | 0.003 |
| Pre-match, propensity-adjusted | --- | --- | 0.83 (0.72–0.96) | 0.010 |
| Propensity-matched | 69% (292/421); 30 (25–35) |
63% (267/421); 41 (34–48) |
0.82 (0.70–0.97) | 0.021 |
| No chronic kidney disease | ||||
| Pre-match, unadjusted | 59% (193/330); 49 (37–62) |
53% (248/467); 71 (60–82) |
0.83 (0.69–1.01) | 0.056 |
| Pre-match, multivariable-adjusted | --- | --- | 1.02 (0.82–1.27) | 0.868 |
| Pre-match, propensity-adjusted | --- | --- | 1.05 (0.85–1.31) | 0.649 |
| Propensity-matched | 58% (119/207); 52 (38–66) |
58% (119/207); 61 (42–81) |
1.03 (0.80–1.33) | 0.826 |
Hazard ratios comparing patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers with patients not receiving those drugs
Figure 2.
Kaplan-Meier plot for all-cause mortality in a propensity-matched cohort of older diastolic heart failure patients with chronic kidney disease receiving and not receiving discharge prescription of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs)
Figure 3.
Association of discharge prescription of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) with all-cause mortality in subgroups of propensity-matched older diastolic heart failure patients with chronic kidney disease; (GFR = glomerular filtration rate)
Of the 309 (73% of 421) matched patients with data on doses, 92 (22%) received target and 217 (51%) received below-target doses of these drugs. HRs for total mortality associated with the use of below-target and target doses were 0.82 (95% CI, 0.67–1.00; p=0.051) and 0.84 (95% CI, 0.63–1.11; p=0.224), respectively. Respective pre-match multivariable-adjusted HRs associated with below-target and target-dose use were 0.80 (95% CI, 0.68–0.95; p=0.011) and 0.79 (95% CI, 0.63–0.99; p=0.042), respectively.
Heart Failure Hospitalization in Diastolic Heart Failure and Chronic Kidney Disease
Patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers had a 6% higher absolute risk and about 7 months longer median time to heart failure hospitalization (HR, 0.98; 95% CI, 0.82–1.18; p=0.816; Table 4). Median time to composite endpoints of all-cause mortality or heart failure hospitalization for patients receiving and not receiving these drugs were 18 (95% CI, 15–21) and 11 (95% CI, 9–14) months, respectively (HR, 0.90; 95% CI, 0.78–1.04; p=0.149).
Table 4.
Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use with Heart Failure Hospitalization in Older Diastolic Heart Failure Patients with and without Chronic Kidney Disease, Before and After Propensity Score Matching
| % (Total Events/Total Patients); Median Time to Event (95% CI) in Months |
||||
|---|---|---|---|---|
| Heart Failure Hospitalization | Hazard Ratio* (95% CI) |
P Value | ||
| Use of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
||||
| No | Yes | |||
| Chronic kidney disease | ||||
| Pre-match, unadjusted | 50% (311/623); 28 (23–34) |
60% (430/717); 31 (27–35) |
1.00 (0.86–1.15) | 0.960 |
| Pre-match, multivariable-adjusted | --- | --- | 0.93 (0.79–1.09) | 0.364 |
| Pre-match, propensity-adjusted | --- | --- | 0.97 (0.83–1.13) | 0.679 |
| Propensity-matched | 52% (218/421); 27 (20–35) |
58% (243/421); 34 (29–34) |
0.98 (0.82–1.18) | 0.816 |
| No chronic kidney disease | ||||
| Pre-match, unadjusted | 47% (155/330); 44 (34–54) |
53% (249/467); 41 (34–48) |
1.10 (0.90–1.34) | 0.363 |
| Pre-match, multivariable-adjusted | --- | --- | 1.03 (0.82–1.31) | 0.793 |
| Pre-match, propensity-adjusted | --- | --- | 1.02 (0.81–1.30) | 0.847 |
| Propensity-matched | 51% (106/207); 43 (35–52) |
49% (102/207); 47 (38–57) |
0.99 (0.76–1.30) | 0.946 |
Hazard ratios comparing patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers with patients not receiving those drugs.
All-Cause Hospitalization in Diastolic Heart Failure and Chronic Kidney Disease
Patients receiving renin-angiotensin inhibitors had a 1% lower relative risk and 3 months longer median time to all-cause hospitalization (HR, 0.81; 95% CI, 0.70–0.94; p=0.005; Table 5). This association remained unchanged for the composite endpoints of all-cause mortality or all-Page 8 of 18 cause hospitalization with median time to events of 5 (95% CI, 4–6) and 3 (95% CI, 2–4) months for those receiving and not receiving therapy, respectively (HR, 0.82; 95% CI, 0.71–0.94; p=0.005).
Table 5.
Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use with All-Cause Hospitalization in Older Diastolic Heart Failure Patients with and without Chronic Kidney Disease, Before and After Propensity Score Matching
| % (Total Events/Total Patients); Median Time to Event (95% CI) in Months |
||||
|---|---|---|---|---|
| All-Cause Hospitalization | Hazard Ratio* (95% CI) |
P Value | ||
| Use of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers |
||||
| No | Yes | |||
| Chronic kidney disease | ||||
| Pre-match, unadjusted | 89% (553/623); 3.1 (2.5–3.7) |
88% (630/717); 5.7 (4.5–6.9) |
0.77 (0.69–0.86) | <0.001 |
| Pre-match, multivariable-adjusted | --- | --- | 0.78 (0.69–0.88) | <0.001 |
| Pre-match, propensity-adjusted | --- | --- | 0.79 (0.70–0.90) | <0.001 |
| Propensity-matched | 89% (374/421); 3.4 (2.5–4.3) |
88% (371/421); 6.1 (4.3–7.9) |
0.81 (0.70–0.94) | 0.005 |
| No chronic kidney disease | ||||
| Pre-match, unadjusted | 86% (285/330); 7.5 (5.9–9.1) | 89% (414/467); 7.3 (5.7–8.9) |
0.95 (0.81–1.10) | 0.459 |
| Pre-match, multivariable-adjusted | --- | --- | 0.93 (0.78–1.11) | 0.424 |
| Pre-match, propensity-adjusted | --- | --- | 0.94 (0.79–1.13) | 0.941 |
| Propensity-matched | 89% (184/207); 7.3 (5.3–9.3) |
86% (178/207); 5.8 (3.3–8.4) |
0.92 (0.75–1.13) | 0.404 |
Hazard ratios comparing patients receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers with patients not receiving those drugs
All-Cause Mortality in Diastolic Heart Failure and Chronic Kidney Disease Stage 3B or Greater
Among the subset of 487 matched patients with diastolic heart failure and chronic kidney disease stage ≥3B, all-cause mortality occurred in 69% (171/247) of those receiving reninangiotensin inhibitors and 76% (182/240) of those not receiving these drugs, with respective median survival times of 32 (95% CI, 25–39) and 22 (95% CI, 15–29) months (HR when the use of these drugs was compared to their non-use, 0.81; 95% CI, 0.66–0.995; p=0.045). Relative to nonuse of these drugs, HRs for all-cause mortality associated with their use in below-target and target doses were 0.84 (95% CI, 0.65–1.08; p=0.176) and 0.70 (95% CI, 0.48–1.03; p=0.067), respectively.
Outcomes in Older Diastolic Heart Failure Patients without Chronic Kidney Disease
A discharge prescription for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers had no association with all-cause mortality (HR, 1.03; 95% CI, 0.80–1.33; p=0.826; Table 3), heart failure hospitalization (HR, 0.99; 95% CI, 0.76–1.30; p=0.946; Table 4) or all-cause hospitalization (HR, 0.92; 95% CI, 0.75–1.13; p=0.404; Table 5) in diastolic heart failure patients without chronic kidney disease.
DISCUSSION
Summary and Relevance of Key Findings
Findings of the current analysis demonstrate that a discharge prescription for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was associated with a significant lower risk of all-cause mortality and all-cause hospitalization in older diastolic heart failure patients with chronic kidney disease, including those with stage 3B or greater chronic kidney disease, but had no association with heart failure hospitalization. These associations were similar regardless of whether patients were receiving these drugs at or above target doses. In contrast, the use of these drugs had no association with outcomes in diastolic heart failure patients without chronic kidney disease. These findings suggest that despite concerns for worsening kidney function, inhibitors of the renin-angiotensin system are safe and beneficial in older patients with diastolic heart failure and chronic kidney disease, a large and heretofore unstudied segment of heart failure population.
Potential Explanations and Mechanisms of the Key Findings
Inhibitors of the renin-angiotensin system improve clinical outcomes in systolic heart failure by reducing ventricular preload and afterload, attenuating myocardial fibrosis, and reducing maladaptive ventricular remodeling.26 However, these drugs did not improve outcomes in ambulatory chronic stable diastolic heart failure patients in clinical trials that excluded patients with chronic kidney disease.10, 11, 27 Although our analysis in those without chronic kidney disease was underpowered, the null associations are consistent with those in clinical trials. A lower total mortality without an associated lower heart failure hospitalization in those with chronic kidney disease receiving renin-angiotensin inhibitors in our study is intriguing. Sudden death, common in heart failure, may preclude hospitalization and drugs that reduce sudden death may improve survival without reducing hospitalization. However, renin-angiotensin inhibitors failed to reduce sudden death in systolic heart failure in clinical trials.28, 29 Instead, they were more effective in reducing death due to pump failure28, 29 Although death due to pump failure is less common in diastolic heart failure, 30 it is more common in advanced heart failure, 31 as in those with chronic kidney disease.2 Further, treatment effect has been shown to be more profound in subsets with advanced disease and poor outcomes.12 Although diastolic heart failure patients with and without chronic kidney disease had similar age (mean, 79 years; Table 1), baseline mortality was higher in those with chronic kidney disease (73% vs. 59% in those without; Table 3). The observed mortality reduction associated with the use of renin-angiotensin inhibition may also in part be explained by slower progression of chronic kidney disease, 32–35 which may be more intrinsic and less cardiorenal syndrome in diastolic heart failure than in systolic heart failure.2 However, we had no data on kidney function during follow-up.
Comparison with Findings from Relevant Published Literature
Inhibition of the renin-angiotensin system has been shown to be associated with a modest improvement in outcomes in systolic heart failure patients with chronic kidney disease.3, 36 To the best our knowledge, this is the first propensity-matched study of clinical effectiveness of these drugs in diastolic heart failure patients with chronic kidney disease, a large, unstudied segment of heart failure population. Although these drugs did not seem to improve outcomes in trial-eligible younger ambulatory diastolic heart failure patients, 10, 11, 27 findings from our study suggest that they may be beneficial in real-world older hospitalized diastolic heart failure patients with chronic kidney disease.
Clinical and Public Health Importance
Over half of older heart failure patients have diastolic heart failure, most of whom also have chronic kidney disease, which is associated with poor outcomes.2 Currently there is no evidence that neurohormonal antagonists improve mortality in diastolic heart failure. If our findings can be replicated in other well-designed propensity-matched inception cohort studies, cumulative data from these studies may provide Level B evidence (derived from single randomized clinical trial or multiple non-randomized studies).37 This is important considering that over half of the current heart failure guideline recommendations are based on Level C evidence (expert opinion, case studies, or standards of care).38 In addition, they may provide hypothesis and preliminary data for a definitive randomized clinical trial.
In the interim, our findings provide important insights into the potential role of these drugs in older patients with diastolic heart failure and chronic kidney disease. The benefit of inhibition of renin-angiotensin system in chronic kidney disease has been documented in various patient populations.32, 34, 35 Findings from our study suggest that this benefit may also extend to those with diastolic heart failure. The prevalence of low systolic blood pressure and elevated serum potassium was not high in our study. However, these drugs should be used with caution in those patients. Considering that the use of these drugs has been shown to be associated with declines in glomerular filtration rates, 39, 40 future studies also need to examine the effect of these drugs on incident dialysis in heart failure patients with chronic kidney disease.
Potential Limitations
Our study has several limitations. As in any non-randomized study, findings of our study may potentially be confounded by imbalances in unmeasured covariates. Findings from our sensitivity analysis suggest that mortality reduction observed in our study was sensitive to a potential unmeasured confounder. However, sensitivity analysis cannot determine if such an unmeasured confounder exists or not. Further, to act as a confounder, an unmeasured covariate would need to be a near-perfect predictor of outcomes, be associated with the exposure, and not be strongly correlated with any of the measured baseline covariates, an unlikely probability. Although an assembly of a balanced matched cohort enhances internal validity, the loss of data during the process may limit external validity. However, our matched associations were similar to those based on pre-match multivariable-adjusted regression models. We had no data on postdischarge adherence to discharge prescriptions, which may have resulted in regression dilution and potential underestimation of the true association.41 We also had no data on cause-specific mortality.
CONCLUSIONS
A discharge prescription for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was associated with a significant reduction in all-cause mortality and all-cause hospitalization in older patients with diastolic heart failure and chronic kidney disease, including those with more advanced chronic kidney disease, but had no association with heart failure hospitalization. Although these drugs have not been shown to improve outcomes in diastolic heart failure, 10, 11, 27, 42 taken together with their benefit in systolic heart failure patients with chronic kidney disease, 3 findings from the current study suggest that renin-angiotensin inhibition may be beneficial in heart failure patients with chronic kidney disease, regardless of ejection fraction. In addition to replicating these findings in other well designed studies, future studies also need to examine the effect of these drugs on incident dialysis in patients with heart failure.
Acknowledgments
Funding/Support: The project described was supported by Grant Numbers R01-HL085561 and R01-HL085561-S from NHLBI/NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or NIH. Dr. Ahmed is also supported by NIH/NHLBI grant R01-HL097047 and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama. Dr. Allman is supported by NIH/NCRR grant 5UL1 RR025777. Dr. Sanders is supported by NIH/NIDDK grant R01-DK46199 and funding from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Authorship: Dr Ahmed conceived the study hypothesis and design in collaboration with coauthors, and wrote the first draft. Dr Ahmed performed statistical analyses in collaboration with Drs Aban, Love, and Patel and Ms Zhang. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. Drs Aban, Ahmed, and Patel and Ms Zhang had full access to the data.
References
- 1.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4:387–399. doi: 10.1016/j.hfc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Fonarow GC, Zhang Y, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. doi: 10.1016/j.amjmed.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A. Association of diastolic dysfunction and outcomes in ambulatory older adults with chronic heart failure. J Gerontol A Biol Sci Med Sci. 2005;60:1339–1344. doi: 10.1093/gerona/60.10.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 11.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 13.Feller MA, Mujib M, Zhang Y, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: Findings from the Alabama Heart Failure Project. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.05.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 16.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 17.Bowling CB, Feller MA, Mujib M, et al. Relationship between Stage of Kidney Disease and Incident Heart Failure in Older Adults. Am J Nephrol. 2011;34:135–141. doi: 10.1159/000328905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawar PP, Jones LG, Feller M, et al. Association between smoking and outcomes in older adults with atrial fibrillation. Arch Gerontol Geriatr. 2012;55:85–90. doi: 10.1016/j.archger.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mujib M, Rahman AA, Desai RV, et al. Warfarin use and outcomes in patients with advanced chronic systolic heart failure without atrial fibrillation, prior thromboembolic events, or prosthetic valves. Am J Cardiol. 2011;107:552–557. doi: 10.1016/j.amjcard.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippatos GS, Ahmed MI, Gladden JD, et al. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. Eur Heart J. 2011;32:712–720. doi: 10.1093/eurheartj/ehq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippatos GS, Desai RV, Ahmed MI, et al. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail. 2011;13:1078–1086. doi: 10.1093/eurjhf/hfr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamopoulos C, Meyer P, Desai RV, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail. 2011;13:200–206. doi: 10.1093/eurjhf/hfq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekundayo OJ, Allman RM, Sanders PW, et al. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53:458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heywood JT, Fonarow GC, Yancy CW, et al. Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy: report from IMPROVE HF. Circ Heart Fail. 2010;3:596–605. doi: 10.1161/CIRCHEARTFAILURE.109.912683. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer JM, Pfeffer MA. Angiotensin converting enzyme inhibition and ventricular remodeling in heart failure. Am J Med. 1988;84:37–44. doi: 10.1016/0002-9343(88)90203-3. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 28.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 29.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 30.Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 31.Carson P, Anand I, O'Connor C, et al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. Journal of the American College of Cardiology. 2005;46:2329–2334. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 33.Hoogwerf BJ. Renin-angiotensin system blockade and cardiovascular and renal protection. Am J Cardiol. 2010;105:30A–35A. doi: 10.1016/j.amjcard.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Hebert LA. Optimizing ACE-inhibitor therapy for chronic kidney disease. N Engl J Med. 2006;354:189–191. doi: 10.1056/NEJMe058295. [DOI] [PubMed] [Google Scholar]
- 35.Dzau VJ, Colucci WS, Williams GH, Curfman G, Meggs L, Hollenberg NK. Sustained effectiveness of converting-enzyme inhibition in patients with severe congestive heart failure. N Engl J Med. 1980;302:1373–1379. doi: 10.1056/NEJM198006193022501. [DOI] [PubMed] [Google Scholar]
- 36.Bowling CB, Sanders PW, Allman RM, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: Insights from the SOLVD Treatment trial. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.12.056. [Epub ahead of print] 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 38.Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC., Jr Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831–841. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- 39.Packer M, Lee WH, Kessler PD, Medina N, Yushak M, Gottlieb SS. Identification of hyponatremia as a risk factor for the development of functional renal insufficiency during converting enzyme inhibition in severe chronic heart failure. Journal of the American College of Cardiology. 1987;10:837–844. doi: 10.1016/s0735-1097(87)80278-4. [DOI] [PubMed] [Google Scholar]
- 40.Packer M, Lee WH, Medina N, Yushak M, Kessler PD. Functional renal insufficiency during long-term therapy with captopril and enalapril in severe chronic heart failure. Ann Intern Med. 1987;106:346–354. doi: 10.7326/0003-4819-106-3-346. [DOI] [PubMed] [Google Scholar]
- 41.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. American journal of epidemiology. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 42.Patel K, Fonarow GC, Kitzman DW, et al. Angiotensin receptor blockers and outcomes in real-world older patients with heart failure and preserved ejection fraction: A propensity-matched inception cohort clinical effectiveness study. Eur J Heart Fail. 2012 doi: 10.1093/eurjhf/hfs101. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]