Abstract
Exosomes are extracellular membrane vesicles whose biogenesis by exocytosis of multivesicular endosomes was discovered in 1983. Since their discovery 30 years ago, it has become clear that exosomes contribute to many aspects of physiology and disease, including intercellular communication. We discuss the initial experiments that led to the discovery of exosomes and highlight some of the exciting current directions in the field.
30 years ago, a paper in JCB (Harding, Heuser and Stahl, 1983) and one in Cell (Pan and Johnstone, 1983)—published within a week of each other—reported that, in reticulocytes, transferrin receptors associated with small ∼50 nM vesicles are literally jettisoned from maturing blood reticulocytes into the extracellular space. The name “exosome” for these extracellular vesicles was coined a few years later by Rose Johnstone, although the term had in fact been used a few years earlier, when referring to other membrane fragments isolated from biological fluids (Trams et al., 1981; the term “exosome complex” has also been used for a totally different entity: namely, the intracellular particle involved in RNA editing [Mitchell et al., 1997]). The promise of these early discoveries has been recognized over the intervening three decades by a nearly explosive growth in the field of exosome biology, resulting in the formation of various societies (International Society for Extracellular Vesicles and The American Society for Exosomes and Microvesicles) and even a dedicated journal (Journal of Extracellular Vesicles), plus numerous international meetings and well over a thousand publications on exosomes, to date. In this comment, we describe how we discovered this new cellular pathway and provide our perspective on the major advances and future directions of this field. For a more detailed analysis of the state of the field and its future challenges, see Raposo and Stoorvogel (in this issue).
Our investigations were initiated when one of us (C.V. Harding), then an MD/PhD student, embarked on a study of endocytosis for his PhD thesis and exploited the expertise of two adjacent laboratories in the Department of Cell Biology and Physiology at Washington University in St. Louis. Work in the Stahl laboratory was then focused on defining the pathways of receptor-mediated endocytosis and recycling (Stahl et al., 1980) as well as determining the general role of acidic intracellular compartments in facilitating ligand delivery to lysosomes (Tietze et al., 1980). Meanwhile, the Heuser laboratory had recently developed the deep-etch technique for electron microscopy (Heuser and Salpeter, 1979) and had already made great strides in visualizing synaptic membrane recycling (Heuser et al., 1979) and the general architecture of endosomes (Heuser, 1980). C.V. Harding decided to focus his thesis work on the transferrin receptor and to use the reticulocyte or maturing red blood cell as a model system because reticulocytes were known to be replete with transferrin receptors. The first paper that grew from this three-way collaboration described the recycling of the transferrin receptor between the plasma membrane and the endocytic compartments of the reticulocyte and delineated the role of these acidic compartments in iron uptake by reticulocytes (Harding and Stahl, 1983). Additional work on the transferrin receptor recycling pathway was published around the same time by several other groups (Ciechanover et al., 1983; Dautry-Varsat et al., 1983; Klausner et al., 1983). In retrospect, all of those studies (as well as advice from Alan Schwartz and Aaron Ciechanover) helped us to begin to grasp the mechanisms of transferrin recycling in reticulocytes.
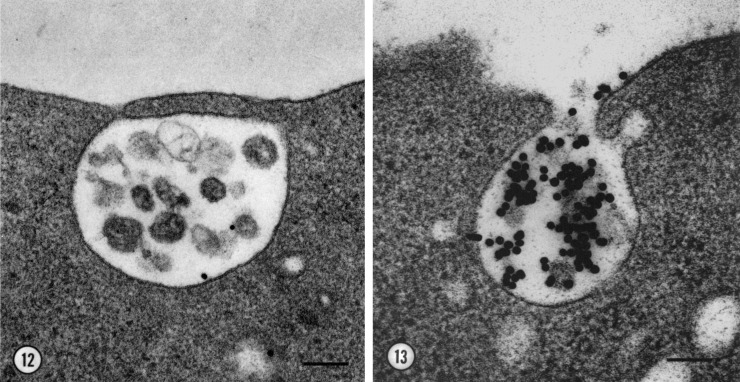
One well-established fact at that time was that reticulocytes lose the machinery necessary for hemoglobin production as they mature into erythrocytes; this includes the loss of the machinery necessary for iron transport via transferrin receptors (van Bockxmeer and Morgan, 1979). Most people presumed that this was simply caused by down-regulation of endocytosis and lysosomal degradation of the transferrin receptors. Our thinking about this was suddenly redirected, however, by two experiments we did to visualize transferrin receptor turnover by monitoring it with colloidal gold-conjugated transferrin (AuTf). We found that AuTf particles were indeed internalized by reticulocyte transferrin receptors with high fidelity. However, we rarely observed any of our gold particles within internal compartments in our rat reticulocytes that might be considered lysosomes—on the basis of their staining for acid phosphatase or aryl sulfatase—indeed, we found that in general, reticulocytes have precious few of these sorts of lysosomes (Harding et al., 1983). To our surprise, internalized AuTf particles were located primarily and predominantly on the many small vesicles that we observed within multivesicular bodies, and we therefore chose to call these organelles multivesicular endosomes (MVEs; Harding et al., 1983). We were even more surprised when we observed MVEs harboring AuTf-labeled vesicles that appeared to have fused with the plasma membrane, as judged by the clear continuity of their two membranes (Fig. 1, left), and by the obvious externalization of their AuTf-labeled vesicles (now known as exosomes; Fig. 1, right). To exclude the possibility that these results were caused by fixation artifacts, we performed electron microscopy on samples that were quick frozen without prior fixation (Fig. 1, right) and observed the same phenomenon. These observations revealed a novel mechanism for the loss of transferrin receptors during maturation of reticulocytes.
Figure 1.
Exocytosis of MVEs releases exosomes containing transferrin receptor. (left) View of an MVE from a fixed reticulocyte sparsely labeled with AuTf. The apparent fusion of the MVE and the plasma membrane may represent incipient MVE exocytosis. Bar, 100 nm. (right) View of MVE exocytosis in a reticulocyte labeled with AuTf, quick frozen without prior fixation, and freeze substituted. Bar, 200 nm. Figure and legend adapted from Harding et al. (1983).
This apparent exocytosis of MVEs was a novel and interesting finding for many reasons. For one, it countered the prevailing view at the time that recycling to the plasma membrane occurred exclusively from early endocytic compartments but not from later endocytic compartments, such as MVEs. If true, MVE exocytosis could provide a mechanistic explanation for the recycling of internalized molecules that enter much more deeply into the endocytic system. The existence of many such molecules was subsequently documented: for instance, the trafficking of lysosomal LAMP-1 to the plasma membrane (Lippincott-Schwartz and Fambrough, 1987) and the trafficking of processed antigens from late endosomes/lysosomes to the plasma membrane during class II major histocompatibility complex (MHC-II) antigen processing (Harding et al., 1991). Even more fundamentally, our first glimpses of MVE exocytosis raised the possibility that cells might use this mechanism as a general way to release membrane vesicles, with significance far beyond the transferrin receptor system, an idea that has since been developed extensively in the exosome field.
Our first images of this MVE externalization event appeared in Harding et al. (1983): an example is shown in Fig. 1. At the very same time, Pan and Johnstone (1983) discovered that sheep reticulocytes released vesicles of rather uniform size that contained transferrin receptor, although they did not directly visualize the production of these vesicles and favored a mechanism of vesicle shedding occurring at the plasma membrane (which produces vesicles that have subsequently been termed “ectosomes” or “microvesicles”). The two simultaneous papers nicely complemented each other, as did subsequent papers from both groups that provided data to further establish the exosome model, e.g., by biochemical purification of exosomes and further electron microscopy studies that confirmed the role of MVE exocytosis in the genesis of exosomes (Harding et al., 1984; Pan et al., 1985). Together, these findings developed a compelling model for a vesicle and receptor shedding pathway, though it took some time for appreciation of their general significance to extend beyond the reticulocyte/transferrin receptor model.
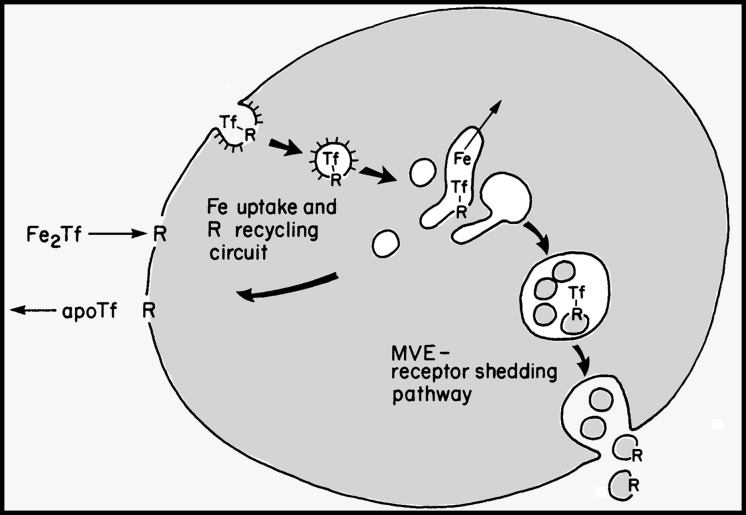
We realized that the MVE–exosome pathway of transferrin receptor shedding was clearly distinct from the pathway for recycling of transferrin receptor to the plasma membrane after delivery of iron by transferrin. By quantifying the localization of intracellular AuTf particles and determining the kinetics of transferrin receptor recycling, we generated a model that included two pathways for trafficking of internalized transferrin receptor in reticulocytes: a fast recycling pathway and a slower shedding pathway. Namely, after endocytosis of transferrin receptors into early endocytic tubules and vesicles, most are rapidly recycled to the cell surface, as detected by their rapid reappearance and susceptibility to surface trypsinization. However, the slower second pathway targeted transferrin receptors to MVE’s, and the rate of transferrin receptor shedding by this pathway matched the rate of transferrin receptor loss from developing reticulocytes (Harding et al., 1984). This finding implicated the exosome release pathway as the major route for loss of transferrin receptor from reticulocytes, a conclusion that fit with the findings of Johnstone’s group. Furthermore, in subsequent years, it has become apparent that other molecules are sorted into vesicles in MVEs for release via the exosome pathway. The model that appeared in the original JCB paper, further detailed in a paper the following year (Fig. 2; Harding et al., 1984), seems to have withstood the test of time.
Figure 2.
Model for routes of transferrin processing in reticulocytes (circa 1983–1984). Transferrin receptors (Tf-R) may recycle or be selectively shed from reticulocytes by trafficking in the following steps. (a) Diferric transferrin binds surface receptors, and receptor–ligand complexes are internalized via coated pits and vesicles. (b) Coat loss and fusion events produce a pleiomorphic class of small, uncoated vesicles and tubules. (c) Iron is removed from transferrin in an acid luminal environment and is transferred into the cytoplasm. (d) Apotransferrin remains bound to its receptor at acid pH, and both receptor and ligand recycle to the plasma membrane, where apotransferrin dissociates from the receptor. Alternatively, transferrin receptors destined to be shed from the cell segregate on inclusion vesicles of MVEs and are released by MVE exocytosis. Model and legend are adapted from Harding et al. (1984) and a precursor model in Harding et al. (1983).
Meanwhile, significant progress has been made in developing an understanding of the cell biology of exosome biogenesis. There is agreement that MVEs have two fates in all cells—to fuse with the lysosomal compartment where membranes and other content are degraded or to fuse with the plasma membrane, resulting in release via the exosome secretion pathway. MVE formation is now rather well understood, necessary components of the endosomal complex required for transport (ESCRT) pathway have been identified (Henne et al., 2011), and provocative studies on the mechanism of inward vesicle budding have been recently published (Hanson et al., 2008; Hurley and Hanson, 2010; Henne et al., 2012). Other studies have pointed to ceramide and other lipids as mediators of exosome biogenesis (Trajkovic et al., 2008). Downstream of MVB formation, certain Rab GTPases appear to direct traffic to the plasma membrane followed by docking and fusion, opening up a range of possibilities for selective regulation. Rab GTPases are known to regulate membrane traffic, and initial work (Vidal and Stahl, 1993) demonstrated that isolated exosomes are enriched in Rab4 and Rab5. Moreover, Savina et al. (2002) implicated Rab11 in exosome secretion, and more recently, Rab27 and Rab35 have been identified as regulatory GTPases (Hsu et al., 2010; Ostrowski et al., 2010). Recent work has demonstrated that elements of the ESCRT pathway, ALIX and VPS4, play a role in the secretion of exosomes, especially those involved in the trafficking of syndecans (Baietti et al., 2012). Lastly, mechanisms that trigger exosome secretion have been studied, and calcium transients have been shown to trigger exosome secretion, suggesting a tightly regulated process (Savina et al., 2005).
Exosomes have become relevant to many fields; we will limit this discussion and focus on a few notable topics in immunology and intercellular communication. In 1996, over a decade after the discovery of exosomes in reticulocytes, Raposo et al. (1996) demonstrated that MHC-II–enriched MVEs (termed “MIIC”) in B lymphocytes fused with the plasma membrane to release exosomes bearing MHC-II, and these exosomes were able to present peptide–MHC-II complexes to activate T cell responses. Soon thereafter, it was shown that dendritic cells produce exosomes (Zitvogel et al., 1998; Théry et al., 2001) with the capacity to stimulate T cell responses (Théry et al., 2002). Macrophages also generate exosomes, and macrophages infected with Mycobacterium tuberculosis (Mtb) generate exosomes that can present Mtb antigen peptide–MHC-II complexes to T lymphocytes (Ramachandra et al., 2010), although these exosomes, like those in some other systems, may be most effective in antigen presentation when attached to the plasma membrane of cells that display them to T cells (Denzer et al., 2000; Théry et al., 2002; Vincent-Schneider et al., 2002). The ability of exosomes to directly stimulate naive T cells may be restricted to exosomes from dendritic cells provided with a maturation stimulus (Segura et al., 2005). The ability of exosomes to stimulate T cell responses generated interest in the potential for exosomes in immunization, particularly in the tumor immunology field, but practical application of exosomes for immunization or tumor immunity remains elusive.
In addition to their ability to affect adaptive immunity by bearing peptide–MHC complexes or antigen that can be processed by antigen-presenting cells for presentation to T cells, exosomes may also bear molecules that stimulate innate immune responses. Macrophages that are infected with Mtb or related mycobacteria produce exosomes containing microbial molecules that may signal via innate immune receptors that regulate antigen-presenting cells and other cells of the immune system (unpublished data; Wearsch, P.A., and J. Athman, personal communication; Bhatnagar and Schorey, 2007; Bhatnagar et al., 2007). Moreover, infection with Mtb (Ramachandra et al., 2010) or inflammasome activation (Qu et al., 2007, 2009) enhances the production of exosomes as well as plasma membrane–derived microvesicles. Although most studies have used mammalian cells as the source of exosomes, nonmammalian cells, including pathogenic parasites such as Leishmania, can secrete exosomes, and pathogen-derived exosomes may regulate host defense and immune responses (Silverman et al., 2010). Thus, exosomes may broadcast signature molecules of infection with pathogens, particularly intracellular pathogens, allowing one infected cell to influence or recruit many other cells, thereby expanding host responses. On the other hand, this route may also allow the dissemination of molecules used by pathogens to subvert immune responses and promote persistence of infection.
There is currently an explosion of interest in the roles of exosomes in intercellular communication. One major aspect of this is the possibility that exosome-associated RNA may be delivered to recipient cells, influencing their RNA expression, proteome, and functions. These functions could be critical in intercellular communication to regulate immune responses or many other types of pathophysiological responses. Much excitement was generated with the discovery of both mRNA and microRNA in exosomes isolated from mouse and human mast cells (Valadi et al., 2007) and the finding that exosomal RNA was delivered to target cells, where it was biologically active. This discovery opened a new field of inquiry. Initial skepticism that the presence of RNA in exosomes may be incidental has now been ruled out by demonstrations showing specificity in exosomal RNA cargo. Exosomes from tumor cells may contain RNA that affects other cells to promote tumor progression (Skog et al., 2008). RNA-bearing exosomes may also regulate cells of the immune system, including the interactions between antigen-presenting cells and T cells (Mittelbrunn et al., 2011). Recent work has demonstrated that the ability to produce exosomes in vitro could be exploited to produce a “recombinant exosome” that incorporates recognition or homing molecules for tissue-specific targeting (Alvarez-Erviti et al., 2011). This area represents an exciting new area with much research activity.
What will the future bring in the field of exosome research? In addition to their importance to fundamental mechanisms of intercellular communication, signaling, and regulation, exosomes and other extracellular vesicles may have important clinical applications in the future (Raposo and Stoorvogel, 2013). There is much interest in the potential for diagnostics based on analysis of exosomes, as they may bear the protein or RNA signatures of pathological or physiological states of their source cells. Because exosomes may traffic from tissue sites to blood, urine, or other body fluids that are easily accessible, they may make such signatures available for diagnostic utilization. Furthermore, the potential for RNA-bearing exosomes to influence or regulate recipient cells suggests the possibility of therapeutic applications for cancer, amelioration of pathological immune responses (e.g., autoimmunity), and other applications. May the next 30 years bring yet more advances!
Acknowledgments
We wish to acknowledge Marilyn Levy and Robyn Roth in the Department of Cell Biology and Physiology, Washington University School of Medicine who provided expert technical assistance during the discovery phase of this work.
Our research (C.V. Harding, J.E. Heuser, and P.D. Stahl) has been supported by grants from the National Institutes of Health.
References
- Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29:341–345 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Ivarsson Y., Depoortere F., Coomans C., Vermeiren E., et al. 2012. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14:677–685 10.1038/ncb2502 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S., Schorey J.S. 2007. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 282:25779–25789 10.1074/jbc.M702277200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S., Shinagawa K., Castellino F.J., Schorey J.S. 2007. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 110:3234–3244 10.1182/blood-2007-03-079152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A.L., Dautry-Varsat A., Lodish H.F. 1983. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J. Biol. Chem. 258:9681–9689 [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H.F. 1983. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 80:2258–2262 10.1073/pnas.80.8.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer K., van Eijk M., Kleijmeer M.J., Jakobson E., de Groot C., Geuze H.J. 2000. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 165:1259–1265 [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Roth R., Lin Y., Heuser J.E. 2008. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J. Cell Biol. 180:389–402 10.1083/jcb.200707031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Stahl P. 1983. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem. Biophys. Res. Commun. 113:650–658 10.1016/0006-291X(83)91776-X [DOI] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. 1983. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97:329–339 10.1083/jcb.97.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. 1984. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 35:256–263 [PubMed] [Google Scholar]
- Harding C.V., Collins D.S., Slot J.W., Geuze H.J., Unanue E.R. 1991. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 64:393–401 10.1016/0092-8674(91)90647-H [DOI] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., Emr S.D. 2011. The ESCRT pathway. Dev. Cell. 21:77–91 10.1016/j.devcel.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., Zhao Y., Emr S.D. 2012. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell. 151:356–371 10.1016/j.cell.2012.08.039 [DOI] [PubMed] [Google Scholar]
- Heuser J. 1980. Three-dimensional visualization of coated vesicle formation in fibroblasts. J. Cell Biol. 84:560–583 10.1083/jcb.84.3.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J.E., Salpeter S.R. 1979. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J. Cell Biol. 82:150–173 10.1083/jcb.82.1.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J.E., Reese T.S., Dennis M.J., Jan Y., Jan L., Evans L. 1979. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J. Cell Biol. 81:275–300 10.1083/jcb.81.2.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., Morohashi Y., Yoshimura S., Manrique-Hoyos N., Jung S., Lauterbach M.A., Bakhti M., Grønborg M., Möbius W., Rhee J., et al. 2010. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 189:223–232 10.1083/jcb.200911018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J.H., Hanson P.I. 2010. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat. Rev. Mol. Cell Biol. 11:556–566 10.1038/nrm2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R.D., Ashwell G., van Renswoude J., Harford J.B., Bridges K.R. 1983. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc. Natl. Acad. Sci. USA. 80:2263–2266 10.1073/pnas.80.8.2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D.M. 1987. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 49:669–677 10.1016/0092-8674(87)90543-5 [DOI] [PubMed] [Google Scholar]
- Mitchell P., Petfalski E., Shevchenko A., Mann M., Tollervey D. 1997. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′—>5′ exoribonucleases. Cell. 91:457–466 10.1016/S0092-8674(00)80432-8 [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M.A., Bernad A., Sánchez-Madrid F. 2011. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2:282 10.1038/ncomms1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P., et al. 2010. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12:19–30 10.1038/ncb2000 [DOI] [PubMed] [Google Scholar]
- Pan B.T., Johnstone R.M. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 33:967–978 10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- Pan B.T., Teng K., Wu C., Adam M., Johnstone R.M. 1985. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101:942–948 10.1083/jcb.101.3.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Franchi L., Nunez G., Dubyak G.R. 2007. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179:1913–1925 [DOI] [PubMed] [Google Scholar]
- Qu Y., Ramachandra L., Mohr S., Franchi L., Harding C.V., Nunez G., Dubyak G.R. 2009. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J. Immunol. 182:5052–5062 10.4049/jimmunol.0802968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra L., Qu Y., Wang Y., Lewis C.J., Cobb B.A., Takatsu K., Boom W.H., Dubyak G.R., Harding C.V. 2010. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect. Immun. 78:5116–5125 10.1128/IAI.01089-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. 2013. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183:1161–1172 10.1084/jem.183.3.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A., Vidal M., Colombo M.I. 2002. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 115:2505–2515 [DOI] [PubMed] [Google Scholar]
- Savina A., Fader C.M., Damiani M.T., Colombo M.I. 2005. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 6:131–143 10.1111/j.1600-0854.2004.00257.x [DOI] [PubMed] [Google Scholar]
- Segura E., Nicco C., Lombard B., Véron P., Raposo G., Batteux F., Amigorena S., Théry C. 2005. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 106:216–223 10.1182/blood-2005-01-0220 [DOI] [PubMed] [Google Scholar]
- Silverman J.M., Clos J., Horakova E., Wang A.Y., Wiesgigl M., Kelly I., Lynn M.A., McMaster W.R., Foster L.J., Levings M.K., Reiner N.E. 2010. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 185:5011–5022 10.4049/jimmunol.1000541 [DOI] [PubMed] [Google Scholar]
- Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr, Carter B.S., Krichevsky A.M., Breakefield X.O. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10:1470–1476 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P.H., Sigardson E., Rodman J.S., Lee Y.C. 1980. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 19:207–215 10.1016/0092-8674(80)90402-X [DOI] [PubMed] [Google Scholar]
- Théry C., Boussac M., Véron P., Ricciardi-Castagnoli P., Raposo G., Garin J., Amigorena S. 2001. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166:7309–7318 [DOI] [PubMed] [Google Scholar]
- Théry C., Duban L., Segura E., Véron P., Lantz O., Amigorena S. 2002. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3:1156–1162 10.1038/ni854 [DOI] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. 1980. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: apparent inhibition of receptor recycling. Biochem. Biophys. Res. Commun. 93:1–8 10.1016/S0006-291X(80)80237-3 [DOI] [PubMed] [Google Scholar]
- Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 319:1244–1247 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- Trams E.G., Lauter C.J., Salem N., Jr, Heine U. 1981. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta. 645:63–70 10.1016/0005-2736(81)90512-5 [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9:654–659 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- van Bockxmeer F.M., Morgan E.H. 1979. Transferrin receptors during rabbit reticulocyte maturation. Biochim. Biophys. Acta. 584:76–83 10.1016/0304-4165(79)90237-X [DOI] [PubMed] [Google Scholar]
- Vidal M.J., Stahl P.D. 1993. The small GTP-binding proteins Rab4 and ARF are associated with released exosomes during reticulocyte maturation. Eur. J. Cell Biol. 60:261–267 [PubMed] [Google Scholar]
- Vincent-Schneider H., Stumptner-Cuvelette P., Lankar D., Pain S., Raposo G., Benaroch P., Bonnerot C. 2002. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 14:713–722 10.1093/intimm/dxf048 [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. 1998. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4:594–600 10.1038/nm0598-594 [DOI] [PubMed] [Google Scholar]