Abstract
Neutrophil recruitment and directional movement toward chemotactic stimuli are important processes in innate immune responses. This study examines the role of Fer kinase in neutrophil recruitment and chemotaxis to various chemoattractants in vitro and in vivo. Mice targeted with a kinase-inactivating mutation (FerDR/DR) or wild type (WT) were studied using time-lapse intravital microscopy to examine leukocyte recruitment and chemotaxis in vivo. In response to keratinocyte-derived cytokine, no difference in leukocyte chemotaxis was observed between WT and FerDR/DR mice. However, in response to the chemotactic peptide WKYMVm, a selective agonist of the formyl peptide receptor, a 2-fold increase in leukocyte emigration was noted in FerDR/DR mice (p < 0.05). To determine whether these defects were due to Fer signaling in the endothelium or other nonhematopoietic cells, bone marrow chimeras were generated. WKYMVm-induced leukocyte recruitment in chimeric mice (WT bone marrow to FerDR/DR recipients or vice versa) was similar to WT mice, suggesting that Fer kinase signaling in both leukocytes and endothelial cells serves to limit chemotaxis. Purified FerDR/DR neutrophils demonstrated enhanced chemotaxis toward end target chemoattractants (WKYMVm and C5a) compared with WT using an under-agarose gel chemotaxis assay. These defects were not observed in response to intermediate chemoattractants (keratinocyte-derived cytokine, MIP-2, or LTB4). Increased WKYMVm-induced chemotaxis of FerDR/DR neutrophils correlated with sustained PI3K activity and reduced reliance on the p38 MAPK pathway compared with WT neutrophils. Together, these data identify Fer as a novel inhibitory kinase for neutrophil chemotaxis toward end target chemoattractants through modulation of PI3K activity.
Introduction
Neutrophil recruitment to the site of inflammation or infection is a key characteristic of innate immune defense. To be recruited from the bloodstream and move to the site of inflammation or infection, a neutrophil must be able to recognize and prioritize various chemoattractant stimuli. Bacterial formylated peptides (fMLP) and complement factors (C5a) are regarded as dominant chemoattractants. However, to reach these end targets, neutrophils must follow concentration gradients of intermediate chemoattractants such as IL-8 or leukotriene B4 (LTB4) (1). Neutrophil movement is mediated by activation of intracellular signaling pathways, including MAPKs (p38 MAPK) and the lipid kinase PI3K pathway that are activated variably in response to different chemoattractants (1). Whereas most neutrophil chemoattractants signal via G protein-coupled receptors, recent studies have identified protein–tyrosine kinases (PTK) as key regulators of chemoattractant-induced neutrophil chemotaxis (2–5).
Fer is a widely expressed member of the group IV family of nonreceptor PTK whose only other member Fes (also called Fps) displays a more tissue-restricted expression pattern (6–9). Fer is composed of an N-terminal F-BAR (Fer/CIP4 homology-Bin/Amphiphysin/Rvs) domain, an extended F-BAR (FX) domain, a central Src homology 2 (SH2) domain, followed by a PTK domain. In addition to promoting oligomerization of Fer (6, 10), the N-terminal F-BAR and FX domains in Fer have recently been linked to membrane targeting Fer via binding of phosphoinositides (4,5-bis-phosphate) and phosphatidic acid (PA), respectively (11, 12). The latter study identified a contribution of phospholipase D and its product PA to Fer activation in vitro, and Fer-induced lamellipodia formation and chemotaxis of polarized epithelial NRK52E cells. Fer also promotes chemotaxis of fibroblasts downstream of integrins and the production of reactive oxygen species (13). The SH2 domain of Fer promotes its recruitment to activated receptor PTKs and phosphorylation of adaptor proteins such as Gab1 and insulin receptor substrate-1 that recruit and activate PI3K (14–16). Recently, the SH2 domain of Fes kinase was shown to positively regulate Fes kinase activity through a SH2-kinase domain interface (17). It is worth noting that these interface residues are conserved in Fer, and suggest that both Fer and Fes PTK activities may be restricted specially and temporally by the presence of their SH2 domain ligands (18).
Understanding the physiological roles of Fer PTK has been greatly advanced by studies of mice with knock-in of a kinase-inactivating mutation in the Fer locus (FerDR/DR mice) (19). Although these mice are viable and overtly normal, we have demonstrated enhanced leukocyte recruitment in response to LPS challenge in vivo (20, 21). These studies suggest that Fer plays a role in limiting leukocyte recruitment across endothelial and epithelial barriers in response to bacterial Ag. In this study, we demonstrate, in vitro and in vivo, that Fer plays a role in leukocyte chemotaxis toward the fMLP-like peptide, WKYMVm, but not toward keratinocyte-derived cytokine (KC). Bone marrow chimeras were used to determine the role of leukocyte and endothelial Fer in WKYMVm-induced chemotaxis. Furthermore, using purified neutrophils from wild-type (WT) and FerDR/DR mice, we observed a role for Fer in limiting neutrophil chemotaxis toward WKYMVm or C5a, but not to intermediate chemoattractants. This correlated with skewed signaling in Fer-deficient neutrophils away from p38 MAPK-based chemotaxis to PI3K-driven chemotaxis. Our data suggest that Fer kinase is a stimulus-specific inhibitory kinase for leukocyte recruitment in vivo and for neutrophil chemotaxis in vitro by modulating PI3K activity.
Materials and Methods
Mice devoid of Fer kinase activity (FerDR/DR) (19) and compound mutant mice lacking Fer, and Fes kinase activities (FesKR/KR/FerDR/DR) were previously described (22). WT 129Sv/J, FerDR/DR, and FesKR/KR/FerDR/DR mice were bred and housed at the University of Calgary in specific pathogen-free environment on standard chow diet. Mice were used between 6 and 10 wk of age and weighed between 20 and 35 g. Mice had access to food and water ad libitum. All experimental procedures were approved by the Animal Care Committee of the University of Calgary and conform to the guidelines established by the Canadian Council for animal care.
Leukocyte recruitment and chemotaxis in vivo
Leukocyte recruitment and chemotaxis in vivo were visualized using intravital microscopy of the cremaster microvasculature, as described previously (23, 24). This thin skeletal muscle preparation can be trans-illuminated and allows for clear viewing of the extravascular space and behavior of emigrated leukocytes. Mice were anesthetized by i.p. injection of xylazine (MTC Pharmaceuticals, Cambridge, ON, Canada) and ketamine hydrochloride (Rogar/STB, Montreal, QC, Canada) in a 10:200 mg/kg ratio. The left jugular vein was cannulated to administer further anesthetic, as required, and the cremaster muscle was exposed with vasculature intact and spread over an optically clear viewing pedestal and secured along the edges with 5-0 suture. The exposed tissue was suffused with bicarbonate-buffered saline (pH 7.4, temperature 37°C). The microvasculature and surrounding tissue were observed through an intravital microscope with a 10× eyepiece (Axioskop 2 Mat Mot; Carl Zeiss Canada, Don Mills, ON, Canada) and a 20× objective lens. A video camera (Hitachi KP-D50 digital color) was used to project the images onto a monitor, and the images were recorded for playback analysis using a time-lapse videocassette recorder (Panasonic AG-TL950).
Recruitment and chemotaxis were induced using fMLP-like chemotactic peptide WKYMVm (Phoenix Pharmaceuticals) at 0.1 μM or KC (R&D Systems), the murine homolog of IL-8/growth-related oncogene-α at a final concentration of 5.2 μM. A chemotactic gradient was created by placing the chemotactic agent, in a 1-mm3 piece of 4% agarose gel, on a preselected avascular area of tissue 350 μm (two monitor screens wide) from a postcapillary venule and superfusing buffer at a very slow rate (<0.2 ml/min) over the gel toward the vessel (23, 24). The agarose gel (4%) was prepared in HBSS:dH2O (50:50). A 100-μl aliquot of this solution was removed, and the chemoattractant was added to this aliquot to achieve the desired final concentration. A small amount of india ink was added to the gel to enable visualization on the cremaster muscle, and the gel was held in place with a cover slip.
Single unbranched venules (25–40 μm in diameter) were selected for study, and intravascular leukocyte kinetics was observed in the same section of venule in 5-min periods at intervals (zero; before gel placement, 30- and 60-min postgel placement) during the experiment. The number of rolling and adherent leukocytes was determined off-line during video playback analysis. Rolling leukocytes were defined as those cells moving at a velocity less than that of erythrocytes within a given vessel. Leukocyte rolling velocity was determined by measuring the time required for a leukocyte to roll along a 100-μm length of venule. Rolling velocity was determined for 20 leukocytes at each time interval. Leukocytes were considered adherent to the venular endothelium if they remained stationary for 30 s or longer. Leukocyte emigration was defined as the number of extravascular leukocytes in the microscopic field of view between the vessel and the gel (a region of ∼200 × 300 mm). Venular diameter was measured on-line using a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX). Centerline RBC velocity (VRBC) was also measured on-line using an optical Doppler velocimeter (Microcirculation Research Institute), and mean RBC velocity was determined as VRBC/1.6. Extravascular leukocyte chemotaxis was time-lapse recorded over 1 h. Leukocyte chemotaxis was determined in WT or FerDR/DR mice or in bone marrow chimeric mice described below.
Bone marrow chimeras
To determine whether Fer kinase activity within the endothelium contributes to emigration and chemotaxis in vivo, bone marrow chimeras were generated, as previously described (25). Chimeric mice were generated in FerDR/DR recipients with WT (129Sv/J) donor bone marrow to generate a mouse with Fer-competent hematopoietic cells and Fer-deficient endothelial cells (endothelium-FerDR/DR). Chimeric mice with Fer deficiency in hematopoietic cells were generated in WT mice receiving FerDR/DR donor bone marrow (leukocyte-FerDR/DR). Briefly, bone marrow was isolated from mice euthanized by spinal cord displacement. Recipient mice were irradiated with 2 doses of 5 Gy (Gammacell 40 [137Cs] gamma-irradiation source; Nordion International, Kanata, ON, Canada) with a 3-h interval between the first and second irradiations. Next, 8 × 106 donor bone marrow cells were injected into the tail vein of recipient irradiated mice. Mice were kept in microisolator cages for 8 wk to allow full humoral reconstitution before use. This protocol previously confirmed that ∼99% of leukocytes in recipient mice were from donor bone marrow (25).
Isolation of bone marrow–derived neutrophils
Neutrophils were isolated from mouse bone marrow, as described in details by Lieber et al. (26). Briefly, femurs and tibias of WT, FerDR/DR, or FesKR/KR/FerDR/DR mice were dissected, and the marrow was flushed with PBS and then centrifuged at 1300 rpm for 6 min. The marrow was layered on a three-step Percoll (Amersham Bioscience) gradient (72, 64, and 52%) and centrifuged at 2600 rpm for 30 min. Neutrophils were taken from the layer between the 64 and 72% Percoll and washed once with PBS before being suspended in RPMI 1640 culture media plus 20% FBS (Life Technologies) at a concentration of 1.0 × 107 cells/ml.
In vitro under-agarose gel assay for neutrophil chemotaxis
The under-agarose assay was performed as described in detail by Heit et al. (1). In brief, three horizontal equidistant wells were formed in 35 × 10-mm culture dishes filled with 1.2% agarose solution. The center well was loaded with different chemoattractants of varying concentration, and the outer wells were loaded with neutrophils. Once loaded, the gels were incubated for 4 h in a 37°C/5% CO2 incubator, to allow sufficient time for neutrophils to migrate the entire distance between the wells. The results were analyzed and recorded by using a video camera attached to a Zeiss Axiovert 135 microscope. Neutrophil chemotaxis was determined as the number of cells migrating toward the source of chemoattractant minus the number of cells migrating away from center well. The following chemoattractants were studied: WKYMVm (Phoenix Pharmaceuticals) 0.01–100 μM; KC (R&D Systems) 0.0125–125 μM; MIP-2 (R&D Systems) 1.25 μM; LTB4 (Calbiochem) 0.01–100 μM; and C5a (Calbiochem) 1 μM. Negative controls were studied by adding 10 μl PBS (vehicle) instead of the chemoattractants in the center well. Chemotaxis in the presence of p38 MAPK inhibitors (SB239063, 10 μM; SB203580, 10 μM; or SKF86002, 30 μM) or PI3K inhibitors (wortmannin, 100 nM; or LY294002, 50 μM) was studied by incubating the neutrophils with the inhibitor or vehicle on ice for 30 min prior to loading them in the agarose gel. These concentrations have been previously shown to be optimal inhibitory concentrations for neutrophil chemotaxis (1, 27).
Western blots
p38 MAPK and Akt phosphorylation was determined in WT and FerDR/DR neutrophils in response to WKYMVm (1 μM) stimulation by immunoblot. Bone marrow–derived neutrophils (5 × 106/condition) were stimulated with WKYMVm at 37°C for 0–15 min or left untreated, and the reaction was stopped with an equal volume of 2× SDS buffer (Sigma-Aldrich) plus 100 μM PMSF, 100 μM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μM pepstatin. The samples were boiled at 90°C for 10 min, and lysates were loaded onto 10% SDS-PAGE gel and run at 50 V overnight. Proteins were transferred to a nitrocellulose membranes and blocked with 2% BSA for 1 h before being incubated overnight at 4°C with 1/1000 dilution of phospho- or total p38 MAPK Ab or pAkt Ab (Ser473) (Cell Signaling) prepared in 2% BSA. The membrane was washed and incubated with 1/10,000 dilution of anti-rabbit HRP-conjugated secondary Ab (Santa Cruz Biotechnology) for 1 h, developed with super signal ECL, and visualized with Kodak x-ray film.
Statistical analysis
Data are expressed as the mean ± SEM. Groups of data were compared using nonparametric Mann-Whitney U test or Kruskal-Wallis one-way ANOVA, followed by a Dunns multiple comparison test. The p values ≤0.05 were considered statistically significant.
Results
Absence of Fer kinase does not alter basal intravascular leukocyte kinetics
Our previous studies have implicated Fer in limiting leukocyte recruitment in response to LPS challenge in cremaster muscle and the gut epithelium (20, 21). In the latter study, mice devoid of Fer kinase activity (FerDR/DR) displayed defects that were partly attributable to altered neutrophil function. To further define the role of Fer within neutrophils, we compared leukocyte recruitment kinetics between WT and FerDR/DR mice in cremaster muscle exposed to neutrophil chemoattractants. As Fer is expressed in both the endothelium and leukocytes, we also compared responses of chimeric FerDR/DR mice (see Materials and Methods). Table I illustrates the mean RBC velocity and vessel diameter in WT, FerDR/DR, and chimeric mice under baseline conditions with no chemoattractant present. Chimeric mice had similar RBC velocity, vessel diameter, and basal leukocyte behavior to WT or FerDR/DR at time 0 (Table I) and these remained constant throughout the experiment (data not shown). Basal leukocyte kinetics (rolling flux, and velocity and adhesion) are indicated in Table II (0 time). As previously shown, circulating WBC counts were similar between WT and FerDR/DR mice (20), and no significant differences in baseline hemodynamics or leukocyte kinetics were observed between WT and Fer mutant mice (Table II) (20).
Table I. Hemodynamic parameters in WT, Fer mutant (FerDR/DR), and FerDR/DR chimeric mice under control conditions.
| WT | FerDR/DR | Endo-FerDR/DR Chimeras | Leuk-FerDR/DR Chimeras | |
|---|---|---|---|---|
| Vessel diameter (μm) | 32.0 ± 0.8 | 30.8 ± 1.5 | 33.1 ± 2.3 | 32.0 ± 1.0 |
| RBC velocity (mms−1) | 5.0 ± 0.5 | 3.3 ± 0.2 | 3.2 ± 0.4 | 3.5 ± 0.3 |
Data are expressed as the mean ± SEM. n = 4 (WT), n = 5 (FerDR/DR), n = 4 (endothelium-FerDR/DR), n = 4 (leukocyte-Fer). For each group, values were taken immediately prior to agarose gel placement on the extravascular tissue. Data were not significantly different immediately postgel placement (data not shown).
Table II. WKYMVm-induced intravascular leukocyte kinetics is not altered in the absence of Fer kinase.
| Rolling Flux (cells/min) |
Rolling Velocity (μm/s) |
Adhesion (cells/100 μm/5 min) |
||||
|---|---|---|---|---|---|---|
| 0 time | 60 min | 0 time | 60 min | 0 time | 60 min | |
| Wild type | 45.5 ± 1.7 | 53.0 ± 17.0 | 43.1 ± 5.4 | 65.0 ± 8.0 | 2.0 ± 0.4 | 10.5 ± 0.5* |
| FerDR/DR | 70.0 ± 12.5 | 62.2 ± 9.7 | 60.7 ± 5.8 | 43.4 ± 7.0 | 0.2 ± 0.2 | 11.0 ± 1.3* |
| Endo-FerDR/DR chimeras | 54.4 ± 8.3 | 55.2 ± 11.9 | 51.5 ± 4.4 | 64.9 ± 7.0 | 0.8 ± 0.4 | 16.0 ± 2.4* |
| Leuk-FerDR/DR chimeras | 61.8 ± 9.4 | 50.3 ± 17.2 | 41.8 ± 5.6 | 43.7 ± 6.0 | 0.8 ± 0.5 | 21.0 ± 1.8*,# |
Leukocyte rolling velocity (μm/s), rolling flux (cells/min), and adhesion (cells/100 μm/5 min) within postcapillary venules of the cremaster were determined using in vivo microscopy. Data are shown for wild-type, Fer-deficient (FerDR/DR), or chimeric (endothelium [Endo]-FerDR/DR and leukocyte [Leuk]-FerDR/DR) mice. Intravascular leukocyte recruitment was induced by setting up a chemotactic gradient using WKYMVm (1 mM) in an agarose gel in the extravascular tissue space 350 μm from the vessel. Leukocyte kinetics were observed immediately following gel placement (0 time) and 60 min later. Data are expressed as mean ± SEM; WT, n = 4; FerDR/DR, n = 5; Endo-FerDR/DR, n = 4; Leuk-FerDR/DR, n = 4.
p < 0.05 significant difference from zero time point within each group, #p < 0.05 significant difference from FerDR/DR mice.
Fer kinase within leukocytes and nonhematopoietic cells contributes to recruitment and chemotaxis toward WKYMVm in vivo
Table II illustrates leukocyte kinetics within the cremaster microcirculation over 60 min following placement of a gel containing WKYMVm in the extravascular space. In WT mice, WKYMVm induced a significant increase (p < 0.05) in leukocyte adhesion within the microvasculature after 60 min. An increase in leukocyte adhesion was also observed in Fer mutant (FerDR/DR or chimeric mice) groups 60 min post-WKYMVm. WKYMVm-induced leukocyte rolling velocity and flux did not differ significantly from data generated in WT mice (Table II). Interestingly, adhesion in chimeric mice with Fer-deficient leukocytes was significantly greater than in whole animal Fer mutants. This suggests that Fer plays a suppressive role on cell adhesion in vivo within leukocytes. A similar trend occurred with Fer-deficient endothelium in mice, but did not reach statistical significance.
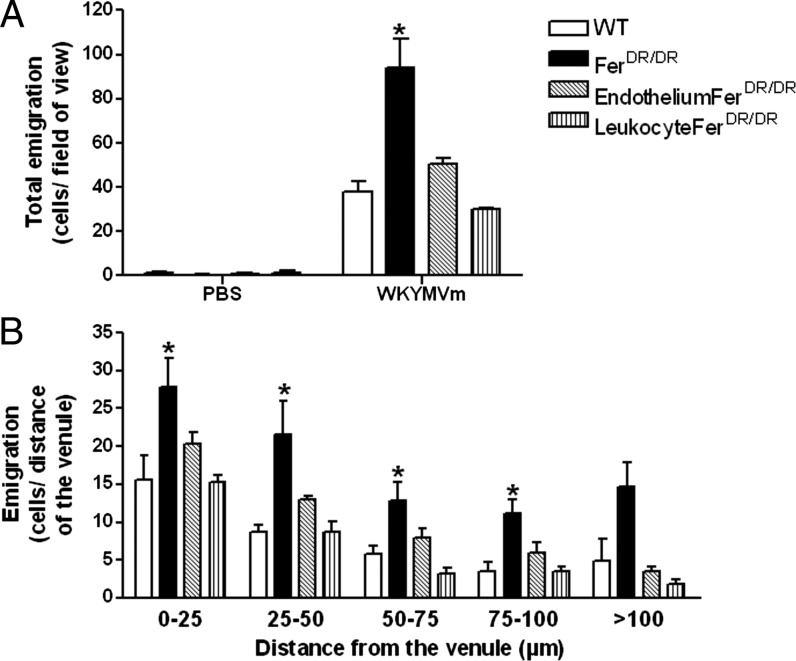
Fig. 1A illustrates the total leukocyte emigration from the blood vessel 60 min after gels containing WKYMVm were placed in the extravascular tissue space. In WT mice, ∼40 cells/field of view are observed in the extravascular space. Interestingly, WKYMVm-induced leukocyte extravasation was significantly enhanced in FerDR/DR mice (Fig. 1A; >2-fold increase; p < 0.05). The enhanced leukocyte recruitment observed in whole animal Fer mutants was reversed in chimeric mice where leukocytes were restored to Fer WT genotype, while retaining Fer deficiency in nonhematopoietic tissues (endothelium-FerDR/DR). Thus, Fer deficiency within nonhematopoietic tissues alone, including endothelial cells, does not account for the enhanced leukocyte extravasation observed in Fer-deficient mice. Similarly, in chimeras where Fer deficiency was present only in leukocytes (leukocyte FerDR/DR), WT levels of transendothelial migration were observed. These data demonstrate that exacerbation of transmigration toward WKYMVm is only observed with the combined Fer deficiency within both leukocytes and the endothelium. Once cells had extravasated, we tracked the distance leukocytes migrated from the venules toward the WKYMVm-containing gel (Fig. 1B). These data demonstrated that cells in all groups were able to chemotax considerable distances (>100 μm) from the vessel within 60 min. However, we observed a significant increase in Fer-deficient mice, compared with WT. Neither chimeric mouse model with partial Fer deficiency phenocopied the Fer-deficient mice, suggesting that Fer limits leukocyte emigration and chemotaxis toward WKYMVm within multiple cell compartments.
FIGURE 1.
WKYMVm-induced leukocyte transmigration and chemotaxis are enhanced in the absence of Fer kinase in vivo. Leukocyte emigration and chemotaxis were induced in vivo by placing WKYMVm (1 mM) in an agarose gel 350 μm from the vessel in the extravascular space. Data were observed in WT mice (open bars), FerDR/DR mice (solid bars), and bone marrow chimeric mice (WT donors into FerDR/DR recipients [EndotheliumFerDR/DR, hatched bars] or FerDR/DR leukocytes into WT recipients [LeukocyteFerDR/DR, vertical hatched bars]). (A) Illustrates total number of emigrated leukocytes observed in the extravascular space (cells/field of view) under control conditions (vehicle), or 60 min after WKYMVm gel placement. (B) Illustrates the number of extravasated cells in each 25-μm increment segment from the blood vessel toward the gel containing WKYMVm. Data are expressed as mean ± SEM, n = 4 (WT), n = 5 (FerDR/DR), n = 4 (Endothelium-FerDR/DR and Leukocyte-FerDR/DR chimeras), *p < 0.05 significant difference from WT mice group.
Fer kinase regulates leukocyte adhesion and transmigration in vivo in a stimulus-specific manner
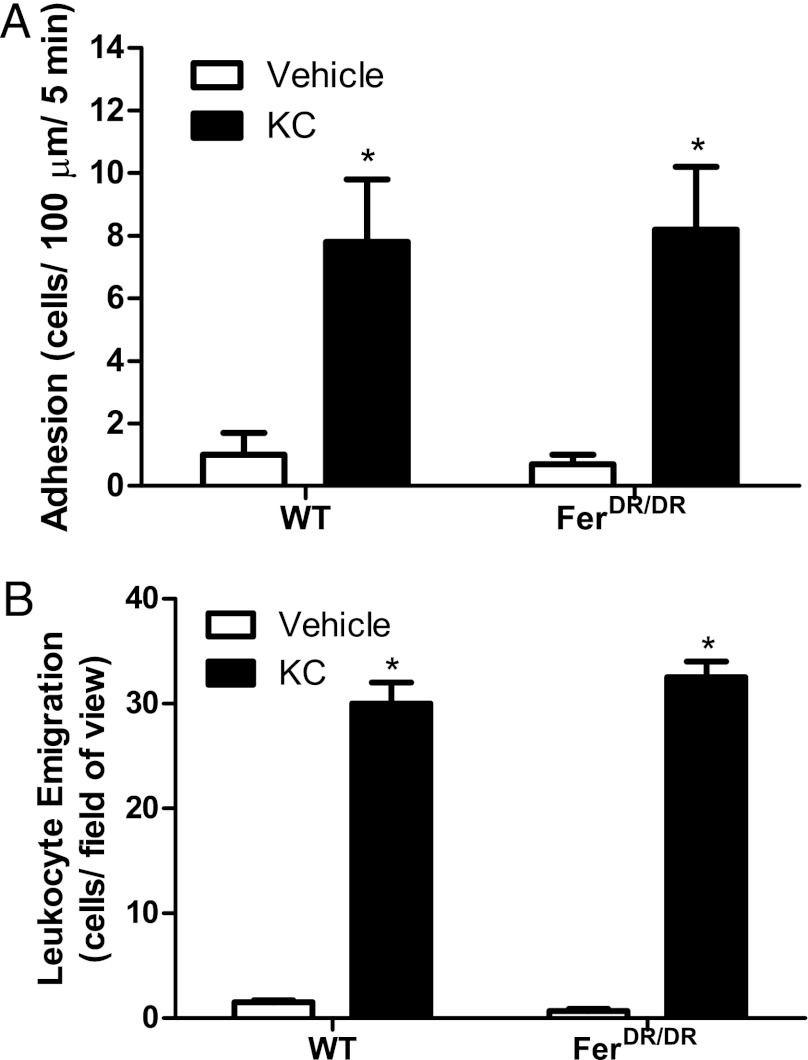
To determine whether Fer kinase suppresses leukocyte recruitment toward all neutrophil chemoattractants, we performed similar experiments using KC protein, which signals via CXCR2 in leukocytes. Within 60 min of placement of a gel containing KC (5.2 μM) or vehicle control in the extravascular space, leukocyte adhesion and emigration in the cremaster were recorded (Fig. 2). As expected, there was a significant increase in adhesion, and leukocyte emigration was noted in response to KC gel versus vehicle control gel in WT mice (Fig. 2). In contrast to results for WKYMVm, we observed no difference in leukocyte adhesion or recruitment between WT and FerDR/DR mice responding to KC. These data suggest that the role of Fer kinase in leukocyte recruitment is stimulus specific, with a potential role in suppressing responses to dominant, but not intermediate chemoattractants.
FIGURE 2.
Fer kinase does not contribute to KC-induced recruitment in vivo. Leukocyte recruitment was induced by placing KC (5.2 μM) in an agarose gel in the extravascular tissue space 350 μm from the vessel. Data are shown during control conditions (vehicle) and at 60-min postgel placement in WT and FerDR/DR mice. (A) Illustrates leukocyte adhesion to the endothelium within the postcapillary venule (cells/100 μm/5 min). (B) Illustrates the total number of emigrated leukocytes (cells/field of view). Data are expressed as mean ± SEM, n = 4 (WT) and n = 5 (FerDR/DR), *p < 0.05 significant difference from vehicle group for each strain.
Fer kinase modulates neutrophil chemotaxis in vitro in a stimulus-specific manner
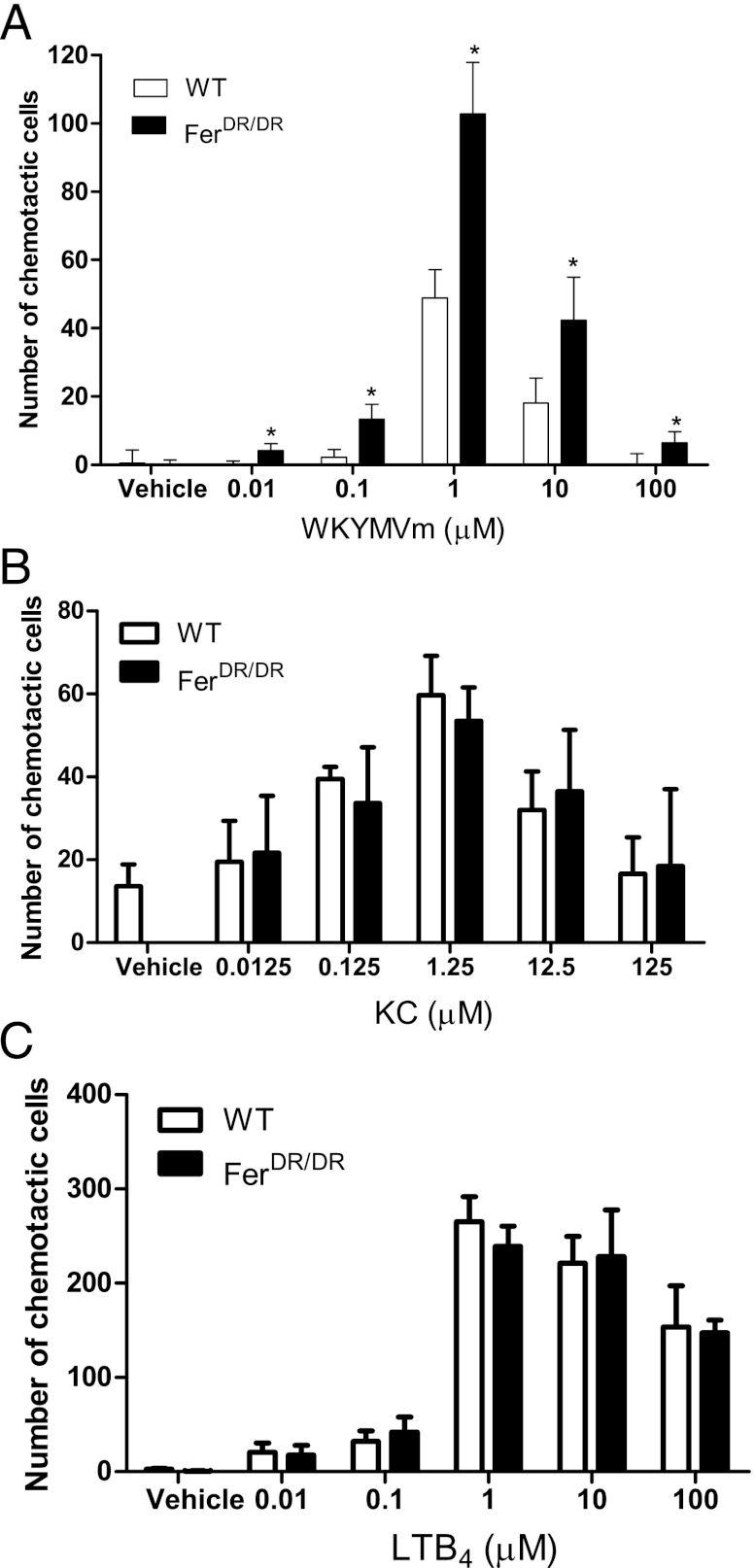
To better define the cell-autonomous role of Fer in regulating leukocyte chemotaxis, we moved to an in vitro under-agarose chemotaxis assay. Bone marrow–derived neutrophils were isolated from WT or FerDR/DR mice, and a dose-response curve for WKYMVm-induced chemotaxis was established. Fig. 3A illustrates the number of neutrophils moving toward 0.01–100 μM WKYMVm, and a classical bell-shape curve was observed in response to increasing concentrations of the chemoattractant using both WT and FerDR/DR neutrophils. The optimal dose of WKYMVm for neutrophil chemotaxis in vitro was observed at 1 μM in both groups. Significantly more FerDR/DR neutrophils moved toward WKYMVm at all concentrations relative to the WT cells. These data demonstrate enhanced leukocyte chemotaxis in vitro, demonstrating the importance of Fer kinase within the leukocyte as a negative regulator of chemotaxis.
FIGURE 3.
Absence of Fer kinase enhances chemotaxis toward end target, but not intermediate chemoattractants in vitro. In vitro neutrophil chemotaxis, determined using the under-agarose gel assay using bone marrow–derived neutrophils from WT (open bars) and Fer mutant mice (FerDR/DR; solid bars). (A) Illustrates the number of chemotactic cells in response to increasing concentrations of WKYMVm (0.01–100 μM). Data are expressed as mean ± SEM, 10–24 plates (n = 5 mice). (B) Illustrates the number of chemotactic cells in response to increasing concentrations of KC (0.0125–125 μM). Data are expressed as mean ± SEM, 9–12 plates (n = 3 mice). (C) Illustrates the number of chemotactic cells in response to increasing concentrations of LTB4 (0.01–100 μM). Data are expressed as mean ± SEM, 9–12 plates (n = 3 mice). *p < 0.05 significant increase over WT neutrophils.
To determine whether Fer kinase modulation of neutrophil chemotaxis was specific to WKYMVm, we studied neutrophil chemotaxis toward various known chemoattractants. Using KC (0.0125–125 μM) as the chemoattractant, a significant increase in chemotaxis over vehicle control was observed for both WT and FerDR/DR neutrophils (Fig. 3B) with a maximal response observed at 1.25 μM. No significant differences were observed between WT and FerDR/DR neutrophils, which recapitulates our results from in vivo chemotaxis assays. Furthermore, no significant differences were observed in chemotaxis of WT or FerDR/DR neutrophils toward LTB4 over 0.01–100 μM range (Fig. 3C). MIP-2 at 1.25 μM induced similar chemotactic response in WT (25.0 ± 5.0 cells) or FerDR/DR (30.0 ± 4.0 cells) neutrophils. These chemoattractants (KC, LTB4, and MIP-2) have been described previously as intermediary chemotactic agents because they direct neutrophils toward the end target chemoattractants (bacterial peptides, complement products). Interestingly, in the absence of Fer kinase, neutrophils exhibit enhanced C5a-induced chemotaxis compared with WT neutrophils (33.0 ± 3.0 versus 17.0 ± 2.0 cells, respectively, p < 0.05). Complement products such as C5a, along with bacterial products (WKYMVm), are considered end target chemoattractants, suggesting that Fer modulates neutrophil chemotaxis toward end target chemoattractants and not toward intermediary chemoattractants.
Fes kinase does not compensate for Fer in neutrophil chemotaxis toward LTB4
The possibility exists that, in the absence of Fer kinase, the closely related group IV PTK Fes (18) compensates for Fer by modulating neutrophil chemotaxis toward intermediate chemoattractants such as LTB4. In separate experiments, we compared LTB4-induced chemotaxis in bone marrow neutrophils from WT, FerDR/DR, and FesKR/KR/FerDR/DR compound mutant mice (lacking both Fes and Fer kinase activities). In Table III, we show that LTB4-induced chemotaxis of neutrophils from FesKR/KR/FerDR/DR mice was similar to that observed for neutrophils from WT and FerDR/DR mice. Although WKYMVm-induced chemotaxis in neutrophils from FesKR/KR/FerDR/DR compound mutant mice trended higher than that of FerDR/DR mutant mice, the differences were not statistically significant. These data suggest that Fes kinase does not compensate for Fer kinase in modulating neutrophil chemotaxis.
Table III. Fes kinase does not contribute to LTB4-induced chemotaxis.
| Number of Chemotactic Cells |
||
|---|---|---|
| WKYMVm | LTB4 | |
| Wild type | 48.9 ± 8.3 | 51.9 ± 6.1 |
| FerDR/DR | 102.7 ± 15.1* | 63.1 ± 7.4 |
| FesKR/KR/FerDR/DR | 159.0 ± 33.4* | 72.1 ± 9.6 |
Neutrophil chemotaxis was determined in vitro using the under-agarose gel assay. Chemotaxis was determined in response to WKYMVm (1 μM) or LTB4 (1 μM) using bone marrow–derived neutrophils from wild-type, Fer mutant (FerDR/DR), or Fps/Fer double-mutant mice (FesKR/KR/FerDR/DR). Data are expressed as mean ± SEM, 23–24 plates (n = 3 mice).
p < 0.05 significantly different from WT neutrophils.
Fer-deficient neutrophils have skewed intracellular signaling pathways driving chemotaxis toward WKYMVm
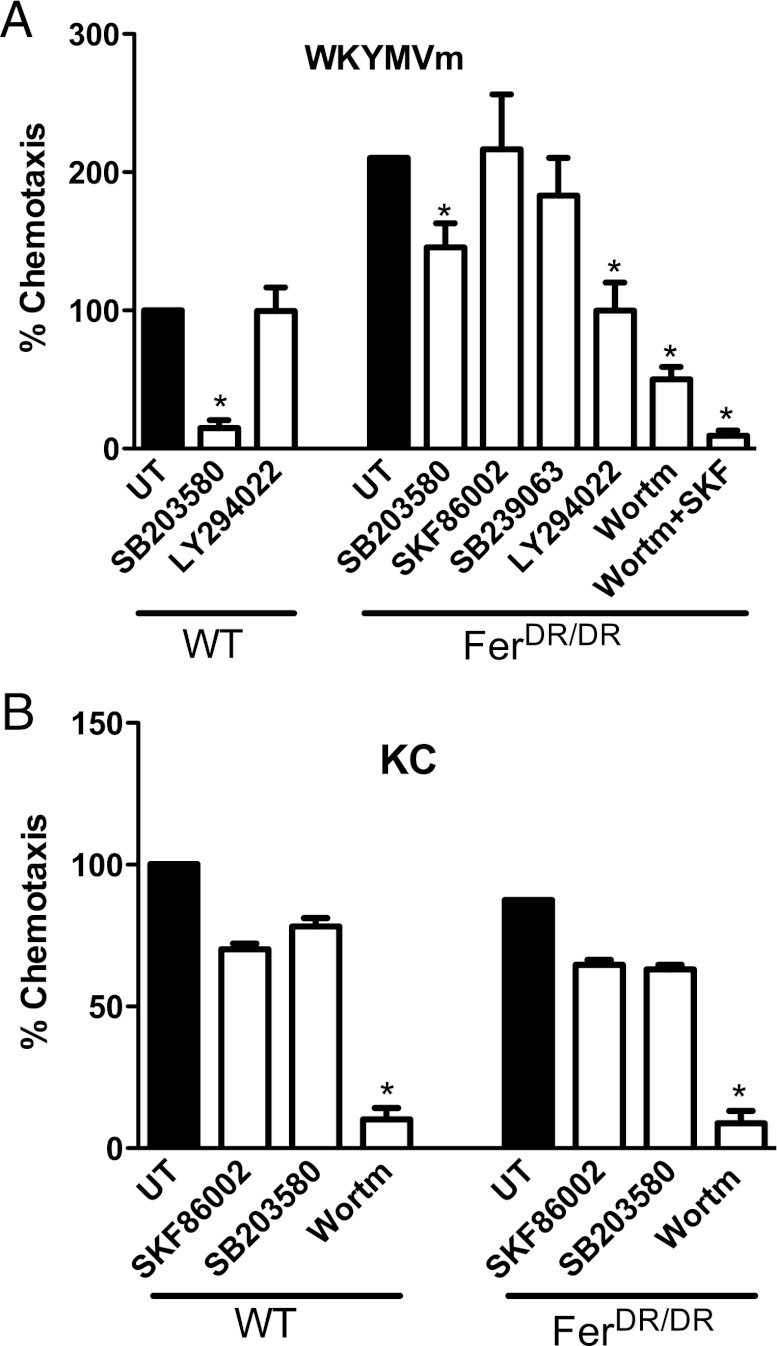
The intracellular signaling pathways used by neutrophils to chemotaxis toward various chemoattractants have been identified previously (1). Neutrophils predominately use the p38 MAPK pathway to chemotaxis toward end target chemoattractants like fMLP peptide (WKYMVm) and the PI3K pathway to move toward intermediate chemoattractants like LTB4 and KC (1). In this study, we used inhibitors to determine whether the intracellular pathways used by neutrophils were altered in the absence of Fer kinase. In WT neutrophils, we observed that WKYMVm-induced chemotaxis was strongly inhibited (>85%) by treatment with the p38 MAPK inhibitor SB203580 (Fig. 4A). In contrast, chemotaxis of WT neutrophils was unchanged by treatment with the PI3K inhibitor LY294022 (Fig. 4A), demonstrating dominance for the p38 MAPK pathway in WKYMVm-induced chemotaxis in WT neutrophils. Interestingly, in the absence of Fer kinase, minimal inhibition of WKYMVm-induced chemotaxis was observed in the presence of SB203580 and no inhibition was observed with two alternative p38 MAPK inhibitors (SKF86002 and SB239063) (Fig. 4A). In contrast, PI3K inhibitors LY294002 and wortmannin inhibited WKYMVm-induced chemotaxis by 53 and 76%, respectively, in FerDR/DR neutrophils, and complete inhibition was induced by a combination of a p38 MAPK inhibitor and a PI3K inhibitor (Fig. 4A). These data suggest that Fer kinase can modulate the intracellular signaling pathways used by neutrophils to chemotax toward WKYMVm. In contrast, WT neutrophils predominately use the PI3K pathway to chemotaxis toward KC, and the dependence on this pathway was unaltered in FerDR/DR neutrophils (Fig. 4B). Together, these results implicate Fer in suppressing a PI3K-dependent pathway of chemotaxis downstream of the WKYMVm/FPR pathway, but not the KC/CXCR2 pathway.
FIGURE 4.
Absence of Fer kinase alters the intracellular signaling pathways used by neutrophils to chemotax toward WKYMVm. In vitro neutrophil chemotaxis was determined using bone marrow–derived neutrophils from WT and Fer mutant (FerDR/DR) mice in the under-agarose gel assay. Chemotaxis was induced toward 1 μM WKYMVm (A) or 1.25 μM KC (B) and expressed as a percentage of the total number of cells moving toward the chemoattractants in untreated (UT) WT groups (taken as 100%; solid bars). The role of p38 MAPK or PI3K pathways in stimulus-induced chemotaxis was determined by pretreating the neutrophils with SB203580 (10 μM), SKF86002 (30 μM), SB239063 (10 μM), LY294002 (50 μM), wortmannin (100 nM), or a combination of wortmannin and SKF86002. Data are expressed as mean ± SEM, 10–16 plates (n = 9 mice). *p < 0.05 significant difference from untreated WT or FerDR/DR neutrophils.
Absence of Fer kinase does not alter WKYMVm-induced p38 MAPK phosphorylation in neutrophils
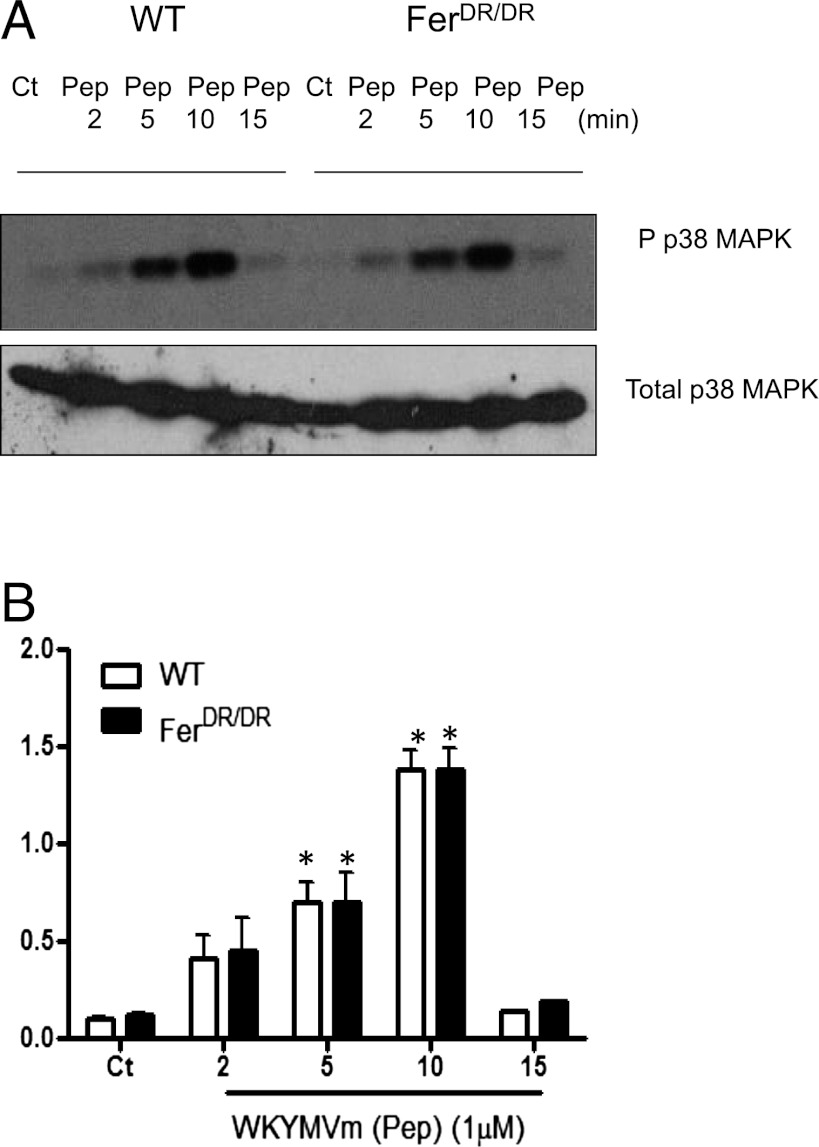
Next, we examined whether p38 MAPK activity is altered in the absence of Fer after WKYMVm stimulation in neutrophils. Fig. 5A illustrates the degree of p38 phosphorylation in WT and FerDR/DR neutrophils in response to WKYMVm (Pep; 1 μM) stimulation over a 15-min time course. WKYMVm stimulation induced an increase in p38 phosphorylation significantly within 5 min in WT neutrophils, which was maximal at 10 min and back to control levels within 15 min (Fig. 5B). A similar pattern of p38 phosphorylation was observed in FerDR/DR neutrophils. These data suggest that p38 MAPK activity is not altered in the absence of Fer kinase in neutrophils in response to WKYMVm stimulation.
FIGURE 5.
WKYMVm-induced p38 MAPK phosphorylation in neutrophils is not altered in the absence of Fer kinase. Bone marrow–derived neutrophils from WT and FerDR/DR mice were stimulated with WKYMVm (Pep; 1 μM) for 2, 5, 10, and 15 min. Lysates were prepared in triplicate, resolved by SDS-PAGE, immunoblotted with anti-pp38 Ab, stripped, and reprobed with anti-p38 Ab. (A) Representative immunoblot of pp38 MAPK and total p38 in each condition. (B) Semiquantitative analysis of p38 MAPK phosphorylation determined by densitometric analysis of n = 3 immunoblots. Data from WT and FerDR/DR neutrophils are shown in open or solid bars, respectively. *p < 0.05 significant difference from control (Ct) for each strain.
PI3K activation is more sustained in Fer-deficient neutrophils stimulated with WKYMVm
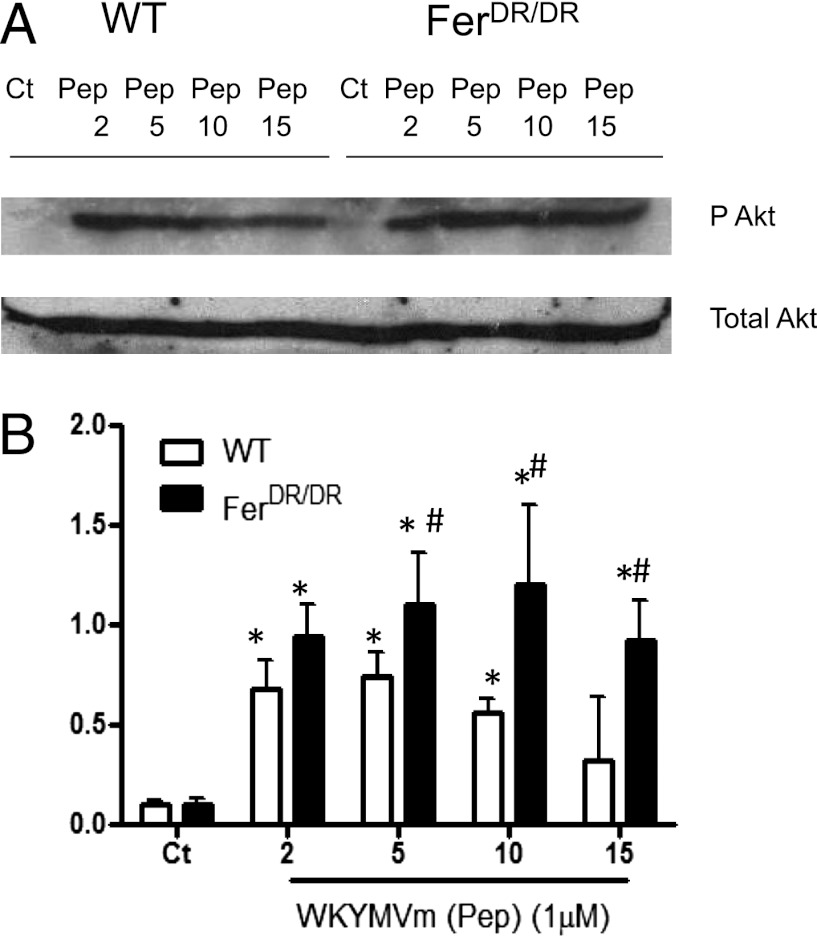
To survey PI3K signaling in WT and FerDR/DR neutrophils, immunoblots were performed using pAkt and total Akt antisera following WKYMVm stimulation (Fig. 6A). In WT neutrophils, a significant increase in Akt phosphorylation was observed within 2 min, and this returned to control levels within 15 min (Fig. 6). In Fer-deficient neutrophils, Akt phosphorylation was increased within 2 min, and this was sustained for the entire time of WKYMVm treatment (Fig. 6). Phosphoinositide-dependent protein kinase 1 (PDK1) is a 63-kDa serine–threonine kinase upstream of Akt, which has been proposed to be involved in cell chemotaxis and migration (28, 29). PDK1 phosphorylation was constitutively expressed in both WT and FerDR/DR neutrophils and was not significantly enhanced after WKYMVm stimulation in either cell type (data not shown). Lastly, we examined pAkt levels in neutrophils after KC stimulation and confirmed that, in the absence of Fer kinase, pAkt levels were similar to those observed in Fer-competent cells (data not shown), confirming that Fer does not participate in PI3K pathway modulation downstream of KC. Taken together, these results suggest that Fer kinase blocks sustained activation of the PI3K pathway within neutrophils activated by end target chemoattractants.
FIGURE 6.
WKYMVm-induced PI3K activation is prolonged in neutrophils in the absence of Fer kinase. Bone marrow–derived neutrophils from WT and FerDR/DR mice were isolated and stimulated with WKYMVm (Pep; 1 μM) for 2–15 min. Lysates were prepared in triplicate, resolved by SDS-PAGE, immunoblotted with anti-pAkt Ab (Ser473), stripped, and reprobed with anti-Akt Ab. (A) Representative immunoblot of phosphorylated and total Akt in each condition. (B) Semiquantitative analysis of Akt phosphorylation determined by densitometric analysis of n = 4 immunoblots. Data are expressed as the mean ± SEM. Data from WT and FerDR/DR neutrophils are shown in open or solid bars, respectively. *p < 0.05 significant difference from control (Ct) of each strain, #p < 0.05 significant difference from WT neutrophils at same stimulation time condition.
Discussion
Previously, we have demonstrated a role for the group IV nonreceptor PTKs, Fer (20, 21) and Fes (30, 31), in leukocyte recruitment in vivo in response to local LPS injection. LPS induces recruitment through the induction of many different chemoattractants. In this study, we wanted to determine whether Fer kinase played a universal role in neutrophil chemotaxis. We demonstrated a unique stimulus-specific role for Fer in modulating leukocyte chemotaxis both in vitro and in vivo toward bacterial-derived chemoattractants. In addition, intracellular signaling pathways used by neutrophils to chemotax are modified by Fer kinase in a stimulus-specific manner.
Using intravital microscopy, we have shown that leukocyte transmigration and chemotaxis toward WKYMVm (but not toward KC) in vivo are significantly increased in Fer mutant mice, compared with WT mice. This enhanced recruitment was only observed with Fer deficiency within whole animal mutants and not observed in chimeric mice (either WT mice transplanted with Fer-deficient leukocytes or vice versa). These results suggest that Fer signaling within both endothelial and hematopoietic cells contributes to leukocyte recruitment in vivo. Diapedesis requires sequential engagement of adhesion/transmigration molecules on both leukocytes and endothelial cells, including integrins and endothelial cell junction adhesion molecules such as PECAM-1. Fer kinase can regulate chemotaxis through integrin engagement and actin-microtubule rearrangement in fibroblasts (13) and can phosphorylate PECAM-1 in endothelial cells (16). Interestingly, chimeric mice deficient in either endothelial or hematopoietic cell PECAM-1 demonstrate a deficiency in neutrophil diapedesis in response to IL-1 (32). Furthermore, the involvement of PECAM-1 in leukocyte transmigration is stimulus dependent and is not involved in IL-8– or LTB4-induced diapedesis (33). Taken together, these data suggest that Fer kinase potentially regulates diapedesis in a stimulus-specific manner in vivo through modulation of homophilic PECAM-1 signaling between leukocytes and endothelial cells.
We were able to replicate this stimulus-specific response in vitro using bone marrow–derived neutrophils and the under-agarose gel assay. We demonstrate a specific role for Fer kinase in chemotaxis toward end target chemoattractants (WKYMVm and C5a), but not toward the intermediate targets (LTB4, KC, and MIP-2). Furthermore, we have presented novel data demonstrating an important role for Fer in modulating the intracellular signaling pathways used by neutrophils in their chemotactic behavior. In this study, we confirmed previously published data that neutrophils use p38 MAPK to chemotax toward the end target chemoattractant WKYMVm, and that the PI3K pathway dominates in chemotaxis toward the intermediate chemoattractant KC (1). Interestingly, in the absence of Fer kinase, the PI3K pathway becomes the dominant pathway used by neutrophils to migrate toward WKYMVm, which enhances chemotaxis efficiency. It has previously been suggested that a crosstalk exists between activated PI3K and p38 MAPK pathways during chemotaxis of neutrophils in a competitive environment. Heit et al. (1) showed that pharmacological inhibition of the p38 MAPK pathway resulted in enhanced PI3K activation and neutrophil chemotaxis. Furthermore, in activated mast cells, Fer kinase was required for sustained p38 MAPK activation and maximal chemotaxis (34). However, the switch in pathway dominance observed in our Fer mutant WKYMVm-stimulated neutrophils is not due to an inhibition of p38 MAPK activation as pp38 levels were similar between WT and Fer-deficient neutrophils. This does not exclude, however, a role for Fer kinase downstream of p38 MAPK on MK2 or its substrate Lsp-1. MK2 deficiency and F-actin polymerization are associated with a lack of PTEN localization to the cell membrane (35). Localization of PTEN, a PIP3 phosphatase, within the cell is required for polarization of PIP3 in the cell pseudopod, which is required for prioritization of chemotactic signals. PTEN redistribution in response to fMLP is p38 dependent (36) and is p38 independent in response to IL-8. In the absence of p38 MAPK or its downstream substrate MK2, Akt levels are increased in response to fMLP. The augmented Akt activation observed in our Fer mutant mice in response to fMLP may be in part due to lack of appropriate p38-dependent PTEN redistribution, with the cell leading to an enhanced chemotactic sensitivity (Fer mutant cells respond to fMLP at lower concentrations).
Alternatively, Fer kinase could act to inhibit or limit PI3K activation in neutrophils upstream of Akt. One possible candidate is PDK1, a 63-kDa protein serine–threonine kinase that consists of N-terminal kinase domain and C-terminal pleckstrin homology domain. PDK1 can be activated by phosphorylation in response to many agonists, including insulin and oxidative stress (37, 38), and several studies have implicated PDK1 in F-actin polymerization and endothelial cell migration (28, 29). In our study, PDK1 phosphorylation was similar in both WT and FerDR/DR neutrophils in response to WKYMVm (data not shown), suggesting that Fer kinase modulates PI3K activity through upstream targets other than PDK1. Studies have shown that Fer kinase can tyrosine phosphorylate the Rac GTPase chaperone protein RhoGDI α (39) and bind to PA (11), increasing its ability to phosphorylate cortactin and Vav2, which promote actin polymerization and cell motility. In fMLP-stimulated neutrophils, PA generated via phospholipase D (PLD) has been implicated in activation of the integrin receptor CDllb/CD18 (Mac-1), which promotes cell adhesion (40). Thus, Fer kinase may be activated by WKYMVm/FPR and C5a/C5aR pathways due to PLD-induced production of PA that is expected to promote Fer localization to the membrane. The PLD/PA axis also leads to activation of CD11b/CD18 integrin receptors, and Fer may signal downstream to regulate the extent of PI3K activation, which promotes Rac activation and actin polymerization during neutrophil chemotaxis (41, 42). A potential mechanism for a suppressive role of Fer in this pathway may involve Fer-induced phosphorylation of ITIMs. In mast cells, Fer and Fes phosphorylate PECAM-1 ITIMs to promote recruitment of protein–tyrosine phosphatase Src homology region 2 domain-containing phosphatase 1 (SHP-1) (43). Directional chemotaxis of neutrophils requires PECAM-1 for recruitment and activation of SHP-1 (44). SHP-1–deficient neutrophils from motheaten mice also displayed increased adhesion, but reduced chemotaxis (45). Future studies will be required to elucidate whether Fer signaling in neutrophils involves signaling to ITIMs, SHP-1, or other substrates that impact on the PI3K pathway of chemotaxis.
Structural similarities between Fer and Fes kinases suggest that they might have similar or even redundant roles in the body. In fact, previous data suggest a redundancy between these two kinases in regulating hematopoiesis, STAT3 activation in macrophages, and signaling pathways downstream of IgE cross-linking and collagen stimulation in mast cells and platelets, respectively (46). Our results suggests that Fes kinase does not compensate for Fer in modulating neutrophil chemotaxis in vitro because LTB4-induced chemotaxis was similar in neutrophils from Fes/Fer compound mutant, Fer mutant, and WT mice. These data suggest that group IV PTK do not participate in LTB4-induced chemotaxis.
In summary, we present novel data, in vitro and in vivo, demonstrating an important role for Fer kinase in leukocyte chemotaxis toward end target chemoattractants, but not toward intermediary chemoattractants. Surprisingly, Fer inhibits PI3K signaling to Akt in neutrophils in response to end target chemoattractants like WKYMVm without inhibiting p38 MAPK activity, although an effect downstream of p38MAPK cannot be ruled out. Inappropriate control of intracellular signaling pathways involved in chemotaxis could lead to a failure to clear bacterial infections. Future studies will be required to determine whether Fer influences the resolution of bacterial infections in mice, due to altered recruitment or function of neutrophils during the immune response.
Acknowledgments
We acknowledge the core facilities available in the Calvin, Phoebe and Joan Snyder Institute for Chronic Diseases.
This work was supported by Canadian Institutes of Health Research Operating Grant RT 71 8852 Fund 60 (to D.-M.M.). D.-M.M. is an Alberta Heritage Foundation for Medical Research Senior Scholar. M.K. was supported by a Ph.D. graduate studentship from Kuwait University.
- KC
- keratinocyte-derived cytokine
- LTB4
- leukotriene B4
- PA
- phosphatidic acid
- PDK1
- phosphoinositide-dependent protein kinase 1
- PLD
- phospholipase D
- PTK
- protein–tyrosine kinase
- SH2
- Src homology 2
- SHP-1
- Src homology region 2 domain-containing phosphatase 1
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Heit B., Tavener S., Raharjo E., Kubes P. 2002. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159: 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sai J., Raman D., Liu Y., Wikswo J., Richmond A. 2008. Parallel phosphatidylinositol 3-kinase (PI3K)-dependent and Src-dependent pathways lead to CXCL8-mediated Rac2 activation and chemotaxis. J. Biol. Chem. 283: 26538–26547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller H., Stadtmann A., Van Aken H., Hirsch E., Wang D., Ley K., Zarbock A. 2010. Tyrosine kinase Btk regulates E-selectin-mediated integrin activation and neutrophil recruitment by controlling phospholipase C (PLC) gamma2 and PI3Kgamma pathways. Blood 115: 3118–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nijhuis E., Lammers J. W., Koenderman L., Coffer P. J. 2002. Src kinases regulate PKB activation and modulate cytokine and chemoattractant-controlled neutrophil functioning. J. Leukoc. Biol. 71: 115–124 [PubMed] [Google Scholar]
- 5.Van Ziffle J. A., Lowell C. A. 2009. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood 114: 4871–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig A. W., Zirngibl R., Greer P. 1999. Disruption of coiled-coil domains in Fer protein-tyrosine kinase abolishes trimerization but not kinase activation. J. Biol. Chem. 274: 19934–19942 [DOI] [PubMed] [Google Scholar]
- 7.Hao Q. L., Heisterkamp N., Groffen J. 1989. Isolation and sequence analysis of a novel human tyrosine kinase gene. Mol. Cell. Biol. 9: 1587–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letwin K., Yee S. P., Pawson T. 1988. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene 3: 621–627 [PubMed] [Google Scholar]
- 9.Pawson T., Letwin K., Lee T., Hao Q. L., Heisterkamp N., Groffen J. 1989. The FER gene is evolutionarily conserved and encodes a widely expressed member of the FPS/FES protein-tyrosine kinase family. Mol. Cell. Biol. 9: 5722–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim L., Wong T. W. 1995. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol. Cell. Biol. 15: 4553–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh T., Hasegawa J., Tsujita K., Kanaho Y., Takenawa T. 2009. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci. Signal. 2: ra52. [DOI] [PubMed] [Google Scholar]
- 12.Tsujita K., Suetsugu S., Sasaki N., Furutani M., Oikawa T., Takenawa T. 2006. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 172: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangrar W., Gao Y., Scott M., Truesdell P., Greer P. A. 2007. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol. Cell. Biol. 27: 6140–6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwanishi M., Czech M. P., Cherniack A. D. 2000. The protein-tyrosine kinase fer associates with signaling complexes containing insulin receptor substrate-1 and phosphatidylinositol 3-kinase. J. Biol. Chem. 275: 38995–39000 [DOI] [PubMed] [Google Scholar]
- 15.Li J., Smithgall T. E. 1998. Fibroblast transformation by Fps/Fes tyrosine kinases requires Ras, Rac, and Cdc42 and induces extracellular signal-regulated and c-Jun N-terminal kinase activation. J. Biol. Chem. 273: 13828–13834 [DOI] [PubMed] [Google Scholar]
- 16.Kogata N., Masuda M., Kamioka Y., Yamagishi A., Endo A., Okada M., Mochizuki N. 2003. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol. Biol. Cell 14: 3553–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filippakopoulos P., Kofler M., Hantschel O., Gish G. D., Grebien F., Salah E., Neudecker P., Kay L. E., Turk B. E., Superti-Furga G., et al. 2008. Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation. Cell 134: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig A. W. 2012. FES/FER kinase signaling in hematopoietic cells and leukemias. Front. Biosci. 17: 861–875 [DOI] [PubMed] [Google Scholar]
- 19.Craig A. W., Zirngibl R., Williams K., Cole L. A., Greer P. A. 2001. Mice devoid of fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol. Cell. Biol. 21: 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCafferty D. M., Craig A. W., Senis Y. A., Greer P. A. 2002. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin. J. Immunol. 168: 4930–4935 [DOI] [PubMed] [Google Scholar]
- 21.Qi W., Ebbert K. V., Craig A. W., Greer P. A., McCafferty D. M. 2005. Absence of Fer protein tyrosine kinase exacerbates endotoxin induced intestinal epithelial barrier dysfunction in vivo. Gut 54: 1091–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senis Y. A., Craig A. W., Greer P. A. 2003. Fps/Fes and Fer protein-tyrosine kinases play redundant roles in regulating hematopoiesis. Exp. Hematol. 31: 673–681 [DOI] [PubMed] [Google Scholar]
- 23.Hickey M. J., Forster M., Mitchell D., Kaur J., De Caigny C., Kubes P. 2000. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J. Immunol. 165: 7164–7170 [DOI] [PubMed] [Google Scholar]
- 24.Cara D. C., Kaur J., Forster M., McCafferty D. M., Kubes P. 2001. Role of p38 mitogen-activated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J. Immunol. 167: 6552–6558 [DOI] [PubMed] [Google Scholar]
- 25.Beck P. L., Li Y., Wong J., Chen C. W., Keenan C. M., Sharkey K. A., McCafferty D. M. 2007. Inducible nitric oxide synthase from bone marrow-derived cells plays a critical role in regulating colonic inflammation. Gastroenterology 132: 1778–1790 [DOI] [PubMed] [Google Scholar]
- 26.Lieber J. G., Webb S., Suratt B. T., Young S. K., Johnson G. L., Keller G. M., Worthen G. S. 2004. The in vitro production and characterization of neutrophils from embryonic stem cells. Blood 103: 852–859 [DOI] [PubMed] [Google Scholar]
- 27.Niggli V., Keller H. 1997. The phosphatidylinositol 3-kinase inhibitor wortmannin markedly reduces chemotactic peptide-induced locomotion and increases in cytoskeletal actin in human neutrophils. Eur. J. Pharmacol. 335: 43–52 [DOI] [PubMed] [Google Scholar]
- 28.Zaru R., Mollahan P., Watts C. 2008. 3-phosphoinositide-dependent kinase 1 deficiency perturbs Toll-like receptor signaling events and actin cytoskeleton dynamics in dendritic cells. J. Biol. Chem. 283: 929–939 [DOI] [PubMed] [Google Scholar]
- 29.Primo L., di Blasio L., Roca C., Droetto S., Piva R., Schaffhausen B., Bussolino F. 2007. Essential role of PDK1 in regulating endothelial cell migration. J. Cell Biol. 176: 1035–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons S. A., Greer P. A. 2006. The Fps/Fes kinase regulates the inflammatory response to endotoxin through down-regulation of TLR4, NF-kappaB activation, and TNF-alpha secretion in macrophages. J. Leukoc. Biol. 80: 1522–1528 [DOI] [PubMed] [Google Scholar]
- 31.Parsons S. A., Mewburn J. D., Truesdell P., Greer P. A. 2007. The Fps/Fes kinase regulates leucocyte recruitment and extravasation during inflammation. Immunology 122: 542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dangerfield J., Larbi K. Y., Huang M. T., Dewar A., Nourshargh S. 2002. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J. Exp. Med. 196: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien C. D., Lim P., Sun J., Albelda S. M. 2003. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood 101: 2816–2825 [DOI] [PubMed] [Google Scholar]
- 34.Craig A. W., Greer P. A. 2002. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol. Cell. Biol. 22: 6363–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y., Hannigan M. O., Kotlyarov A., Gaestel M., Wu D., Huang C. K. 2004. A requirement of MAPKAPK2 in the uropod localization of PTEN during FMLP-induced neutrophil chemotaxis. Biochem. Biophys. Res. Commun. 316: 666–672 [DOI] [PubMed] [Google Scholar]
- 36.Heit B., Robbins S. M., Downey C. M., Guan Z., Colarusso P., Miller B. J., Jirik F. R., Kubes P. 2008. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat. Immunol. 9: 743–752 [DOI] [PubMed] [Google Scholar]
- 37.Biondi R. M. 2004. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem. Sci. 29: 136–142 [DOI] [PubMed] [Google Scholar]
- 38.Kikani C. K., Dong L. Q., Liu F. 2005. “New”-clear functions of PDK1: beyond a master kinase in the cytosol? J. Cell. Biochem. 96: 1157–1162 [DOI] [PubMed] [Google Scholar]
- 39.Fei F., Kweon S. M., Haataja L., De Sepulveda P., Groffen J., Heisterkamp N. 2010. The Fer tyrosine kinase regulates interactions of Rho GDP-dissociation inhibitor α with the small GTPase Rac. BMC Biochem. 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powner D. J., Pettitt T. R., Anderson R., Nash G. B., Wakelam M. J. 2007. Stable adhesion and migration of human neutrophils requires phospholipase D-mediated activation of the integrin CD11b/CD18. Mol. Immunol. 44: 3211–3221 [DOI] [PubMed] [Google Scholar]
- 41.Cathcart M. K. 2009. Signal-activated phospholipase regulation of leukocyte chemotaxis. J. Lipid Res. 50: S231–S236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banno Y., Takuwa Y., Akao Y., Okamoto H., Osawa Y., Naganawa T., Nakashima S., Suh P. G., Nozawa Y. 2001. Involvement of phospholipase D in sphingosine 1-phosphate-induced activation of phosphatidylinositol 3-kinase and Akt in Chinese hamster ovary cells overexpressing EDG3. J. Biol. Chem. 276: 35622–35628 [DOI] [PubMed] [Google Scholar]
- 43.Udell C. M., Samayawardhena L. A., Kawakami Y., Kawakami T., Craig A. W. 2006. Fer and Fps/Fes participate in a Lyn-dependent pathway from FcepsilonRI to platelet-endothelial cell adhesion molecule 1 to limit mast cell activation. J. Biol. Chem. 281: 20949–20957 [DOI] [PubMed] [Google Scholar]
- 44.Wu Y., Stabach P., Michaud M., Madri J. A. 2005. Neutrophils lacking platelet-endothelial cell adhesion molecule-1 exhibit loss of directionality and motility in CXCR2-mediated chemotaxis. J. Immunol. 175: 3484–3491 [DOI] [PubMed] [Google Scholar]
- 45.Kruger J., Butler J. R., Cherapanov V., Dong Q., Ginzberg H., Govindarajan A., Grinstein S., Siminovitch K. A., Downey G. P. 2000. Deficiency of Src homology 2-containing phosphatase 1 results in abnormalities in murine neutrophil function: studies in motheaten mice. J. Immunol. 165: 5847–5859 [DOI] [PubMed] [Google Scholar]
- 46.Greer P. 2002. Closing in on the biological functions of Fps/Fes and Fer. Nat. Rev. Mol. Cell Biol. 3: 278–289 [DOI] [PubMed] [Google Scholar]