Abstract
Background/Aim
Zeng Sheng Ping (ZSP) is a traditional herbal remedy used to prevent progression and growth of neoplastic lesions. It has been shown to inhibit Notch2 expression in a murine lung cancer model, leading us to investigate its therapeutic potential in Notch-dependent brain tumors.
Materials and Methods
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), apoptosis, and quantitative real-time polymerase chain reaction (RT-PCR) analyses were performed in glioma and medulloblastoma cell lines, and morphological analyses in DAOY flank xenografts.
Results
ZSP inhibited brain tumor growth in vitro, in part by apoptotic induction. Down-regulation of the Notch2 receptor, the pathway target Hairy/Enhancer of Split homolog 1 (Hes1), and the stem cell markers Nestin and CD133 was also observed. Reductions in tumor mass and increases in the necrotic fraction of DAOY xenografts in mice treated with oral ZSP were also observed, but these were not significant.
Conclusion
ZSP can block brain tumor growth and the expression of Notch pathway members and stem cell markers in vitro.
Keywords: ACAPHA, antitumor-B, Zeng Sheng Ping, Notch, medulloblastoma, glioblastoma
Medulloblastoma and glioblastoma are the most common types of malignant cancer arising in the central nervous systems (CNS) of children and adults, respectively (1). Mortality is high, and more effective therapeutic agents are urgently needed. Complementary and alternative treatments are increasingly being used by oncology patients, but the mechanisms of action and efficacy of such therapies are often poorly understood. In this study, we focus on the traditional herbal remedy Zeng Sheng Ping (ZSP, also known as ACAPHA and antitumor B), for which preliminary preclinical (2, 3) and clinical (4) data have been published for solid tumors arising outside the CNS.
ZSP is composed of six herbs (Sophora tonkinensis, Polygonum bistorta, Prunella vulgaris, Sonchus brachyotus, Dictamnus dasycarpus and Dioscorea bulbifera), and was shown in different Chinese clinical studies to reduce the progression of histopathologically confirmed pre-cancerous esophageal squamous dysplasia to carcinoma by 48–52% compared to placebo (5–7). In a phase III study involving 449 patients with esophageal epithelial hyperplasia, ZSP reduced the progression of disease from 24.8% to 3.3% (4). ZSP has also been shown to reduce the incidence of bladder cancer in rat models by 91% over 13 months (2), and the incidence of oral squamous cell carcinoma in two animal models, as well as reduce oral lesions in human patients with oral leukoplakia (3). Finally, a 2004 study performed in the United States found that ZSP blocked progression of lung tumors in a mouse model harboring a dominant-negative p53 mutation and/or loss of one p16 (Ink4a) allele (8). Oligonucleotide-array analysis of ZSP-treated and untreated lung lesions identified expression alterations in members of the Notch pathway, including down-regulation of the Notch2 receptor in treated carcinomas.
Brain tumors have also been shown to have active Notch signaling, with important roles played by Notch2 and other pathway members in medulloblastoma and glioblastoma, and suppression of pathway activity inhibiting tumor proliferation and survival (9–15). Current therapeutic approaches targeting Notch have focused on inhibiting gamma-secretase activity, but these can be associated with significant toxicity in the gut and other organs (16). We therefore sought to determine if ZSP might inhibit the growth of malignant brain tumors.
Materials and Methods
Zeng Sheng Ping
Powdered ZSP/ACAPHA was provided by Global Cancer Strategies Ltd (British Columbia, Canada). The herbal mixture was analyzed using high performance liquid chromatography (HPLC) in the Medicinal Chemistry Core at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center to determine the concentration of matrine, a major alkaloid previously identified in ZSP (17). Pure matrine for use as an analytical standard was obtained from Sigma-Aldrich (St. Louis, MO, USA), and the amount of matrine in the ZSP used was found to be 0.08%. For cell culture studies, ZSP was extracted in 1 ml sterile phosphate buffered saline (PBS) per 300 mg of powder at room temperature for 1 h. The mixture was then centrifuged at 13,000 rpm (15,700 g) for 10 min at 4°C, and the resulting supernatant sterile-filtered through a 0.22 μm filter. A series of dilutions in cell culture media (1:2000, 1:1000, 1:500, 1:200 and 1:100) was used for subsequent in vitro experiments.
Cell lines and in vitro assays
The medulloblastoma cell line DAOY, adherent glioblastoma cell line U87, and glioblastoma-derived neurosphere lines HSR-GBM1 JHH-GBM10 and JHH-GBM14 were obtained and cultured as previously described (18). For growth assays, approximately 2×103 viable cells were seeded in each well of a 96-well plate, then assayed in different concentrations of ZSP using CellTiter (MTS) assays according to manufacturer’s instructions (Promega, Madison, WI, USA). Growth assays for each line were performed two to three times, and the growth curves and statistical analyses shown include combined data from all experimental replicates. Apoptosis assays were performed using the Guava Annexin V assay kit and GUAVA-PCA flow cytometry system (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. Each experiment represents a minimum of 2×103 gated events from each of three wells. RNA was extracted with Qiagen’s RNeasy kit (Valencia, CA, USA) and gene expression was assayed by RT-PCR analysis performed in triplicate with SYBR Green reagent (Applied Biosystems, Foster City, CA, USA) on an I-Cycler IQ real-time detection system (Bio-Rad, Hercules, CA, USA) as previously described (18, 19).
Xenograft assays
Flank xenografts were established using 2×105 viable DAOY cells mixed 1:1 with Matrigel (BD Biosciences) in a total volume of 200 μl injected subcutaneously into each flank of 4-week-old female nude mice (nu/nu). Animal studies were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. ZSP powder was suspended at 50 mg/ml in fresh chocolate milk to facilitate voluntary ingestion, and sonicated in a bath sonicator for 5 min each day prior to use. Mice were assigned randomly in groups of four to receive oral doses of 0, 50 or 100 mg/kg/day. In the early treatment group, mice received their first dose of ZSP 2 hours before tumor cells were injected, while mice in the late treatment group received ZSP beginning 10 days after tumor injection. Estimates of volume were made using the following formula: width2 × length × 0.52 (20). To calculate areas of necrosis, whole tumor images were joined using the ‘Photomerge’ algorithm in Adobe Photoshop CS3 (San Jose, CA, USA) and total and necrotic tumor area were measured using the histogram function. Mitotic counts in ten random ×400 high powered fields were performed in each tumor by a pathologist (K.D.R.) masked with respect to the treatment group.
Statistics
All in vitro experiments were performed in triplicate, and repeated at least twice unless otherwise noted. GraphPad Prism 5 software (San Diego, CA, USA) was used for all statistical analyses. Values are graphed as the mean ± standard error (SEM) unless otherwise noted, and statistical differences were determined using Student’s two-tailed t-test. A p-value of ≤0.05 was considered significant. The in vivo xenograft experiment was performed once and statistical differences determined with Student’s two-tailed t-tests and two-way ANOVA. p-values indicated in the results section were obtained from t-tests. One outlier that had a difference in tumor volume of more than two standard deviations from the mean in the early treatment group was omitted.
Results
ZSP slows growth of malignant brain tumor cells in culture
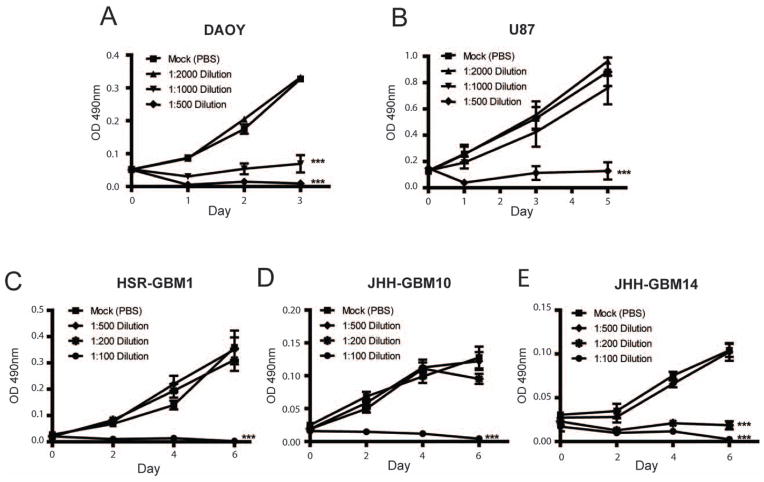
Higher concentrations of ZSP inhibited growth of the medulloblastoma cell line DAOY and the glioblastoma cell line U87 (Figure 1A and B). MTS assays revealed a statistically significant growth arrest over three and five days, respectively, in DAOY and U87 adherent cultures treated with aqueous ZSP extract diluted 1:500, and a less pronounced reduction in DAOY growth using a 1:1000 dilution. ZSP also slowed the growth of NIH-3T3 cells in vitro (data not shown), suggesting these effects were not specific to cancer cells in culture, although previous clinical data have shown that it is safe for consumption by humans (6, 7, 21).
Figure 1.
Zeng Sheng Ping (ZSP) inhibits brain tumor cell growth. ZSP treatment reduced tumor cell mass as measured by MTS assay in the medulloblastoma cell line DAOY (Figure 1A), as well as the glioblastoma cell lines U87 (B), HSR-GBM1 (C), JHH-GBM10 (D) and JHH-GBM14 (E). ***p≤ 0.0001 relative to mock-treated control.
It has been suggested that cancer stem cells play an important role in maintaining long- term tumor growth, and glioblastoma cultures growth as neurospheres in serum-free media are often used to assess stem cell phenotype (14, 19, 22–25). We therefore also used ZSP extract to treat several established glioblastoma neurosphere cultures (18, 26), and found that higher concentrations of ZSP (1:200 or 1:100 dilution) were required to significantly inhibit the growth of all three lines tested as compared to adherent brain tumor cells (Figure 1C–E). This may be due to the known resistance of cancer stem cells to chemotherapeutic agents (27, 28).
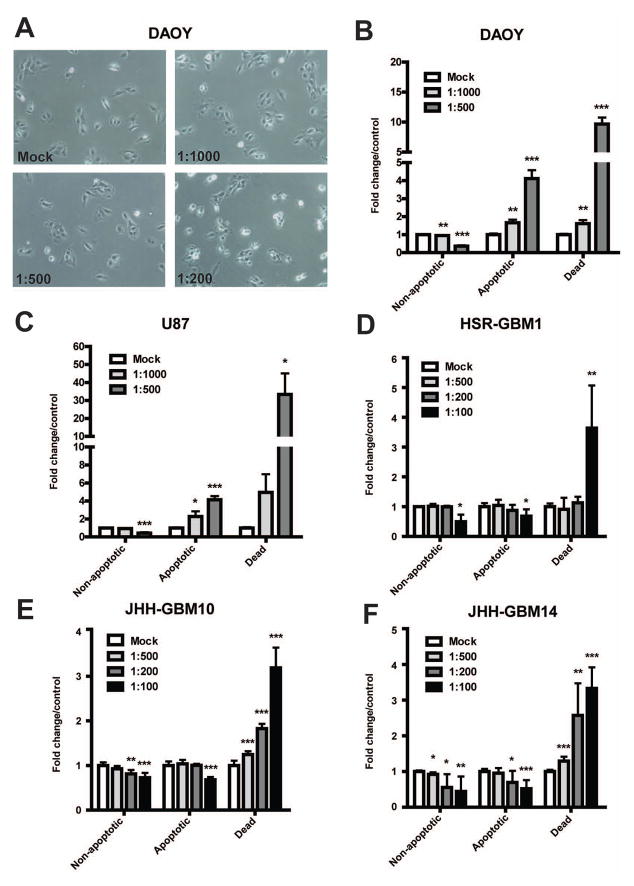
Microscopic examination of treated cultures revealed increased numbers of dead cells beginning soon after ZSP extract was added. Figure 2A shows that 4 hours following ZSP treatment, cell shrinkage began to occur at a 1:500 dilution which was even more pronounced at the more concentrated 1:200 dilution. To quantify the induction of cell death by ZSP, treated cells were collected after 24 hours and flow cytometric analysis was performed. There was a significant, dose-dependent increase in apoptosis of the medulloblastoma cell line DAOY beginning at a 1:1000 dilution (Figure 2B), and of U87 glioblastoma cells at a 1:1000 dilution (Figure 2C). We observed an even greater induction of cell death as measured with the viable dye 7-Aminoactinomycin D (7-AAD), a component of the GUAVA annexin V assay kit, which stains cells that could have died from either apoptosis or necrosis. This suggests that induction of both apoptotic and non-apoptotic cell death plays a role in the loss of viable cells in these cultures. In the neurosphere lines HSR- GBM1 (Figure 2D), JHH-GBM10 (Figure 2E) and JHH-GBM14 (Figure 2F) ZSP did not induce apoptosis, but the percentage of dead cells increased in a dose-dependent manner following treatment with ZSP at concentrations which had reduced overall growth.
Figure 2.
ZSP induces apoptotic cell death in a subset of brain tumor cell lines. After adherent medulloblastoma and glioblastoma cell lines DAOY (A and B) and U87 (C) were subjected to ZSP treatment, a dose-dependent increase in the percentage of apoptosis was observed. In the glioblastoma neurosphere lines HSR-GBM1 (D), JHH-GBM10 (E), and JHH-GBM14 (F), the percentage of dead cells increased in a dose-dependent fashion. *p≤0.05, **p≤0.001 and ***p≤0.0001 relative to mock-treated control.
ZSP inhibits Notch signaling and reduces expression of stem cell markers
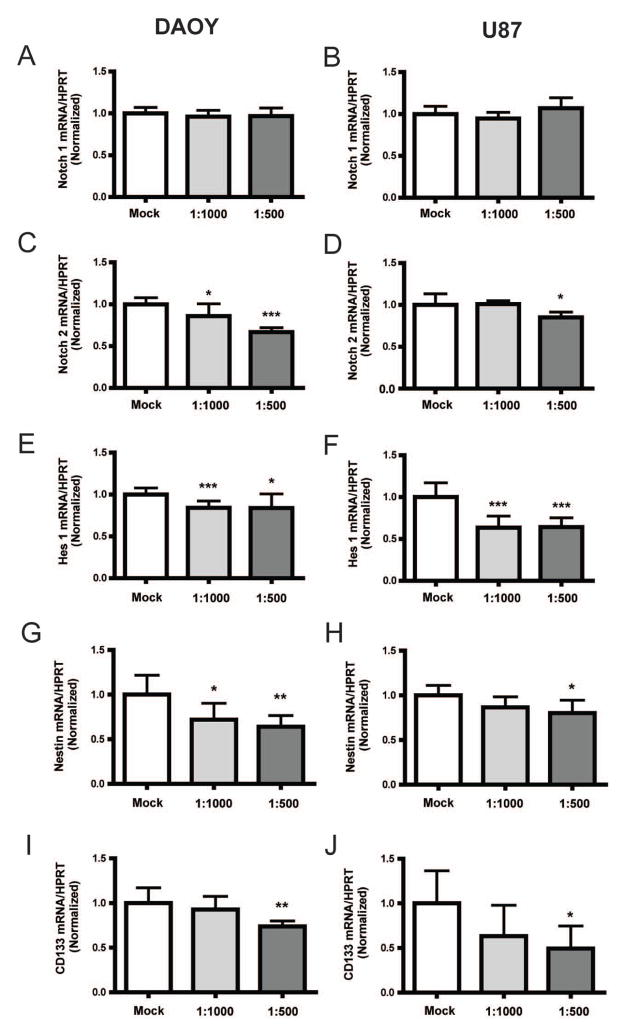
Zhang and colleagues (8) previously demonstrated chemopreventive ability of ZSP against lung tumor in an A/J mouse model harboring a dominant-negative p53 and/or Ink4a/Arf deletion. In their microarray analysis of the murine tumors, Notch2 was one of the genes that were significantly down-regulated by ZSP. As brain tumors have previously been shown to have dysregulated Notch2 activity (29, 30), we examined if ZSP also modulated Notch in brain tumor cells. The brain tumor cell lines DAOY and U87 were treated with different concentrations of ZSP for 4 hours [a time-point where cell shrinkage starts to occur (Figure 2A)], and transcript levels of Notch receptors (Notch1 and 2) as well as the pathway’s downstream effector Hes1 were measured using quantitative PCR. While Notch1 mRNA was not affected (Figure 3A and B), Notch2 was down-regulated in a dose-dependent manner in DAOY medulloblastoma cells and at the highest ZSP concentration in U87 glioma cells (Figure 3C and D). The Notch pathway target Hes1 was also reduced in these cell lines at lower concentrations (Figure 3E and F). DAOY cells treated with ZSP at the 1:500 dilution for 2 days also showed a significant reduction in transcripts of the Notch target, Hes5 (data not shown).
Figure 3.
ZSP inhibits Notch signaling and cancer stem-like marker expression in DAOY and U87 cells. Transcript levels of the Notch1 receptor remain unchanged (A and B) while those of Notch2 receptor were down-regulated following ZSP treatment in DAOY (C) and U87 (D) cells. Hes1, a Notch downstream target, also showed dose-dependent reductions (E and F). In addition, transcript levels of stem-like cancer markers, Nestin and CD133, also demonstrated statistical significant reductions at higher concentrations (G–J). HPRT: hypo-xanthine phosphoribosyltransferase. *p≤0.05, **p≤0.01 and ***p≤0.0001 relative to the mock-treated control.
We have previously shown that notch blockade can affect the expression of genes associated with stem cell phenotype in malignant brain cancer (14, 15). Therefore, we measured levels of the stem cell markers nestin and CD133 following ZSP treatment. As shown in Figure 3G, there was a reduction of nestin mRNA transcripts in DAOY following ZSP treatment at the 1:1000 and 1:500 dilutions. For U87 cells, there was a statistical significant reduction at the 1:500 dilution (Figure 3H). Similarly, CD133 transcripts were reduced at the 1:500 dilution for both DAOY and U87 cell lines (Figure 3I and J).
ZSP treatment of medulloblastoma xenografts
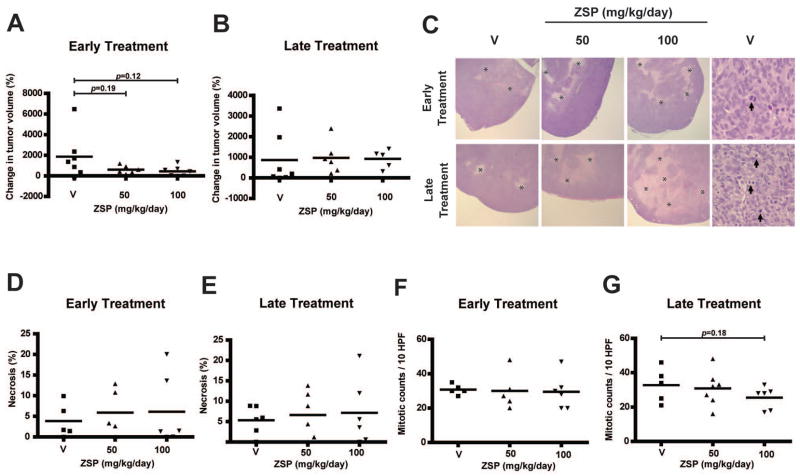
In order to investigate if ZSP prevents medulloblastoma growth in vivo, we used a DAOY flank xenograft model in athymic (nude) mice. Two therapeutic paradigms were tested: early treatment beginning the day of tumor cell injection, and late treatment beginning after flank tumors had formed. The former was designed to model treatment of minimal residual disease remaining after conventional surgery and radiation therapy, while the latter reflects the potential efficacy of ZSP against larger tumor masses. In the early treatment trial, the growth in mean tumor volume over 9 weeks was progressively reduced by 45% and 60% using 50 and 100 mg/kg/day doses of oral ZSP (Figure 4A), but these reductions were not statistically significant (p=0.19 and p=0.12, respectively). No reduction in growth was noted when treatment was initiated in larger tumors over 8 weeks (Figure 4B). All tumors were formalin fixed, paraffin embedded and examined microscopically by two pathologists after animals were sacrificed. The xenografts were primitive, embryonal lesions with extensive mitotic activity and variably sized regions of necrosis (Figure 4C). Interestingly, in both the early and late treatment groups, a 34% to 87% increase in the amount of tumor necrosis was noted. A decrease in the mitotic count per high-powered field of up to 22% was also noted in animals receiving ZSP (Figure 4D–G). However, neither of these changes was statistically significant.
Figure 4.
ZSP and medulloblastoma xenograft growth. In vivo oral ZSP treatment in nude mice with flank DAOY xenografts led to a reduction in tumor growth in the early treatment model (A) but the results were not statistically significant. No growth reduction was seen in the late treatment model (B). Microscopic examination revealed necrotic areas (*) in the xenografts (C, left six panels, original magnification ×40), which increased in size with treatment, but again these changes were not significant (D and E). Mitotic figures were also common (C, right two panels, arrows. original magnification ×400), but blinded counts showed only a non-significant reduction in this marker of proliferation (F and G). HPF: High power field; V: vehicle control
Discussion
ZSP is a traditional Chinese herbal medicine. Preliminary preclinical (2, 3) and clinical (4) studies from China suggest that ZSP may be effective in preventing progression of precancerous lesions in the upper aerodigestive tract. Through these clinical studies, ZSP has accumulated a long history of safety in several thousand users over more than 25 years. A phase II clinical trial by British Columbia Cancer Agency and National Cancer Institute is currently investigating the ability of ZSP in preventing lung cancer in former smokers with bronchial intraepithelial neoplasia (ClinicalTrials.gov identifier: NCT00522197). This was based in part on preclinical work in a murine lung cancer model in which both tumor growth and Notch2 expression were inhibited by ZSP (8). However, thus far, there has been no report on the ability of ZSP to prevent progression of malignant brain cancer, which is also known to grow in response to Notch2 activation (10, 14). In this study, we sought to investigate if ZSP is effective in glioblastoma and medulloblastoma cell line models in vitro and in vivo.
We found that ZSP reduced growth of glioblastoma and medulloblastoma cells via induction of cell death, partly through apoptotic mechanisms. ZSP was also found to reduce the expression of Notch2 transcripts as well as the Notch target Hes1 in DAOY (Figure 3C and E) and U87 (Figure 3D and F) cells, indicating that ZSP can modulate Notch activity in multiple tumor types. Because Notch has been shown to be an important player in tumorigenesis through induction of cancer cell proliferation and/or survival [reviewed in (31)], it is also possible that some of the effects of ZSP on tumor pathobiology are mediated by Notch blockade. There are several reports supporting a role of Notch in stem-like cancer cells in glioblastoma (14, 32), as well as breast cancer (33, 34). Blocking the Notch pathway in embryonal brain tumors was also found to deplete stem-like cells and subsequent engraftment in mice (15). Targeting the cancer stem-like population via Notch blockade could thus have significant therapeutic implications (35). Other tumors dependent on Notch activity might therefore also be tested for sensitivity to ZSP.
While ZSP showed promising in vitro effects on tumor survival and growth, and the size of xenografts was reduced to some extent with early treatment, the in vivo reductions were not statistically significant. Increases in the percentage of necrotic xenograft tissue were also not statistically significant. One potential reason for this is the lower dose of ZSP given to the animals in our study as compared to the prior report on murine lung cancer (8). We chose these lower doses as they more closely paralleled those used in human trials. More studies are necessary before we can prove or refute the effectiveness of ZSP in chemoprevention or treatment of malignant brain tumors.
Acknowledgments
We thank Angelo Vescovi and Francesco DiMeco for their kind gift of HSR-GBM1 cells, and Global Cancer Strategies Ltd for providing ZSP. We also thank Dr. Eli Bar for technical assistance, and Dr. Xing Fan for performing preliminary studies. This work was supported by National Institute of Health R21 ATOO3893 (C.G.E.)
Footnotes
Conflict of Interests
The Authors declare that they have no conflict of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. 4. Lyon: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan X. Inhibitory effect of antitumor-B and retinamide on precancerous lesions of the bladder in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1993;15(1):71–73. (in Chinese) [PubMed] [Google Scholar]
- 3.Sun Z, Guan X, Li N, Liu X, Chen X. Chemoprevention of oral cancer in animal models, and effect on leukoplakias in human patients with Zeng Sheng Ping, a mixture of medicinal herbs. Oral Oncol. 2010;46(2):105–110. doi: 10.1016/j.oraloncology.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Wang J. Results of phase III clinical trial of zeng sheng-ping in the treatment of patients with esophageal epithelial hyperplasia. Zhonghua Zhong Liu Za Zhi. 2000;22(6):510–512. (in Chinese) [PubMed] [Google Scholar]
- 5.Lin P, Zhang J, Rong Z, Han R, Xu S, Gao R, Ding Z, Wang J, Feng H, Cao S. Studies on medicamentous inhibitory therapy for esophageal precancerous lesions--3- and 5-year inhibitory effects of antitumor-B, retinamide and riboflavin. Proc Chin Acad Med Sci Peking Union Med Coll. 1990;5(3):121–129. [PubMed] [Google Scholar]
- 6.Ding Z, Gao F, Lin P. Long-term effect of treating patients with precancerous lesions of the esophagus. Zhonghua Zhong Liu Za Zhi. 1999;21(4):275–277. (in Chinese) [PubMed] [Google Scholar]
- 7.Hou J, Lin PZ, Chen ZF, Ding ZW, Li SS, Men FS, Guo LP, He YT, Qiao CY, Guo CL, Duan JP, Wen DG. Field population-based blocking treatment of esophageal epithelia dysplasia. World J Gastroenterol. 2002;8(3):418–422. doi: 10.3748/wjg.v8.i3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Wang Y, Yao R, Li J, Yan Y, La Regina M, Lemon WL, Grubbs CJ, Lubet RA, You M. Cancer chemopreventive activity of a mixture of Chinese herbs (antitumor B) in mouse lung tumor models. Oncogene. 2004;23(21):3841–3850. doi: 10.1038/sj.onc.1207496. [DOI] [PubMed] [Google Scholar]
- 9.Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4(11):e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Kesari S, Rooney C, Strack PR, Shen H, Wu L, Griffin JD. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1(8):822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106(3):417–427. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- 12.Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, Maric D, Eberhart CG, Fine HA. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65(6):2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XP, Zheng G, Zou L, Liu HL, Hou LH, Zhou P, Yin DD, Zheng QJ, Liang L, Zhang SZ, Feng L, Yao LB, Yang AG, Han H, Chen JY. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008;307(1–2):101–108. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- 14.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28(1):5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 16.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, Zhang L, Higgins GA, Parker EM. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279(13):12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 17.Gao G, Law FC. Physiologically based pharmacokinetics of matrine in the rat after oral administration of pure chemical and ACAPHA. Drug Metab Dispos. 2009;37(4):884–891. doi: 10.1124/dmd.108.023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16(24):6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177(3):1491–1502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390(6658):404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 21.Lin P, Chen Z, Hou J, Liu T, Wang J. Chemoprevention of esophageal cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1998;20(6):413–418. (in Chinese) [PubMed] [Google Scholar]
- 22.Laywell ED, Kukekov VG, Steindler DA. Multipotent neurospheres can be derived from forebrain subependymal zone and spinal cord of adult mice after protracted postmortem intervals. Exp Neurol. 1999;156(2):430–433. doi: 10.1006/exnr.1999.7029. [DOI] [PubMed] [Google Scholar]
- 23.Kornblum HI, Yanni DS, Easterday MC, Seroogy KB. Expression of the EGF receptor family members ErbB2, ErbB3, and ErbB4 in germinal zones of the developing brain and in neurosphere cultures containing CNS stem cells. Dev Neurosci. 2000;22(1–2):16–24. doi: 10.1159/000017423. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Martin M. Brain tumour stem cells: implications for cancer therapy and regenerative medicine. Curr Stem Cell Res Ther. 2008;3(3):197–207. doi: 10.2174/157488808785740370. [DOI] [PubMed] [Google Scholar]
- 26.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 27.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 28.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 29.Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64(21):7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 30.Sivasankaran B, Degen M, Ghaffari A, Hegi ME, Hamou MF, Ionescu MC, Zweifel C, Tolnay M, Wasner M, Mergenthaler S, Miserez AR, Kiss R, Lino MM, Merlo A, Chiquet-Ehrismann R, Boulay JL. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer Res. 2009;69(2):458–465. doi: 10.1158/0008-5472.CAN-08-2610. [DOI] [PubMed] [Google Scholar]
- 31.Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22(42):6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, Rich JN, Sullenger BA. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28(1):17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farnie G, Clarke RB. Mammary stem cells and breast cancer-role of Notch signalling. Stem Cell Rev. 2007;3(2):169–175. doi: 10.1007/s12015-007-0023-5. [DOI] [PubMed] [Google Scholar]
- 34.Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C, Chieco P, Bonafe M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25(3):807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- 35.Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, Miele L. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16(12):3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]