Abstract
γ-H2AX (activated histone 2AX) and pChk2 (activated checkpoint kinase 2), which are DNA damage response molecules, are produced in irradiated cells and may be signature molecules of radiation exposure. We investigated their use as potential biomarkers to identify individuals exposed to ionizing radiation. We collected exfoliated oral epithelial cell samples from 100 healthy individuals undergoing routine dental radiographic examination (2.34 cGy) both before and after the radiograph using a non-invasive technique. The expression levels of pChk2 and γ-H2AX in oral cells were assessed by immunohistochemical assay. Both biomarkers showed statistically significant increases in levels of expression after the radiation exposure (P < 0.001). This suggests that pChk2 and γ-H2AX may serve as sensitive indicators of low-dose radiation exposure.
INTRODUCTION
A large segment of a population may be exposed to ionizing radiation during an accident or in a nuclear bioterrorist event. Such potential events require an efficient method to conduct mass scale screening within a few days after the exposure. A rapid and reliable radiation dosimetric modality is critical to the effort to identify victims, assess radiation doses, and deliver immediate medical intervention (1). Collecting large numbers of samples within a few days of exposure may be expedited if the procedure is non-invasive. A high-throughput technique to process the samples in a short period will have additional benefit.
Ionizing radiation is known to induce DNA damage, especially DNA double-strand breaks (DSBs) (2). The proteins selectively expressed after DSBs occur may serve as markers of exposure to ionizing radiation. The cellular response to DNA damage is largely coordinated by ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related protein (ATR), and DNA-protein kinase (DNA-PK), the serine/threonine kinases that are activated early in the damage response and phosphorylate many key effectors of the response, including histone 2AX (H2AX) and checkpoint kinase 2 (Chk2) (2–4). ATM-dependent phosphoactivation of the H2AX (γ-H2AX, phosphorylation of serine 139) and Chk2 (pChk2, phosphorylation of threonine 68) initiates cellular responses to DNA damage (2–4). γ-H2AX together with pChk2 facilitates DNA repair processes, at least in part by phosphorylation of the BRCA1 tumor suppressor, an event required for homology-directed repair of DSBs (4). In this study, we examined the γ-H2AX and pChk2 expression levels in oral exfoliative cells collected non-invasively using a mouthwash technique.
MATERIALS AND METHODS
Patients and Cell Sample Collection
Exfoliated oral epithelial cells were collected from consenting participants before and after exposure to ionizing radiation and with approval from the Columbia University Institutional Review Board. We recruited 100 individuals, 55 males and 45 female, from the Columbia University Dental Radiology clinic who were to receive routine dental radiographs (full mouth series). The participants’ ages ranged from 20 to 77, the median being 47 years. The ethnic background consisted of Hispanic (n = 67), white (n = 27), black (n = 5) and Asian (n = 1). The dental radiographs consist of 18 F-speed intraoral films, equivalent to a radiation dose of approximately 2.34 cGy. We collected exfoliative oral cells from the consenting participants before and 20 min after exposure to ionizing radiation from the radiographs. As a comparison, oral cell samples were collected sequentially from 10 nonirradiated subjects 20 min apart.
The exfoliative oral epithelial cells were collected using a mouthwash technique as described previously (5). Each participant was asked to rinse vigorously with 25 ml of a commercial mouthwash solution containing 15% alcohol for 40 s and to expectorate into a cup. The samples were transferred into a 15-ml tube within 4 h of collection and centrifuged (Thermo IEC) at 1000 rpm for 10 min. The pellet containing the oral cells was then resuspended in 100–200 μl phosphate-buffered saline (PBS). A 10-μl aliquot was taken and diluted with 90 μl PBS. Five smear slides were prepared by placing 10–20 μl (~1000 cells) on the blank glass slide. The slides were checked to ensure that the number of cells was sufficient and allowed to air dry. To fix the cells, 70% cold acetone was applied for 10 min. Slides were stored at −80°C until an immunohistochemical assay was performed.
Immunohistochemical Assay
The expression levels of pChk2 and γ-H2AX were assessed by IHC assay following published protocols (6). The fixed slides were washed three times in PBS and incubated in 0.3% H2O2 with PBS for 10 min. Blocking solution was then applied for 20 min. The slides were incubated overnight with anti-pChk2 (1:100, Cell Signaling, Danvers, MA) and for 1 h with anti-γ-H2AX (1:50, Upstate Biotechnology, Lake Placid, NY). The primary antibodies were then labeled with 50 μl of horseradish peroxidase (HRP)-conjugated secondary antibody (Envision+RSystem Labelled Polymer-HRP, Anti-rabbit-HRP, DakoCytomation, Denmark) and washed in PBS. Color was developed using Dako DAB for 10 min. Oral epithelial tissues with known pChk2 and γ-H2AX expression were used with each batch as the positive and negative controls.
Quantification of Marker Expression and Statistical Analysis
The relative intensity of nuclear staining in 25–30 cells from an average of five to six randomly selected high-power fields (400× magnification) was quantified using the Cell Measurement Program software package (Cell Analysis System CAS 200 optical microscope, Becton Dickinson, San Jose, CA). Nuclear intensity was assessed based on the object average absorbance multiplied by 1000. The average staining intensity (mean, median) and standard deviation (SD) were calculated for two markers before and after the radiation exposure. ANOVA and the paired t test were conducted to assess the difference in marker expression levels. Pearson’s correlation coefficient was used to evaluate pairwise correlation between the distributions of the two markers. Data management and statistical analysis were carried out using the SAS software version 9.0 (SAS Institute Inc., Cary, NC). Two-tailed values of P < 0.05 were deemed statistically significant.
RESULTS
Patient Characteristics and the Adequacy of Samples Collected
The oral cell samples were collected from 100 subjects before and after the exposure to 2.34 cGy radiation. From each participant, an average of 100,000 cells was collected (range: 10,000 to 1,000,000). Approximately 40% of each sample were oral epithelial cells with small ovoid nuclei and abundant cytoplasm and were deemed adequate for analysis. The remaining 60% were of the surface keratin, anucleated squames, inflammatory cells and amorphous debris. Of the 200 samples collected from 100 subjects, four samples from three subjects had insufficient numbers of cells for analysis and were excluded from the study. Subsequently, the analysis was performed for 97 subjects (196 samples).
Ionizing Radiation Induces pChk2 and γ-H2AX Expression
The intensity of the nuclear staining in the exfoliative oral epithelial cells was analyzed (Fig. 1). Both biomarkers showed significantly increased levels of expression after the radiation exposure (Fig. 2). The mean intensity for pChk2 was 0.114 before irradiation (SD = 0.035) and 0.139 after irradiation (SD = 0.038). For γ-H2AX, the mean intensity before irradiation was 0.105 (SD = 0.033) and 0.125 after irradiation (SD = 0.052). A diffuse pattern of staining was observed. When two sequentially collected samples from nonirradiated subjects were analyzed, a smaller and statistically insignificant change in the mean pChk2 intensity was observed from 0.085 (SD = 0.042) to 0.087 (SD = 0.034). For γ-H2AX, the intensity changed from 0.087 (SD = 0.023) to 0.095 (SD = 0.023), which was statistically significant (P = 0.043). However, for both pChk2 and γ-H2AX, the increase in marker expression was significantly greater for the irradiated cases compared to the nonirradiated controls (P = 0.0007 for pChk2 and P = 0.0486 for γ-H2AX).
FIG. 1.

Photomicrograph of immunohistochemical staining of exfoliative oral cells. Panel A: pChk2; panel B: γ-H2AX.
FIG. 2.
Box plot of biomarker expression levels before and after radiation exposures. Before and after indicate the status of radiation exposure. Staining intensity indicates the biomarker expression level. Boxes are the interquartile ranges of the distribution (25th–75th percentile); horizontal lines within the boxes are medians, horizontal lines outside the boxes are the 5th and 9th percentiles, and black dots are outliers.
Individual Trend of Marker Expression
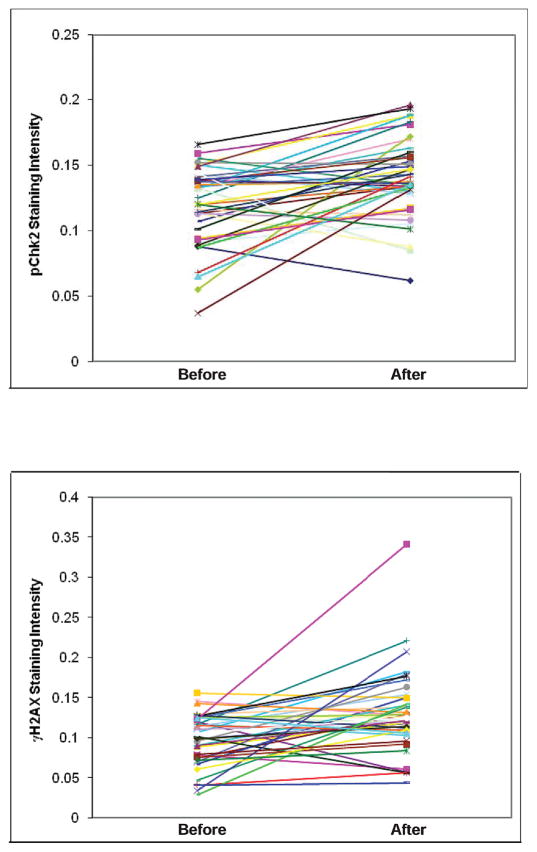
Most participants expressed higher levels of each marker after the exposure to ionizing radiation (Fig. 3). When we analyzed by individual study subject, increases in pChk2 after the radiation exposure were observed in 70 individuals. The greatest difference in the pChk2 staining intensity before and after the exposure for a subject was 0.134, a change from 0.143 to 0.277. In 27 individuals, a decrease in pChk2 expression level was observed; the difference in absolute values ranged from 0.001 to 0.108. For γ-H2AX, increases in expression were observed in 67 individuals, the greatest difference for an individual being 0.173, a change from 0.034 to 0.207 before and after the irradiation, respectively. In 30 individuals, a decrease in expression levels of γ-H2AX was observed; the difference in absolute values ranged from 0.002 to 0.107. A similar change in expression levels was observed for nonirradiated controls. For pChk2, one subject showed no change, three showed decreases, and six showed increases in marker expression. For γ-H2AX, one subject showed no change, two showed decreases, and seven showed increases in marker expression.
FIG. 3.
Comparison of pChk2 and γ-H2AX expression levels in each participant before and after the exposure to ionizing radiation.
Correlation between Two Biomarkers
There were no statistically significant correlations between pChk2 and γ-H2AX expression levels. The correlation coefficients before and after the radiation exposure were 0.13 (P = 0.19) and 0.19 (P = 0.06), respectively. This suggested that the two biomarkers were independently indicative of the exposure without any additive predictive power. A concordant increase or decrease in both pChk2 and γ-H2AX expression was observed in only 47 cases. In the remaining cases, one marker showed a decrease in expression while the other showed an increase. When both markers showed increased expression, the magnitudes of the increases were not concordant. For example, the case with the highest increase in pChk2 expression level (0.134) showed only a marginal increase in γ-H2AX level (0.002). Similarly, half of the controls collected from nonirradiated individuals showed discordant changes in the values for pChk2 and γ-H2AX.
DISCUSSION
We tested the potential utility of a non-invasive and high-throughput modality for rapid identification of individuals exposed to ionizing radiation by linking a mouthwash sample collection technique to a high-throughput immunohistochemical assay for data interpretation. The mouthwash method does not require the presence of a healthcare professional and oral cells may be self-collected, which is an efficient way of gathering samples from a large population. The immunohistochemical assays are high-throughput modalities that can test hundreds of samples simultaneously, and the results can be available 1 day after the sample collection. Biomarker level assessment on non-invasively collected samples by a high-throughput modality is suitable for mass-scale screening during an accident or in a nuclear bioterrorist event.
With regard to sample collection, the mouthwash technique is a non-invasive means of obtaining exfoliative oral cells for radiation dosimetry studies. By rinsing the mouth vigorously, we were able to obtain a heterogeneous mixture of cells from various epithelial layers. Small amounts of anucleated squames and keratotic materials were invariably included in each sample. Since we were measuring the expression of markers in the nucleus of oral epithelial cells, anucleated squames and parakeratins were not included in the intensity interpretation. Our results show that the exfoliative oral cells obtained by the mouthwash technique can serve as appropriate samples for biomarker analysis.
Activation and increased expression of γ-H2AX and pChk2 are observed in cells exposed to ionizing radiation. Bartkova and his colleagues demonstrated that there is selective expression of γ-H2AX and pChk2 in the cells with DNA damage but not in undamaged tissue (7). Rothkamm and Lobrich showed that there is nuclear expression of γ-H2AX in response to DSB after ionizing radiation doses as low as 0.1 cGy (2). Marchetti et al. (1) performed a comprehensive review of the literature and found that the phosphoactivation of H2AX is initiated as early as 6 min after radiation exposure and increases in a dose-dependent manner between 0.3 cGy and 100 Gy. Similarly, pChk2 nuclear expression is detected 25 min after the exposure and is observed in the range of 0.5–100 Gy (2). The time window of detection after ionizing radiation exposure is 0.1–48 h for γ-H2AX and 0.25–32 h for pChk2 (1). Consistent with these findings, statistically significant increases in expression levels were observed in our study for both biomarkers 20 min after exposure to 2.34 cGy of ionizing radiation.
DNA DSBs are central to carcinogenesis and initiate the DNA damage response (4, 8). Expression of DNA damage response molecules such as γ-H2AX and pChk2 was observed in precancerous and carcinomatous oral lesions but not in normal tissue (4, 6). An individual’s γ-H2AX and pChk2 expression levels may be falsely elevated, unrelated to radiation exposure, if the subject has precancerous and cancerous oral lesions. However, considering the extremely low prevalence of such lesions in the general population (approximately 1%), it is expected to have minimal impact on the data collected (9).
We observed a decrease, rather than an increase, in marker expression after irradiation in approximately 30% of the cases. In addition, there was discordance between the two markers, pChk2 and γ-H2AX, which are functionally linked and thus are expected to show similar trends in expression. To further investigate this seemingly illogical result, we collected control samples from 10 nonirradiated individuals as a comparison. The mean pChk2 expression during the initial collection was 0.085 and after 20 min was 0.087. For γ-H2AX, the mean expression was 0.087 initially and 0.095 after 20 min. The mean difference in the expression levels for pChk2 and γ-H2AX was 0.002 and 0.008, respectively, which are significantly less than those for the irradiated cases (0.025 and 0.020 for pChk2 and γ-H2AX, respectively). Similar to the irradiated subjects, expression of pChk2 showed decreases in three and γ-H2AX in two nonirradiated controls. Approximately a half of the nonirradiated individuals showed discordant values between pChk2 and γ-H2AX similar to those observed in the irradiated subjects.
Our initial speculation regarding the unexpected decrease in the marker expression after irradiation as well as the discordance between the functionally linked markers γ-H2AX and pChk2 was that our data reflect individual variations in the protein kinetics and turnover. However, this is unlikely because both histone and Chk2 proteins are rather stable, with long half-lives and slow turnover (10). A more likely explanation for the variation in the data is that since we are measuring the phospho-modification of the proteins, the data may reflect the activity of relevant kinases, especially ATM (for Chk2) and ATR and DNA-PK (for γ-H2AX). Any stress that affects these kinase activities in individuals may contribute to the variability in the data obtained. Discrepancies in data may also be due to technical errors such as sample processing because the time from sample collection to fixation of the cells was not always the same for all samples. The difference in the time from irradiation to cell collection may also have contributed to variation in data because it was difficult to collect the cells at exactly 20 min for all participants.
We recognize several limitations of our study. The data are limited by the relatively small sample size. Moreover, the samples were collected after exposure to a set dose of 2.34 cGy, and the dose response was not assessed. Thus the ranges of doses that will induce detectable pChk2 and γ-H2AX expression in exfoliative oral cells are unknown. Furthermore, the time window for reliable detection of marker expression has not been determined. The high rate of false negatives and the discrepancy rate between the two markers that are intimately linked also make this modality too unpredictable at present to serve as an indicator of low-dose radiation exposure for individuals. Our data nevertheless demonstrate that it is feasible to measure biomarker expression in exfoliated oral cells to assess radiation exposure status. Based on this proof-of-concept study, in a future investigation, we can assess the dose response and the time course for the biomarkers and define a dose and time range that yield highest sensitivity.
Acknowledgments
This work was supported by a Pilot Projects Grant (A. Yoon) provided through the Columbia University Center for Radiological Research U19AI067773 (D. Brenner) from the NIH/NIAID, a K12 Career Development Award (A. Yoon) provided through Clinical and Translational Science Award KL2 RR024157-01 (H. Ginsberg) from the NIH/NCRR, and P30 ES009089 (R. Santella) from the NIH/NIEHS. We thank Qiao Wang for technical assistance.
References
- 1.Marchetti R, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82:605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- 2.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smilenov LB, Brenner DJ, Hall EJ. Modest increased sensitivity to radiation oncogenesis in ATM heterozygous versus wild-type mammalian cells. Cancer Res. 2001;61:5710–5713. [PubMed] [Google Scholar]
- 4.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 5.Yarborough A, Zhang YJ, Hsu TM, Santella RM. Immunoperoxidase detection of 8-hydroxydeoxyguanosine in aflatoxin B1-treated rat liver and human oral mucosal cells. Cancer Res. 1996;56:683–688. [PubMed] [Google Scholar]
- 6.Yoon AJ, Shen J, Santella RM, Zegarelli DJ, Chen R, Weinstein IB. Activated checkpoint kinase 2 expression and risk for oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:2768–2772. doi: 10.1158/1055-9965.EPI-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartkova J, Bakkenist CJ, Rajpert-DeMeyts E, Skakkebaek NE, Sehested M, Lukas J, Kastan MB, Bartek J. ATM activation in normal human tissues and testicular cancer. Cell Cycle. 2005;4:838–845. doi: 10.4161/cc.4.6.1742. [DOI] [PubMed] [Google Scholar]
- 8.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 9.Neville BW, Damm DD, Allen CM, Bouquot JE. Epithelial pathology. In: Schrefer J, Rudolph P, Alvis K, McKinley L, Forest E, Ramirez J, editors. Oral and Maxillofacial Pathology. 3. Saunders; Philadelphia: 2004. pp. 362–452. [Google Scholar]
- 10.Lukas C, Barkova J, Latella L, Falck J, Mailand N, Schroeder T, Sehested M, Lukas J, Bartek J. DNA damage-activated kinase chk2 is independent of proliferation or differentiation yet correlates with tissue biology. Cancer Res. 2001;61:4990–4993. [PubMed] [Google Scholar]