Synopsis
Necrotizing enterocolitis (NEC) is a leading cause of neonatal morbidity and mortality and preventative therapies that are both effective and safe are urgently needed. Current evidence from therapeutic trials suggests that probiotics are effective in decreasing NEC in preterm infants and probiotics are currently the most promising therapy on the horizon for this devastating disease. However, concerns regarding safety and optimal dosing have limited the widespread adoption of routine clinical use of probiotics in preterm infants. In addition, prebiotics and postbiotics may be potential alternatives or adjunctive therapies to the administration of live microorganisms, although studies demonstrating their clinical efficacy in preventing NEC are lacking. This review summarizes the current evidence regarding the use of probiotics, prebiotics and postbiotics in the preterm infant, including its therapeutic role in preventing NEC.
Keywords: Necrotizing Enterocolitis, Probiotic Bacteria, Premature Infants, Very Low Birth Weight
Introduction: Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is a leading cause of neonatal morbidity and mortality and the most common gastrointestinal emergency in neonates [1, 2]. NEC develops in approximately 1 out of 10 infants born at less than 29 weeks gestation [3]. Approximately 20% to 30% of VLBW (birth weight ≤ 1500 grams) infants who develop NEC die [4] and those infants who survive the disease are at risk for long-term complications, including neurodevelopmental impairment, short bowel syndrome, and impaired growth [5]. This results in considerable health costs, with the financial impact of affected infants in the United States estimated to be between $500 million to $1 billion per year [6]. Finally, NEC is particularly poignant for families because it affects preterm infants who have survived the initial postnatal period only to develop a new and life-threatening illness.
NEC is characterized by intestinal injury, inflammation and necrosis. Despite investigative efforts, the underlying pathogenesis remains unclear. Leading hypotheses implicate a multifactorial pathophysiology, which includes host factors, inflammatory propensity of the immature gut, enteral feeding, and abnormal bacterial colonization [6–8] (Box 1). NEC can rapidly progress from early clinical signs to extensive intestinal necrosis within hours, limiting the effectiveness of therapeutic intervention. As such, approaches to prevent NEC have become a focus of research efforts and preventative therapies for NEC are urgently needed [9].
Box 1. Risk Factors Influencing NEC Predisposition.
-
Prematurity
-
Enteral feeding
-
Abnormal bacterial colonization
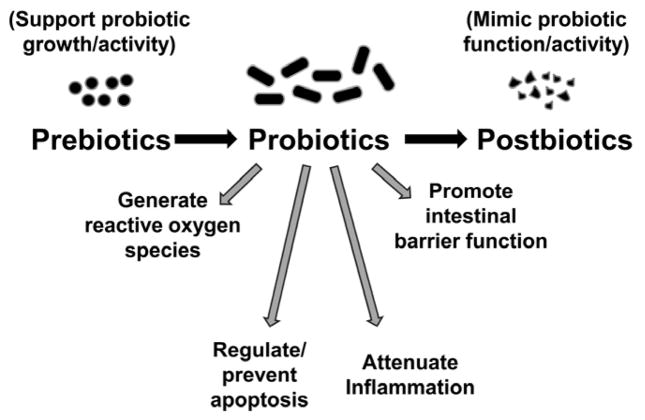
Probiotics are the most promising treatment on the horizon for this devastating disease. However, as we review in this article, further study is needed before probiotics can be routinely recommended as a preventative therapy. In addition, prebiotics and postbiotics remain potential alternatives or adjunctive therapies to the use of live microorganisms. Herein, we review the role of microorganisms in intestinal heath and disease in neonates and the current evidence supporting prebiotic, probiotic and postbiotic use in preterm infants. In addition, we discuss the potential mechanisms of action of probiotic organisms and weigh the risks and benefits of therapy. Important terminology is defined in Box 2.
Box 2. Key Definitions.
Probiotics are supplements or foods that contain viable microorganisms that alter the microflora of the host.
Prebiotics are supplements or foods that contain a nondigestible ingredient that selectively stimulates the growth and/or activity of indigenous bacteria.
Postbiotics are non-viable bacterial products or metabolic byproducts from probiotic microorganisms that have biologic activity in the host.
Synbiotic is a product that contains both probiotics and prebiotics.
Role of intestinal microorganisms in intestinal development and health
The neonatal intestine is the largest interface of the host to the external environment. Ideally, the intestine protects the vulnerable neonate from pathogens and toxins while allowing harmonious habitation of commensal bacteria. In addition, the intestine must protect itself from bacterial pathogens that cause inflammation while encouraging the growth and habitation of commensal bacteria, which can attenuate inflammation and maintain intestinal homeostasis [10]. Importantly, uncontrolled inflammation can lead to tissue injury and necrosis and is thought to play an important role in the pathogenesis of NEC [6].
The immature neonatal gut in the preterm infant has even greater challenges as it transitions from a sterile lumen absent of microbes to the fully realized “bioreactor” of the mature adult gut. Very soon after birth, a newborn infant’s intestine begins to acquire a diverse set of commensal bacteria. These bacteria eventually grow to outnumber their host by a factor of ten to one [11–13]. The infant/host provides an hospitable, temperature-stable, nutrient-rich environment for bacteria while receiving, in return, benefits from the commensal bacteria, which include digestion, absorption, and storage of nutrients [14, 15], intestinal homeostasis and protection against injury [13, 16], and the development and control of epithelial immune responses and function [17, 18]. However, this postnatal transition from a sterile to commensal-rich intestine may become disrupted or delayed in infants born prematurely.
Abnormal bacterial colonization in preterm infants
Premature infants, when compared to their term counterparts, are more likely to be colonized with fewer, more virulent organisms [19]. In addition, they have delayed acquisition of commensal bacteria, particularly Bifidobacteria [20]. Why does this occur? Several explanations are likely (Figure 1). Preterm infants and their mothers are commonly exposed to antibiotic therapy, both of which have been associated with an increased risk of NEC [21–24]. In addition, since preterm infants are often delivered by cesarean and experience delayed enteral feeding, they are less likely to acquire commensal flora perinatally from passage through the birth canal or from human milk feeding. This may lead to decreased colonization of beneficial probiotic bacteria, including Bifidobacteria, Lactobacillus and Bacteroides [25, 26]. The hospital environment, with its preponderance of pathogenic organisms [27], may also negatively affect the intestinal colonization of beneficial commensal bacteria. Ongoing studies investigating the intestinal microbiome in premature neonates will provide greater insight into the role of microbial diversity on the development of NEC [28–30]. Since abnormal bacterial colonization likely plays a role in the pathogenesis of NEC, probiotic bacteria may exert their beneficial effects by restoring or supplying the essential commensal strains necessary for protection against intestinal inflammation and injury. Prebiotics and postbiotics may also accomplish this goal, through the promotion of commensal growth or by mirroring commensal activity, respectively.
Figure 1.
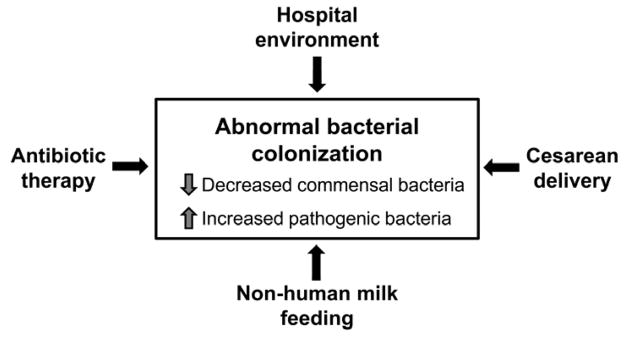
Factors influencing abnormal intestinal bacterial colonization in preterm infants.
Mechanisms of Action
Probiotics
What is the mechanism of action for probiotics? In preterm infants, probiotic supplementation can allow acquisition of normal commensal flora in a host where this process has been delayed or support the transition to an intestinal microbiome with beneficial microbes, particularly in hosts where this process has been disrupted. Several mechanisms of probiotic action may explain how their therapeutic use can help prevent NEC. These mechanisms include enhancement of epithelial barrier function, competitive exclusion of pathogens, and direct anti-inflammatory effects on epithelial signaling pathways [31–33]. At the cellular level, probiotics have a number of important effects (Figure 2): 1) attenuation of NF-κB activation, a major pro-inflammatory pathway [34]; 2) upregulation of cytoprotective genes [13, 35]; 3) prevention of apoptosis and cell death [35, 36]; 4) generation of reactive oxygen species important in cell signaling [37, 38]; 5) Induction of the expression of tight junction proteins necessary for barrier function [39, 40]. Whether live microorganisms, instead of killed or inactivated bacteria or bacterial products, are required for these beneficial effects remains an important area of study and recent data suggest that bacterial products, in the absence of viable organisms, may have similar effects on signaling pathways [41] and barrier function [39]. These bacterial products, which we broadly characterize as postbiotics, are discussed in a separate section below.
Figure 2.
Mechanisms of action of probiotics at the cellular level in intestinal epithelia.
Since the preterm gut demonstrates delayed commensal colonization and low bacterial diversity, it may be particularly amenable to therapeutic manipulation by probiotic administration. In keeping with this idea, a number of clinical studies have demonstrated the benefit of probiotic administration in reducing the incidence and severity of NEC (Table 1). Most of these trials have used strains of probiotics from the Genus Lactobacillus or Bifidobacteria, although the treatment regimen, including dose and duration of therapy vary widely (Table 2). In addition, concerns regarding the risks of therapy, optimal species or combination of species, duration of therapy and dosing remain unanswered. The therapeutic use of probiotics is discussed below.
Table 1.
Summary of Probiotic Therapy Trials in Preterm Infants
| Study Authors | Year Published | Number of Infants | BW and/or GA | Primary Outcome of NEC |
|---|---|---|---|---|
| Kitajima et al.[86] | 1997 | 91 | <1500g | No |
| Dani et al.[87] | 2002 | 585 | <1500g or <33wk | Yes |
| Costalos et al.[88] | 2003 | 87 | 28–32wk | No |
| Bin-Nun et al.[89] | 2005 | 145 | <1500g | Yes |
| Lin et al.[90] | 2005 | 367 | <1500g | Yes |
| Manzoni et al.[91] | 2006 | 80 | <1500g | No |
| Mohan et al.[92] | 2006 | 38 of 69* | <37wk | No |
| Stratiki et al.[93] | 2007 | 41 of 75* | 27–36wk | No |
| Lin et al.[69] | 2008 | 434 | <1500g & <34wk | Yes |
| Samanta et al.[68] | 2009 | 186 | <1500g & <32wk | Yes |
| Rouge et al.[94] | 2009 | 94 | <1500g & <32wk | No |
| Manzoni et al.[67] | 2009 | 319 of 472 | <1500g | No |
| Underwood et al.[48] | 2009 | 90 | 750–2000g & <35wk | No |
| Mihatsch et al.[95] | 2010 | 183 | <1500g and <30wk | No |
| Braga et al.[96] | 2011 | 231 | 750–1499g | Yes |
| Sari et al.[97] | 2011 | 221 | <1500g or <33wk | Yes |
| Fernandez-Carrocera et al.[98] | 2012 | 150 | <1500g | Yes |
Abbreviations: NEC, necrotizing enterocolitis; BW, birth weight; GA, gestational age; Data from individual studies referenced and from Deshpande et al.[66].
Only infants <34wk and <1500g included.
Table 2.
Summary of Probiotic Strains Used in Therapeutic Trials in Preterm Infants
| Probiotic Genus | Species | Dose (CFU/day) | Reference |
|---|---|---|---|
| Bifidobacterium | bifidus | 1 × 109 a | [48], [89] |
| 2 × 109 a | [69] | ||
| 5 × 109 a | [68] | ||
|
| |||
| breve | 0.5 × 109 | [86] | |
| 3.5 × 107–3.5 × 109 a | [96] | ||
|
| |||
| infantis | 2.76 × 107 a | [98] | |
| 1 × 109 a | [48], [89] | ||
| 5 × 109 a | [68] | ||
| NR | [90] | ||
|
| |||
| lactis | 2 × 107 b | [93] | |
| 1.6–4.8 × 109 | [92] | ||
| 1.2 × 1010 | [95] | ||
|
| |||
| longus | 4 × 108 a | [94] | |
| 1 × 109 a | [48] | ||
| 5 × 109 a | [68] | ||
|
| |||
| Lactobacillus | acidophilus | 1 × 109 a | [48], [98] |
| 2 × 109 a | [69] | ||
| 5 × 109 a | [68] | ||
| NR | [90] | ||
|
| |||
| casei | 1 × 109 a | [98] | |
| 3.5 × 107–3.5 × 109 a | [96] | ||
|
| |||
| rhamnosus GG | 4 × 108 | [94] | |
| 4.4 × 108 a | [98] | ||
| 1 × 109 | [48] | ||
| 6 × 109 | [67], [87], [91] | ||
|
| |||
| plantarum | 1.76 × 108 a | [98] | |
|
| |||
| sporogenes | 3.5 × 108 | [97] | |
|
| |||
| Streptococcus | theromphilus | 6.6 × 105 a | [98] |
| 1 × 109 a | [89] | ||
|
| |||
| Saccharomyces | boulardii | 2 × 109 per kg | [88] |
Abbreviations: CFU, colony forming units; NR, not reported in CFU per day
Indicates therapeutic use in combination with other probiotic species
Dose is per gram of milk powder of preterm formula
Prebiotics
Another potential therapeutic strategy to prevent NEC that may carry less infectious risk is prebiotic therapy. Prebiotics are non-digestible dietary products, which improve intestinal health by promoting selective growth of beneficial commensal bacteria [42]. The most common prebiotics are oligosaccharides, which are found in human milk. Prebiotics have been added to infant formulas in Japan for over two decades and there is increasing use of prebiotics in the commercial formula market in the United States although concerns regarding their efficacy remain [43, 44]. Several studies have demonstrated increased Bifidobacteria and decreased pathogenic bacteria in the stool of preterm infants fed prebiotic-containing formula with short-chain galacto-oligosaccharides (GOS) and/or fructo-oligosaccharides (FOS) as compared to control infants [45–47]. Similar effects of increased Bifidobacteria colonization were seen with the use of synbiotics, which combine prebiotics with probiotics [48]. In addition to promoting the growth of beneficial commensals, prebiotic therapy may also improve intestinal motility and gastric emptying, resulting in improved feeding tolerance [49, 50]. Evidence suggests that this effect of prebiotics on gastrointestinal motility is mediated by bacterial metabolites such as short-chain fatty acids[51], and, therefore, may actually be a postbiotic effect rather than a direct effect of the prebiotic. Term infants exhibit decreased stool pH and increased stool short-chain fatty acids when fed prebiotic containing formula, reportedly comparable to that seen in breastfed infants [52]. In addition, preliminary evidence suggests a positive effect on overall immune function [53]. While no serious adverse effects of prebiotic therapy were noted in the previously mentioned studies, prebiotic therapy is associated with several gastrointestinal side effects. These include flatulence, bloating, and diarrhea, which can be reversed by stopping treatment [42].
Postbiotics
Using bacterial products or metabolites remains an exciting, yet largely unexplored, therapeutic option for preventing NEC. Postbiotics aim to mimic the beneficial therapeutic effects of probiotics while avoiding the risk of administering live microorganisms to preterm infants with immature intestinal barriers or impaired immune defenses. Many commensal bacteria produce butyrate, a short-chain fatty acid produced by the catabolism of undigested carbohydrates in the intestine. Butryate is a major energy source for the colon and has an important role in intestinal growth and differentiation [54, 55], inflammatory suppression [56–58] (including suppression through generation of reactive oxygen species)[59], and regulation of apoptosis [60, 61]. It has been used with limited success in inflammatory bowel disease [62]. Perhaps this and other small molecule products of the normal flora are at least partially responsible for the beneficial effects of the normal flora (and exogenous pro- and prebiotics), and could be employed as a more controllable and safer therapeutic surrogate. Heat-killed probiotics may also function, in the broad sense, as postbiotics. Heat-killed microorganisms retain important bacterial structures that may exert biologic activity in the host. We recently reported that heat-killed LGG has similar beneficial efficacy as live probiotics in accelerating intestinal barrier maturation in a murine model of immature intestines [39]. More importantly, while administration of higher doses of live LGG led to increased mortality in immature neonatal mice, administration of heat-killed LGG did not.
Therapeutic Options
Numerous probiotic organisms have been studied in preterm infants, at varied dosages and durations of therapy (Table 2). The diversity of use has made selecting an optimal probiotic regimen difficult. Several studies have used single agents, while a number of larger trials have used a combination of probiotics. Dosing varies among probiotic species and ranges from 105 to 1010 CFU per day. In addition, the actual probiotic composition may vary from what is advertised [48]. Most studies randomized infants and/or initiated therapy within a week after birth and duration of therapy typically lasted beyond 1 month of age with several studies continuing therapy until hospital discharge. The two most commonly used probiotic strains were from the genera Bifidobacteria and Lactobacillus. A meta-analysis by Wang et al. [63], including a number of recent trials conducted in China, compared the relative efficacy of these strains by pooling studies that used these probiotic agents and found that Bifidobacteria, Lactobacillus and a combination of the two strains had similar efficacy in the prevention of NEC (Table 3). Of note, monitoring for adverse effects varied greatly among the studies, particularly in those where NEC was not a primary outcome measure.
Table 3.
Comparison of the Efficacy of Probiotic Strains
| Outcome | Probiotic | Total Patients (n) | Pooled Relative Risk (95% CI) |
|---|---|---|---|
| NEC | Lactobacillus | 1205 | 0.37 (0.19–0.73) |
| Bifidobacteria | 976 | 0.30 (0.16–0.58) | |
| Both | 1403 | 0.33 (0.19–0.58) | |
| Death | Lactobacillus | 1205 | 0.61 (0.38–0.97) |
| Bifidobacteria | 340 | 0.74 (0.18–2.97) | |
| Both | 1312 | 0.47 (0.26–0.87) | |
| Sepsis | Lactobacillus | 1205 | 0.79 (0.46–1.36) |
| Bifidobacteria | 340 | 0.84 (0.29–2.41) | |
| Both | 1312 | 0.90 (0.60–1.36) |
Abbreviations: NEC, necrotizing enterocolitis; CI, confidence interval Adapted from Wang et al.[63]
For prebiotics, several oligosaccharides including fructose, galactose, lactulose, inulin, or a combinations of these are potential therapeutic options for clinical use [64]. GOS, FOS and a combination of the two (GOS-FOS) have been used clinically in preterm infants and studies have shown increased Bifidobacteria stool colony counts in prebiotic treated infants, although none of these studies evaluated NEC as a primary outcome measure [65].
Clinical Outcomes
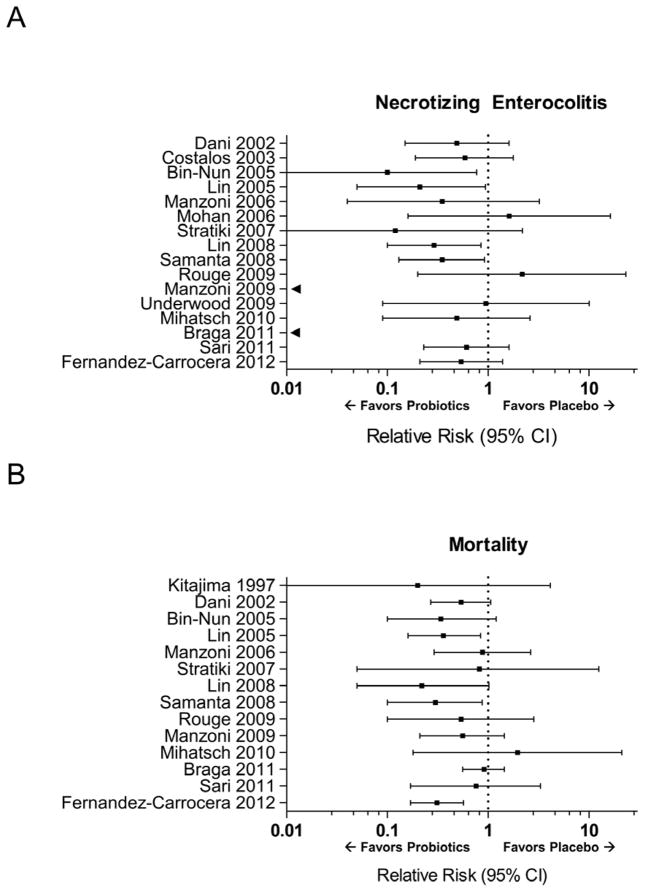
Clinical trials have consistently demonstrated trends in favor of probiotic treatment, compared to placebo, in reducing the incidence of NEC (Figure 3A). Four of these studies demonstrated a statistically significant benefit and two recent meta-analyses of therapeutic trials have both shown similar pooled relative risks in favor of probiotics for the prevention of NEC. Deshpande et al. evaluated 11 trials and found an overall pooled relative risk (RR) of 0.35 (95% CI 0.23–0.55) for NEC in infants randomized to probiotic therapy compared to controls [66]. Wang et al., as mentioned previously, included more recent trials, and found a similar pooled RR of 0.33 (95% CI 0.24–0.46) from a total of 20 studies [63]. Of note, only 8 of 17 trials we identified had NEC listed as a primary outcome measure and the dose and species of probiotics used varied widely (Tables 1 and 2). Importantly, the previously mentioned meta-analyses of also demonstrated a decreased risk of death (Figure 3B) in infants randomized to probiotic treatment compared to control. It is likely that the mortality benefit is through a reduction in NEC- and sepsis-related deaths, although the causes of death have not been routinely evaluated in clinical trials.
Figure 3.
Plots demonstrate the relative risk of necrotizing enterocolitis (NEC) (panel A) and mortality (panel B) in the probiotic treatment arm, compared to placebo, of therapeutic trials in preterm infants. Error bars reflect 95% confidence interval (CI) for relative risk estimates. Two studies had no NEC events in the probiotic treatment arm and estimates, without 95% CI, are indicated by black arrows. Data from individual studies referenced as well as from Deshpande et al.[66] and Wang et al.[63].
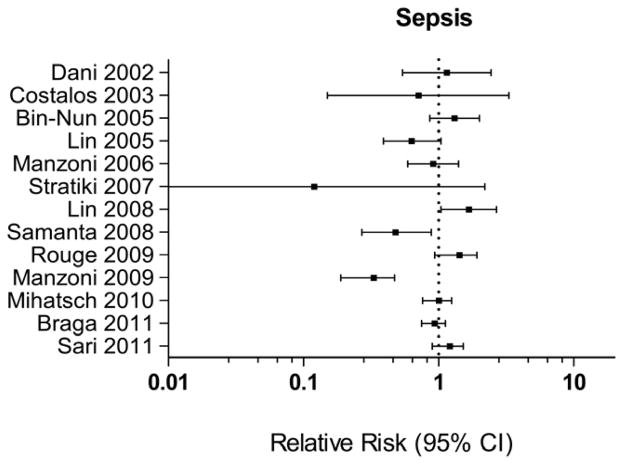
However, the benefit for probiotics in the prevention of sepsis (Figure 4) is not as clear. Two randomized trials, including a large trial by Manzoni et al., have shown a benefit of probiotics in reducing late-onset sepsis [67, 68]. However, Manzoni et al. used the probiotic LGG in combination with lactoferrin and did not evaluate probiotic treatment alone. Other studies have shown no benefit and one study showed an increased risk of sepsis with a RR 1.67 (95% CI 1.04–2.67), raising concerns regarding the risk of sepsis with probiotic therapy [69].
Figure 4.
Plot demonstrates the relative risk of sepsis in the probiotic treatment arm, compared to placebo, of therapeutic trials in preterm infants. Error bars reflect 95% confidence interval for relative risk estimates. Data from individual studies referenced as well as from Deshpande et al.[66] and Wang et al.[63].
While prebiotics and postbiotics are potential alternatives or supplements to probiotic therapy, no clinical trials have studied the therapeutic use of these substances for the prevention of NEC. Therefore, we can only speculate on the potential benefit of prebiotics and postbiotics in the preterm infant until more definitive data are available regarding their effects on important clinical outcomes of prematurity, such as NEC, sepsis or death.
Complications and Concerns
Although the clinical efficacy of probiotics in preventing NEC is promising, concerns regarding the complications associated with therapy have mitigated widespread use. Probiotic use in premature infants could expose intestinal epithelia with poor defenses and a tendency towards inflammation to a microbial challenge too soon, resulting in inflammation, injury or sepsis. Several reports of probiotic-associated sepsis [70–72] have raised concerns regarding routine clinical use of live bacteria in hosts, such as premature infants, who have immature epithelial barrier defenses. The American Academy of Pediatrics Committee on Nutrition - Section On Gastroenterology, Hepatology, And Nutrition [73] and the ESPGHAN Committee on Nutrition [44] both have highlighted the need for large, well-designed clinical research studies before widespread use is adopted. The use of prebiotics or postbiotics may decrease these concerns regarding safety. However, no data from trials of prebiotics or postbiotics for the prevention of NEC in preterm infants are currently available. In addition, these agents are likely to encounter similar issues regarding optimal dosing as seen with probiotic therapy.
Summary
NEC remains a devastating complication of prematurity and new preventative therapies are urgently needed. Probiotic therapy has demonstrated promising efficacy for the prevention of NEC, as evidenced by a very strong treatment effect in favor of probiotic therapy in two recent meta-analyses [63, 66]. However, safety and dosing concerns continue to temper widespread use and these concerns are likely to remain until large, multicenter trials adequately designed to address safety are completed. In addition, the use of prebiotics or postbiotics may be potential alternatives to the use of live probiotic organisms, although the therapeutic benefit for these products in preventing NEC remains largely unknown. Further studies are needed to guide clinicians on the use of probiotics, prebiotics and postbiotics for the prevention of NEC.
Key Points.
Current evidence suggests that probiotics are effective in decreasing NEC in preterm infants.
Concerns regarding safety and optimal dosing have limited the routine clinical use of probiotics in preterm infants.
Prebiotics and postbiotics are potential alternatives or adjunctive therapies to the administration of live microorganisms, although studies demonstrating their clinical efficacy in preventing NEC are currently lacking.
Acknowledgments
Grant support: Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number UL1TR000454 (R.M.P.) and NIH R01 HD059122 (P.W.D). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors do not have any relevant disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henry MC, Moss LR. Necrotizing enterocolitis. Annu Rev Med. 2009;60:111–24. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- 2.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–5. doi: 10.1016/j.jpedsurg.2009.02.013. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 5.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 6.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–64. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Patel RM, Lin PW. Developmental biology of gut-probiotic interaction. Gut Microbes. 2010;1:186–95. doi: 10.4161/gmic.1.3.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, et al. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–4. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 10.Collier-Hyams LS, Neish AS. Innate immune relationship between commensal flora and the mammalian intestinal epithelium. Cell Mol Life Sci. 2005;62:1339–48. doi: 10.1007/s00018-005-5038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 12.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–8. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 13.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 14.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 16.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 19.Kosloske AM. Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:2–7. doi: 10.1111/j.1651-2227.1994.tb13232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F167–73. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm premature rupture of membranes. Cochrane Database Syst Rev. 2001:CD001058. doi: 10.1002/14651858.CD001058. [DOI] [PubMed] [Google Scholar]
- 23.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 26.Westerbeek EA, van den Berg A, Lafeber HN, Knol J, Fetter WP, van Elburg RM. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr. 2006;25:361–8. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J Pediatr. 1978;93:288–93. doi: 10.1016/s0022-3476(78)80523-x. [DOI] [PubMed] [Google Scholar]
- 28.Mshvildadze M, Neu J. The infant intestinal microbiome: Friend or foe? Early Hum Dev. 2010 doi: 10.1016/j.earlhumdev.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156:20–5. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the Role of Intestinal Microbes in the Pathogenesis of Necrotizing Enterocolitis. Pediatrics. 2010 doi: 10.1542/peds.2009-3149. [DOI] [PubMed] [Google Scholar]
- 31.Teitelbaum JE, Walker WA. Nutritional impact of pre- and probiotics as protective gastrointestinal organisms. Annu Rev Nutr. 2002;22:107–38. doi: 10.1146/annurev.nutr.22.110901.145412. [DOI] [PubMed] [Google Scholar]
- 32.Mack DR, Lebel S. Role of probiotics in the modulation of intestinal infections and inflammation. Curr Opin Gastroenterol. 2004;20:22–6. doi: 10.1097/00001574-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–3. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 35.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res. 2008;64:511–6. doi: 10.1203/PDR.0b013e3181827c0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1118–27. doi: 10.1152/ajpgi.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47:1205–11. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K, et al. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007;26:4457–66. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626–35. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G940–9. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Russell WM, Douglas-escobar M, Hauser N, Lopez M, Neu J. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res. 2009;66:203–7. doi: 10.1203/PDR.0b013e3181aabd4f. [DOI] [PubMed] [Google Scholar]
- 42.Ouwehand AC, Derrien M, de Vos W, Tiihonen K, Rautonen N. Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol. 2005;16:212–7. doi: 10.1016/j.copbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Ghisolfi J. Dietary fibre and prebiotics in infant formulas. Proc Nutr Soc. 2003;62:183–5. doi: 10.1079/pns2002228. [DOI] [PubMed] [Google Scholar]
- 44.Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011;52:238–50. doi: 10.1097/MPG.0b013e3181fb9e80. [DOI] [PubMed] [Google Scholar]
- 45.Knol J, Boehm G, Lidestri M, Negretti F, Jelinek J, Agosti M, et al. Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paediatr Suppl. 2005;94:31–3. doi: 10.1111/j.1651-2227.2005.tb02152.x. [DOI] [PubMed] [Google Scholar]
- 46.Boehm G, Lidestri M, Casetta P, Jelinek J, Negretti F, Stahl B, et al. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:F178–81. doi: 10.1136/fn.86.3.F178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev. 2007;83:335–9. doi: 10.1016/j.earlhumdev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Underwood MA, Salzman NH, Bennett SH, Barman M, Mills DA, Marcobal A, et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J Pediatr Gastroenterol Nutr. 2009;48:216–25. doi: 10.1097/MPG.0b013e31818de195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Indrio F, Riezzo G, Raimondi F, Bisceglia M, Cavallo L, Francavilla R. Effects of probiotic and prebiotic on gastrointestinal motility in newborns. J Physiol Pharmacol. 2009;60 (Suppl 6):27–31. [PubMed] [Google Scholar]
- 50.Indrio F, Riezzo G, Raimondi F, Francavilla R, Montagna O, Valenzano ML, et al. Prebiotics improve gastric motility and gastric electrical activity in preterm newborns. J Pediatr Gastroenterol Nutr. 2009;49:258–61. doi: 10.1097/MPG.0b013e3181926aec. [DOI] [PubMed] [Google Scholar]
- 51.Labayen I, Forga L, Gonzalez A, Lenoir-Wijnkoop I, Nutr R, Martinez JA. Relationship between lactose digestion, gastrointestinal transit time and symptoms in lactose malabsorbers after dairy consumption. Aliment Pharmacol Ther. 2001;15:543–9. doi: 10.1046/j.1365-2036.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- 52.Boehm G, Stahl B, Jelinek J, Knol J, Miniello V, Moro GE. Prebiotic carbohydrates in human milk and formulas. Acta Paediatr Suppl. 2005;94:18–21. doi: 10.1111/j.1651-2227.2005.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 53.Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, Stahl B, et al. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr Suppl. 2005;94:22–6. doi: 10.1111/j.1651-2227.2005.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 54.Tsukahara T, Iwasaki Y, Nakayama K, Ushida K. Stimulation of butyrate production in the large intestine of weaning piglets by dietary fructooligosaccharides and its influence on the histological variables of the large intestinal mucosa. J Nutr Sci Vitaminol (Tokyo) 2003;49:414–21. doi: 10.3177/jnsv.49.414. [DOI] [PubMed] [Google Scholar]
- 55.Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr. 2004;28:210–22. doi: 10.1177/0148607104028004210. discussion 22–3. [DOI] [PubMed] [Google Scholar]
- 56.Kanauchi O, Andoh A, Iwanaga T, Fujiyama Y, Mitsuyama K, Toyonaga A, et al. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J Gastroenterol Hepatol. 1999;14:1173–9. doi: 10.1046/j.1440-1746.1999.02025.x. [DOI] [PubMed] [Google Scholar]
- 57.Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. J Biol Chem. 2001;276:44641–6. doi: 10.1074/jbc.M105170200. [DOI] [PubMed] [Google Scholar]
- 58.Venkatraman A, Ramakrishna BS, Shaji RV, Kumar NS, Pulimood A, Patra S. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2003;285:G177–84. doi: 10.1152/ajpgi.00307.2002. [DOI] [PubMed] [Google Scholar]
- 59.Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS. The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol. 2009;182:538–46. doi: 10.4049/jimmunol.182.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avivi-Green C, Polak-Charcon S, Madar Z, Schwartz B. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol Res. 2000;12:83–95. doi: 10.3727/096504001108747558. [DOI] [PubMed] [Google Scholar]
- 61.Mentschel J, Claus R. Increased butyrate formation in the pig colon by feeding raw potato starch leads to a reduction of colonocyte apoptosis and a shift to the stem cell compartment. Metabolism. 2003;52:1400–5. doi: 10.1016/s0026-0495(03)00318-4. [DOI] [PubMed] [Google Scholar]
- 62.Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care. 2004;7:563–7. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg. 2012;47:241–8. doi: 10.1016/j.jpedsurg.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 64.Sherman PM, Cabana M, Gibson GR, Koletzko BV, Neu J, Veereman-Wauters G, et al. Potential roles and clinical utility of prebiotics in newborns, infants, and children: proceedings from a global prebiotic summit meeting, New York City, June 27–28, 2008. J Pediatr. 2009;155:S61–70. doi: 10.1016/j.jpeds.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 65.Srinivasjois R, Rao S, Patole S. Prebiotic supplementation of formula in preterm neonates: a systematic review and meta-analysis of randomised controlled trials. Clin Nutr. 2009;28:237–42. doi: 10.1016/j.clnu.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125:921–30. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 67.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 68.Samanta M, Sarkar M, Ghosh P, Ghosh J, Sinha M, Chatterjee S. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr. 2009;55:128–31. doi: 10.1093/tropej/fmn091. [DOI] [PubMed] [Google Scholar]
- 69.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 70.Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, Nanba Y, et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J Pediatr. 2010;156:679–81. doi: 10.1016/j.jpeds.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 71.Guenther K, Straube E, Pfister W, Guenther A, Huebler A. Severe sepsis after probiotic treatment with Escherichia coli NISSLE 1917. Pediatr Infect Dis J. 2010;29:188–9. doi: 10.1097/INF.0b013e3181c36eb9. [DOI] [PubMed] [Google Scholar]
- 72.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–81. doi: 10.1542/peds.2004-2137. [DOI] [PubMed] [Google Scholar]
- 73.Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–31. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 74.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci U S A. 2004;101:7404–8. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–8. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–61. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 77.van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed. 2003;88:F52–5. doi: 10.1136/fn.88.1.F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174–80. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 79.Nowicki PT, Nankervis CA. The role of the circulation in the pathogenesis of necrotizing enterocolitis. Clin Perinatol. 1994;21:219–34. [PubMed] [Google Scholar]
- 80.Berseth CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. 1989;115:646–51. doi: 10.1016/s0022-3476(89)80302-6. [DOI] [PubMed] [Google Scholar]
- 81.Anderson DM, Kliegman RM. The relationship of neonatal alimentation practices to the occurrence of endemic necrotizing enterocolitis. Am J Perinatol. 1991;8:62–7. doi: 10.1055/s-2007-999344. [DOI] [PubMed] [Google Scholar]
- 82.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111:529–34. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 83.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 84.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007:1–186. [PMC free article] [PubMed] [Google Scholar]
- 85.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 86.Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997;76:F101–7. doi: 10.1136/fn.76.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. 2002;82:103–8. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 88.Costalos C, Skouteri V, Gounaris A, Sevastiadou S, Triandafilidou A, Ekonomidou C, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev. 2003;74:89–96. doi: 10.1016/s0378-3782(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 89.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–6. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 90.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 91.Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis. 2006;42:1735–42. doi: 10.1086/504324. [DOI] [PubMed] [Google Scholar]
- 92.Mohan R, Koebnick C, Schildt J, Schmidt S, Mueller M, Possner M, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. J Clin Microbiol. 2006;44:4025–31. doi: 10.1128/JCM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007;83:575–9. doi: 10.1016/j.earlhumdev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Rouge C, Piloquet H, Butel MJ, Berger B, Rochat F, Ferraris L, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2009;89:1828–35. doi: 10.3945/ajcn.2008.26919. [DOI] [PubMed] [Google Scholar]
- 95.Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology. 2010;98:156–63. doi: 10.1159/000280291. [DOI] [PubMed] [Google Scholar]
- 96.Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr. 2011;93:81–6. doi: 10.3945/ajcn.2010.29799. [DOI] [PubMed] [Google Scholar]
- 97.Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr. 2011;65:434–9. doi: 10.1038/ejcn.2010.278. [DOI] [PubMed] [Google Scholar]
- 98.Fernandez-Carrocera LA, Solis-Herrera A, Cabanillas-Ayon M, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2013 Jan;98(1):F5–9. doi: 10.1136/archdischild-2011-300435. [DOI] [PubMed] [Google Scholar]