Abstract
Introduction: Tamoxifen is associated with a twofold increased risk of thromboembolic events. Third generation aromatase inhibitors (AIs), such as letrozole, anastrozole, and exemestane have therefore replaced tamoxifen in the adjuvant therapy of hormone receptor-positive breast cancer. A retrospective review was performed in patients who underwent delayed microvascular breast reconstruction and received tamoxifen at the time of surgery in order to assess the risk of both minor and major flap complications including thromboembolic events.
Patients and methods: Twenty-nine patients who underwent delayed microsurgical breast reconstruction with autologous tissue between 2006 and 2012 were included in the study. The overall complication rates were compared between patients who did versus those who did not receive tamoxifen at the time of microsurgical breast reconstruction.
Results: Breast reconstruction was performed with a DIEP flap in 25 patients and with a TRAM flap in 4 patients. Overall, the complication rate was 37.9% (n=11) consisting of 5 major (including one total flap loss) and 6 minor complications. In patients receiving tamoxifen (n=5), we observed one minor complication and one major complication with a total flap loss due to thrombus formation at the anastomosis site. In one patient pulmonary embolism occurred without association to tamoxifen. The number of thromboembolic events was equivalent in both groups (p=0.642). No increase of major (p=0.858) or minor (p=0.967) complications in the tamoxifen group could be observed. Taking the overall complication rate into account there was no statistically difference between the two groups (p=0.917).
Conclusion: In our study we could not observe an increased risk for thromobembolic events in patients receiving tamoxifen while undergoing autologous microvascular breast reconstruction.
Keywords: Tamoxifen, breast cancer, adjuvant therapy, microvascular breast reconstruction
Abstract
Aromatasehemmer der dritten Generation haben mittlerweile Tamoxifen in der neoadjuvanten und adjuvanten Therapie des hormonrezeptorpositiven Brustkrebses postmenopausaler Frauen abgelöst. Tamoxifen ist mit einem ca. zweifach erhöhten Risiko für thrombembolische Komplikationen (tiefe Beinvenenthrombose, Lungenembolie) assoziiert. Vor dem Hintergrund einer aktuellen und im Februar 2012 im Journal of Plastic and Reconstructive Surgery erschienenen retrospektiven Studie mit Nachweis einer erhöhten Komplikationsrate freier Lappentransplantate zur Brustrekonstruktion unter Tamoxifen-Einnahme haben wir unser Patientenkollektiv nach mikrochirurgischer Brustrekonstruktion retrospektiv auf Minor- und Major-Komplikationen einschließlich thrombembolischer Ereignisse insbesondere vor dem Hintergrund einer perioperativen Tamoxifeneinnahme untersucht. Eingeschlossen wurden insgesamt 29 Patientinnen nach mikrochirurgischer autologer Brustrekonstruktion. Es wurden mikrovaskuläre Lappenkomplikationen und Lappenverluste von Patientinnen ohne und mit Einnahme von Tamoxifen und Aromatasehemmern zum Zeitpunkt der mikrochirurgischen Brustrekonstruktion verglichen. 25 Patientinnen (86,2%) erhielten eine DIEP-Lappenplastik und 4 (13,8%) eine TRAM-Lappenplastik. Fernerhin verglichen wir die Rate allgemeiner thrombembolischer Ereignisse unter Berücksichtigung sonstiger Nebendiagnosen und Risikofaktoren.
Von insgesamt 29 Patientinnen standen zum Zeitpunkt der mikrovaskulären Brustrekonstruktion 3 unter einer Aromatasehemmer-Therapie und 5 unter einer Tamoxifen-Therapie. Insgesamt traten in 11 Fällen (37,9%) postoperative Komplikationen auf, in 5 Fällen handelte es sich hierbei um Major-Komplikationen (mit einem Lappenverlust) und in 6 Fällen um Minorkomplikationen.
Unter Tamoxifeneinnahme kam es in einem Fall zu einer Minorkomplikation, und in einem anderen Fall zu einem thrombembolisch bedingten Lappenverlust.
Insgesamt kam es postoperativ zu einem thrombembolischen Ereignis in Form einer Lungenembolie ohne Assoziation zur Tamoxifen-Therapie.
Die Anzahl thrombembolischer Ereignisse stellten sich mit und ohne Tamoxifen-Einnahme im Rahmen der Mamma-Rekonstruktion durch freie DIEP- oder TRAM-Lappenplastik als äquivalent dar (p=0,642). Weder die Komplikationsraten an Major-Komplikationen (p=0,858) noch an Minor-Komplikationen (p=0,967) waren unter Tamoxifen-Einnahme signifikant erhöht. Somit zeigte sich auch die allgemeine Komplikationsrate zwischen beiden Gruppen als nicht signifikant unterschiedlich (p=0,917).
Introduction
Breast cancer is the most common malignancy affecting women in Germany with an annual incidence of approximately 72,000 cases [1]. Early diagnosis and adequate therapy have led to a survival rate that exceeds 90% [2]. A broad arsenal of diagnostic and therapeutic approaches, such as early detection methods, sentinel lymph node biopsy, adjuvant radio- and chemotherapy, and breast-conserving surgery, have increased the overall survival rate in breast cancer patients [2]. In the case of estrogen-receptor-positive tumors, there is a strong evidence for recurrence and mortality reduction after five years of treatment with tamoxifen [3].
Third-generation aromatase inhibitors (AIs) are the preferred treatment for hormone receptor-positive breast cancer in postmenopausal women. They suppress aromatase activity by reversible (anastrozole and letrozole) or irreversible (exemestane) binding to the aromatase enzyme [4], [5]. They are associated with fewer adverse events than former generations, which includes hot flushes, loss of libido, vaginal dryness, fatigue, joint stiffness, arthralgias and loss of bone mineral density with subsequent increased risk of fracture [4], [5].
In contrast to the aforementioned AIs, tamoxifen, a selective estrogen receptor modulator, is known to be associated with increased rates of thromboembolic events, including deep venous thrombosis and pulmonary embolism [6]. Therefore third generation aromatase inhibitors (AIs) have replaced Tamoxifen in the adjuvant therapy of hormone receptor-positive breast cancer [7]. De Pinho Pessoa et al. showed a tamoxifen-induced intima hyperplasia in rats, and many clinical studies describe an increased risk for venous and arterial thrombosis under tamoxifen therapy [8], [9], [10], [11].
The Deep Inferior Epigastric Perforator or DIEP flap can be considered a gold standard for immediate or delayed breast reconstruction with free tissue transfer. For patients in which perforator vessels are difficult to dissect, the free Transverse Rectus Abdominis Myocutaneus or TRAM flap still represents a viable option. In any case, the success of free tissue transfer depends particularly on a patent venous and arterial anastomosis. A thrombosis within the anastomosed vessels leads to flap compromise or flap failure.
The current study assesses whether tamoxifen application is associated with a greater risk for microvascular flap complications in patients undergoing microvascular breast reconstruction. We reviewed minor and major complications in patients after delayed microvascular breast reconstruction, including thromboembolic events.
Patients and methods
All patients undergoing free microvascular tissue transfer for breast reconstruction after breast cancer related mastectomy between 2006 and 2012 at a single Plastic Surgery Department were reviewed. Deep Inferior Epigastric Perforator and TRAM flaps are routinely performed for delayed breast reconstruction at this department. Patients were divided in the following groups:
Tamoxifen group: patients who took tamoxifen during the month before breast reconstruction.
No tamoxifen (control) group: Patients who had never used tamoxifen or who had not taken tamoxifen during the month before breast reconstruction
Complications were divided into minor and major complications. Minor complications were defined as seroma, hematoma and fat necrosis without compromised flap perfusion. Major complications were defined as flap compromise due to venous congestion or arterial insufficiency at the anastomosis site and thrombosis of the microvascular bed with the need for immediate reexploration to prevent partial or total flap loss. Furthermore, deep venous thrombosis and pulmonary embolism were defined as major complications. Data analyis was performed using the Chi-square-test. A p-value less than 0.05 was considered statistically significant. SPSS statistical software package 20.0 for Windows (SPSS Inc., Chicago, Ill, USA) was used for statistical analysis.
Results
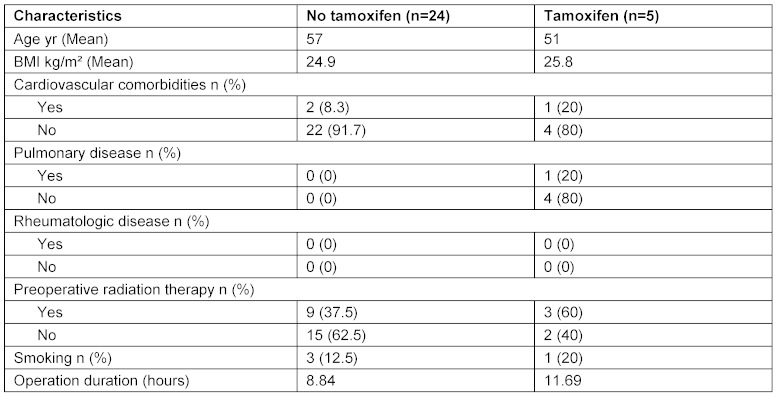
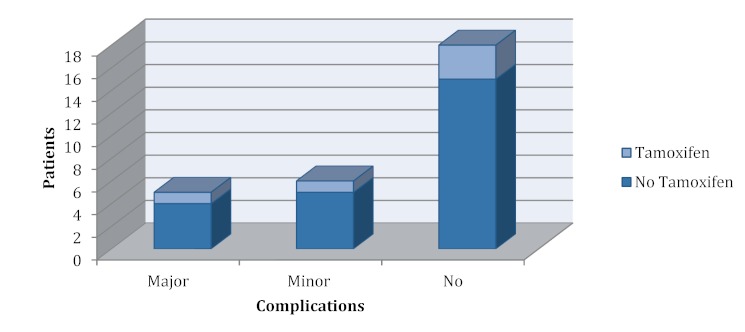
Twenty-nine patients who underwent breast reconstruction with free tissue transfer between 2006 and 2012 were included in the study. A total of 5 patients received tamoxifen during the month before surgery (tamoxifen group), 24 patients received no tamoxifen (no tamoxifen group). Three patients in the latter group received an aromatase inhibitor. A DIEP flap was used in 25 patients and a TRAM flap in 4 patients. Table 1 (Tab. 1) outlines the demographics of the two groups. A total of 11 complications, all of which occurred in patients with a DIEP flap breast reconstruction, were recorded. These 11 complications were divided into 5 major complications, including one case of complete flap loss, and 6 minor complications. Two complications occurred in the tamoxifen group and 9 complications were recorded in the no tamoxifen group, including a systemic event in terms of pulmonary embolism. No significant differences between the groups were observed in terms of cardiovascular comorbidities (p=0.436). The risk for thromboembolic (p=0.642) occurrence of major (p=0.858) and minor complications (p=0.967) events also did not differ significantly between the groups. All results are summarized in Figure 1 (Fig. 1).
Table 1. Comorbidities in patients receiving and not receiving tamoxifen.
Figure 1. Frequency distribution of complications in the tamoxifen and no tamoxifen group.
Discussion
This study was encouraged by the recently published work of Kelley et al. in the Journal of Plastic and Reconstructive Surgery who demonstrated an increased risk of microvascular flap complications in patients undergoing microvascular breast reconstruction [12]. In contrast to this study, we did not observe an increase of microvascular flap complications in patients receiving tamoxifen within 28 days before microsurgical breast reconstruction [12]. Additionally, it appeared that tamoxifen did not increase the risk of pulmonary embolism during or after delayed microvascular breat reconstruction. Tamoxifen, a selective estrogen receptor modulator, uses multiple mechanisms to block the effects of estrogen on vascular endothelium. Estrogen, which is located in the vascular endothelium, mediates its beneficial vascular effects by binding to the nuclear estrogen receptor resulting in arterial and venous vasodilatation via nitric oxide and cyclooxygenase pathway [12], [13]. Furthermore, it activates intracellular pathways resulting in relaxation of vascular rings and inhibition of proliferation in cultured vascular smooth muscle cells [13]. As a consequence of this interaction, we also expected an increased rate of microvascular flap complications in the tamoxifen group but could not confirm this result in our patient population. This could partly be caused by the very small sample size of our study. One also has to consider the fact that in the retrospective study of Kelley et al. there was a significantly higher incidence of flap loss in patients with less common and technically more challenging flaps (e.g., superior and inferior gluteal artery perforator flap) compared with patients who received abdominal flaps. This aspect was emphasized by Joseph Disa in his discussion of Kelley’s results, calling for retrospective and especially prospective studies to increase the level of evidence of the data [14].
Another result of Kelley’s study we could not confirm was a higher risk of microvascular complications in patients with cardiovascular comorbidities receiving tamoxifen. In our study, patients receiving tamoxifen were younger than patients not receiving tamoxifen. Age did not affect the risk of complications in women receiving tamoxifen, although it is known that premenopausal women are at high risk for adverse effects, whereas in postmenopausal women acute vascular responses decline with age [2], [15].
An analysis of multiple study variables, such as comorbidities and surgical management, implies a much higher study population. We therefore also postulate the need for a prospective multi-center-study with a large amount of patiens that focuses on a few selected clinically significant variables. Due to the fact that vascular responsiveness to estrogen may differ significantly between various vascular locations, it seems that this study should also focus on only one region of flap origin, such as the abdomen (DIEP-, TRAM-, SIEP flaps) [8], [16].
Conclusions
In comparison to the retrospective study of Kelley et al. our study is limited by the small sample size. Although we could not show an increased risk of flap failure or other microvascular flap complications in patients receiving tamoxifen at the time of delayed microsurgical breast reconstruction, we support the suggestion of Kelley and colleagues to pause tamoxifen for one month before surgery in order to avoid potential adverse effects of tamoxifen on the vascular endothelium of a microsurgically reconstructed breast. This treatment algorithm is supported by our oncologists who do not expect any adverse effects related to the pause in tamoxifen treatment.
Further prospective and randomized studies have to address the question when tamoxifen should be paused before surgery and when it should be given again. They should also focus on only one region of flap origin, such as the abdomen (DIEP-, TRAM-, SIEP flaps), in order to exclude further variables. While reconstructive and microsurgical methods are mostly standardized, anticoagulative protocols and practices lack sufficient evidence. The influence of perioperative anticoagulative protocols on the outcome of free flap surgery should therefore also be considered in future studies.
Notes
Competing interests
The authors have no financial and personal relationships with other people or organisations that could inappropriately influence (bias) this study.
References
- 1.Krebs in Deutschland. Berlin: Robert Koch-Institut; Available from: http://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_inhalt.html. [Google Scholar]
- 2.Diagnostik, Therapie und Nachsorge des Mammakarzinoms der Frau. AWMF Leitlinie Registernummer 032 - 045OL. Düsseldorf: AWMF online; Available from: http://www.awmf.org/leitlinien/detail/ll/032-045OL.html. [Google Scholar]
- 3.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998 May 16;351(9114):1451–1467. doi: 10.1016/S0140-6736(97)11423-4. Available from: http://dx.doi.org/10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- 4.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007 Dec;61(12):2051–2063. doi: 10.1111/j.1742-1241.2007.01587.x. Available from: http://dx.doi.org/10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winer EP. Optimizing endocrine therapy for breast cancer. J Clin Oncol. 2005 Mar;23(8):1609–1610. doi: 10.1200/JCO.2005.01.005. Available from: http://dx.doi.org/10.1200/JCO.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez RK, Sørensen HT, Pedersen L, Jacobsen J, Lash TL. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer. 2009 Oct;115(19):4442–4449. doi: 10.1002/cncr.24508. Available from: http://dx.doi.org/10.1002/cncr.24508. [DOI] [PubMed] [Google Scholar]
- 7.Bundred NJ. The effects of aromatase inhibitors on lipids and thrombosis. Br J Cancer. 2005 Aug;93 Suppl 1:S23–S27. doi: 10.1038/sj.bjc.6602692. Available from: http://dx.doi.org/10.1038/sj.bjc.6602692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Pinho Pessoa BB, Menezes Cavalcante BB, Maia MP, Ribeiro Filho HH, Koike MK, De Pinho Pessoa SG, Porto Pinheiro LG, De Souza Montero EF. Effect of tamoxifen on arterial microvascular anastomosis. Microsurgery. 2007;27(4):286–288. doi: 10.1002/micr.20357. Available from: http://dx.doi.org/10.1002/micr.20357. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh N, Tripathi SP. Thrombosis of tibial arteries in a patient receiving tamoxifen therapy. Cancer. 1995 Sep;76(6):1006–1008. doi: 10.1002/1097-0142(19950915)76:6<1006::AID-CNCR2820760614>3.0.CO;2-K. Available from: http://dx.doi.org/10.1002/1097-0142(19950915)76:6<1006::AID-CNCR2820760614>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.McDonald CC, Alexander FE, Whyte BW, Forrest AP, Stewart HJ. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomised trial. The Scottish Cancer Trials Breast Group. BMJ. 1995 Oct;311(7011):977–980. doi: 10.1136/bmj.311.7011.977. Available from: http://dx.doi.org/10.1136/bmj.311.7011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson CB, Lambert HE, Scott RD. Subclavian and axillary vein thrombosis following radiotherapy for carcinoma of the breast. Clin Radiol. 1987 Jan;38(1):95–96. doi: 10.1016/S0009-9260(87)80425-7. Available from: http://dx.doi.org/10.1016/S0009-9260(87)80425-7. [DOI] [PubMed] [Google Scholar]
- 12.Kelley BP, Valero V, Yi M, Kronowitz SJ. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg. 2012 Feb;129(2):305–314. doi: 10.1097/PRS.0b013e31823ae86c. Available from: http://dx.doi.org/10.1097/PRS.0b013e31823ae86c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008 Jun;60(2):210–241. doi: 10.1124/pr.107.08002. Available from: http://dx.doi.org/10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disa JJ. Discussion. Tamoxifen increases the risk of microvascular flap complications in patients undergoing microvascular breast reconstruction. Plast Reconstr Surg. 2012 Feb;129(2):315–316. doi: 10.1097/PRS.0b013e31823aeccd. Available from: http://dx.doi.org/10.1097/PRS.0b013e31823aeccd. [DOI] [PubMed] [Google Scholar]
- 15.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007 Aug;27(8):1782–1787. doi: 10.1161/ATVBAHA.107.145383. Available from: http://dx.doi.org/10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 16.Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007 Jun;49(6):1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. Available from: http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]