Summary
Although tight junctions (TJ) have been extensively studied in simple epithelial cells, it is still unknown whether their organization is coupled to cell differentiation in stratified epithelia. We studied the expression of TJ in RCE1(5T5) cells, an in vitro model which mimics the sequential steps of rabbit corneal epithelial differentiation. RCE1(5T5) cells expressed TJ components which were assembled once cells constituted differentiated epithelia, as suggested by the increase of transepithelial electrical resistance (TER) which followed a similar kinetic to the expression of the early differentiation marker Pax-6. TJ were functional as indicated by the establishment of an epithelial barrier nonpermeable to ruthenium red or a biotin tracer. In immunostaining experiments, TJ were located at the superficial cells from the suprabasal layers; Western blot and RT-PCR suggested that TJ were composed of claudins (cldn) -1, -2, -4, cingulin (cgn), occludin (ocln) and ZO-1. Semi-quantitative RT-PCR and TER measurements showed that TJ became organized when cells began to form a 3–5 layers stratified epithelium; TER increased once cells reached confluence, with a time course comparable to the raise in the expression of cgn, cldn-2 and -4. Nevertheless, cldn-1, -2, ZO-1 and ocln were present in the cells from the beginning of cultivation, suggesting that TER increases mainly depend on TJ assembly. While EGF increased epithelial barrier strength, retinoic acid disrupted it, increasing paracellular flux about 2-fold; this effect was concentration dependent and completely reversible. Our results suggest that TJ assembly is tightly linked to the expression of corneal epithelial terminal phenotype.
Keywords: Corneal epithelium, Tight junction, Retinoic acid, Claudin, Occludin, Cingulin, Cell differentiation, Cell culture
Introduction
Epithelial tissues are composed of a continuous sheet of cells that line the cavities and surfaces of structures throughout the body. In general, epithelia constitute a selective, polarized barrier to the vectorial exchange of substances between the compartments separated by them. This is due to a gasket-like seal which restricts the movement of substances through the paracellular pathway. Such seal is the result of an adhesive structure established between adjacent epithelial cells, known as the tight junction (TJ), or zonula occludens (ZO) (reviewed by Anderson, 2001; Stevenson and Keon, 1998).
TJ carry out several functions. They participate in cell polarization and permeability barrier functions (Anderson, 2001; Stevenson and Keon, 1998), besides to play a role in cell signaling, in the control of the organization of the cytoskeleton, and in the activity of some transcription factors (Paris et al., 2008; Buchert et al., 2009; Remue et al., 2010).
TJ are located at the apex of the cell and are composed of more than 40 different types of proteins with adhesive, scaffolding, cytoskeletal and regulatory roles (reviewed by Chiba et al., 2008; Furuse, 2010). Among their major transmembrane components there are proteins which mediate intercellular adhesion, such as different claudin (cldn) isoforms, occludin (ocln), tricellulin and junctional adhesion molecule-A (Tsukita et al., 2001; Ikenouchi et al., 2005; Hartsock and Nelson, 2008; Paris et al., 2008; Anderson and Van Itallie, 2009). On the other hand, essential cytosolic components of the TJ are the intracellular scaffold proteins members of the ZO protein family (Tsukita et al., 2001; Feldman et al., 2005; Hartsock and Nelson, 2008; Paris et al., 2008; Anderson and Van Itallie, 2009), in addition to plaque proteins such as symplekin, cingulin (cgn) and 7H6 (Balda and Matter, 2008; Denker and Nigam, 1998). Besides the structural proteins of TJ, exist a number of regulatory proteins involved in signal transduction (Paris et al., 2008), and in transcriptional and post-transcriptional regulation (Aho et al., 2009; Matter and Balda, 2007; Kavanagh et al., 2006; Jaramillo et al., 2004; Balda et al., 2003).
In epithelial and endothelial cells, TJ show a composition that depends upon the distinct permeability functions of these tissues (Elkouby-Naor and Ben-Yosef, 2010; Furuse, 2010; Amasheh et al., 2011). So far, physiology and regulation of TJ have been mainly studied in epithelial cell monolayers, most often those formed by kidney cell lines such as MDCK and LLC-PK1 (Sabath and Denker, 2006; Prozialeck et al., 2006), or intestine epithelial cells such as Caco-2 (Peng et al., 2009; Buzza et al., 2010). These studies have led to understand part of the mechanisms that regulate the assembly and permeability of epithelial TJ.
However, assembly of the TJ complex in stratified epithelia has not been analyzed as extensively as in simple epithelia. The accumulated evidence shows that the formation of adherens junctions and desmosomes precedes that of TJ at the granular layer of epidermis (Pummi et al., 2001), which seem to be functional at this epidermal layer (Kirschner et al., 2012). Although data from different laboratories suggest possible link between TJ assembly and keratinocyte differentiation, it is not clear if TJ formation is coupled or regulated to cell differentiation or vice versa.
In corneal epithelium the permeability barrier is generated by TJ formed between the superficial cells (McLaughlin et al., 1985; Wang et al., 1993; Sugrue and Zieske, 1997). Such distribution would also insinuate that the expression of terminal phenotype shares common elements with the regulation of TJ assembly in corneal epithelium. This possible relation confers high relevance to the study of corneal zonula occludens. Thus far, the analysis of both human and rat corneal epithelium shows that TJs contain ocln, ZO-1, ZO-2, and cldn-1, -2, -3, -4, -7, -9, -14 and -15 as components of the intercellular adhesion complex (Yi et al., 2000; Ban et al., 2003a; Ban et al., 2003b; Elkouby-Naor and Ben-Yosef, 2010). In addition, cldn-10 is specifically induced in vitro in corneal epithelial cells (Ban et al., 2003a).
To analyze the relationships between the expression of the differentiated phenotype and TJ assembly, we determined whether RCE1(5T5) cells, a cell line which mimics the sequential steps of corneal epithelial cell differentiation in a similar manner to primary cell cultures (Castro-Muñozledo, 1994; Tamariz et al., 2007; Castro-Muñozledo, 2008; García-Villegas et al., 2009), express and assemble functional TJs. We demonstrate that epithelia formed by RCE1(5T5) cells express TJ components which are assembled once cells constitute a differentiated, stratified epithelium. The functional TJ complexes were located at the superficial cells from the suprabasal layers, and contain cldn-1, -2 and -4, besides ZO-1, ocln and cgn. Results also show that TJs are assembled only when cells begin to stratify, following a time course similar to the raise in the expression of cldn-4 and cgn proteins, and to the expression of Pax-6 which is an early differentiation marker. In contrast, cldn-1, -2, ZO-1 and ocln are present from the beginning of cell cultivation and their changes did not correlate with the emergence of a functional epithelial barrier. Since large-scale junctional rearrangements play crucial roles in normal epithelial morphogenesis and epithelial barrier breakdown in many pathological conditions (Ivanov et al., 2005; Cavey and Lecuit, 2009; Turner, 2009), we examined the effects of EGF and all-trans retinoic acid (RA) on the epithelial barrier, and found that epithelial barrier is modulated by RA in a reversible manner. Together, our results suggest a possible correlation between the expression of cldn-2 and -4 and cgn with the assembly of functional TJs. Moreover, the results suggest that TJ assembly is tightly coordinated with the expression of terminal phenotype of corneal epithelium. The mechanisms involving such relation require further investigation.
Results
RCE1(5T5) cells assemble morphological and functional TJ complexes
To explore whether RCE1(5T5) cells are a good model to study TJ assembly and its coordination with the differentiation process, we confirmed the presence of the ZO complex in the cultured epithelial sheets. Since epithelial sheets are constituted after cells reach confluence at 6th day in cell culture, we carried out electron microscopy analyses of two day confluent (8 days in cell culture) and mature four day post-confluent cultured epithelia (10 days in cell culture). Examination showed the typical small direct contact sites of the two plasma membranes at the most suprabasal layer of the cultured epithelia (Fig. 1A–C), and suggesting the presence of TJ. This was confirmed by the use of ruthenium red to test whether TJ were present, showing that the tracer diffused all the way through the intercellular space until a TJ complex stopped additional penetration of the compound to deeper epithelial layers (Fig. 1C, arrow).
Fig. 1.
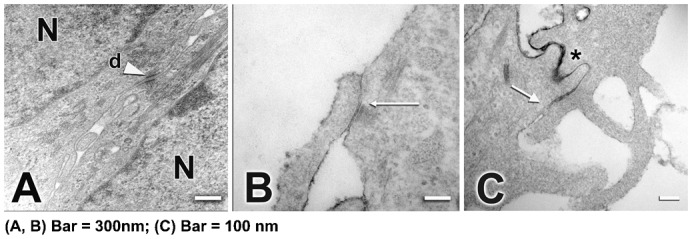
Ultrastructure of 4 day (A–C) post-confluent cultured epithelia formed by RCE1(5T5) cells. It is shown the presence of desmosomes (d) between cells (A, arrowhead). TJ were observed at the upper layers of the epithelial sheet, mainly in 4-day confluent epithelia as small direct contact sites between the plasma membranes of two adjacent cells (B, arrows). (C) Note that diffusion of ruthenium red throughout the intercellular space (asterisk) was stopped at TJ location (C, arrow). N, nucleus. In A,B, Bar = 300 nm; in C, Bar = 100 nm.
To further demonstrate that TJ in RCE1(5T5) epithelia were functional, we first measured trans-epithelial electrical resistance (TER), which is a direct measure of the epithelial resistance to passive ion flow. The cells were plated into cell culture inserts as described (see Materials and Methods) and grown up to four days after confluence, when they had already formed a 3–4 layered epithelium, and TER values were determined. Positive control consisted in confluent MDCK cells, which constitute epithelia with hermetic TJ (Anderson and Van Itallie, 2009); confluent 3T3 fibroblasts, which do not possess TJ, were used as background for measurements. While MDCK epithelial sheets showed TER values of 4436±2153.4 Ω/cm2, the epithelia formed by RCE1(5T5) cells showed TER values of 291±65.7 Ω/cm2.
Since results could also be explained as a consequence of the existence of cell layers instead the assembly of TJ, we tested paracellular permeability of the cultured epithelia by the use of a biotin tracer assay (Umeda et al., 2006). The RCE1(5T5) epithelia were incubated with EZ-Link sulfo-NHS-LC-biotin (MW = 556.59) (see Materials and Methods), and immunostained for cldn-1 to visualize TJ complexes. The tracer was detected either with FITC-streptavidin or Texas Red-streptavidin. When the tracer assay was carried out on confluent epithelia (Fig. 2), we could detect the biotinylation of proteins only at epithelial cell surface but not at the cellular spaces between the different cell layers of the cultured epithelium, regardless of the time in cell culture (Fig. 2A–H), suggesting that the tracer was stopped by functional TJ complexes. Together, these results show that RCE1(5T5) cells constitute a 3–4 layered epithelium, with morphological and functional TJ located at the upper layer of the cultured epithelium, similar to those found in the normal corneal epithelium.
Fig. 2. Paracellular permeability of the cultured corneal epithelia was examined by use of the low molecular weight tracer, EZ-Link sulfo-NHS-LC-biotin.
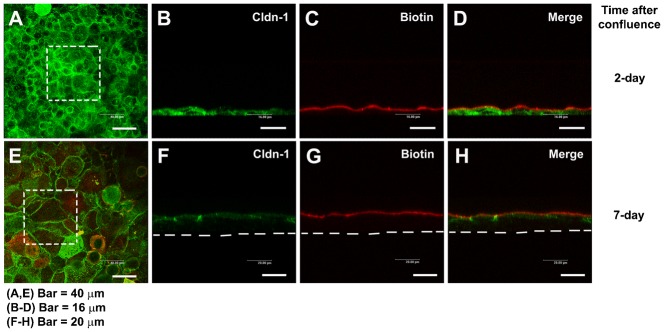
The tracer did not penetrate the epithelial sheet neither 1 day after confluence (7 days in cell culture) (A–D), nor in 7 day confluent epithelia (E–H), as indicated by the (C,G) lack of staining of biotinylated proteins at cell–cell boundaries in the lower layers of the epithelium. (D,H) Maximal projections of the merged channels, transverse optical sections of cultures stained for cldn-1 to immunolocalize TJ (green channel; B,D,F,H). Proteins biotinylated with the tracer are stained in red (C,D,G,H). (A,E) show the aspect of cldn-1 in xyz maximal projections of the stained cell cultures. Dashed squares show the fields examined in the transverse sections. Dashed lines indicate the basal side of the cultured epithelia. In A,E, Bar = 40 µm; in B–D, Bar = 16 µm; in F–H, Bar = 20 µm.
Expression of TJ components in RCE1(5T5) epithelia
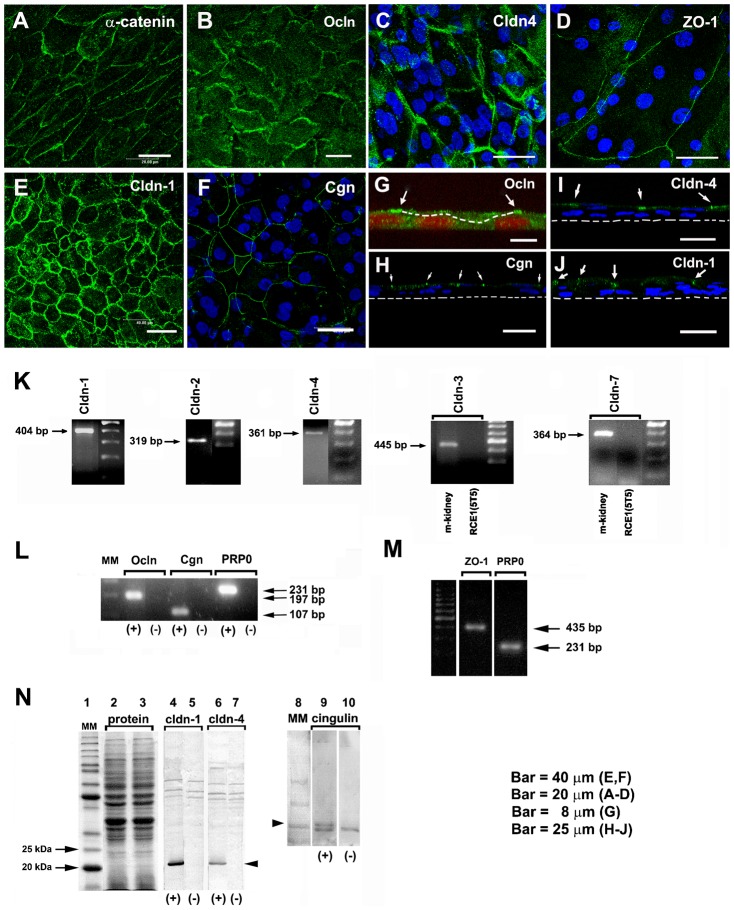
Given that different epithelia have distinct permeability properties according to the expression and distribution of the different types of cldns (Van Itallie and Anderson, 2006), and in view of the above results, we tested the expression of cldns, ocln, ZO-1 and cgn by immunostaining, Western blot and end point RT-PCR in 7-day post-confluent (12–14 days in cell culture) RCE1(5T5) epithelia. As shown in Fig. 3A, α-catenin, an essential protein in the assembly of adherent and TJ, was associated to cell membrane and cytoplasmic compartments; antibodies against cldn-1 (Fig. 3E,J), cldn-4 (Fig. 3C,I), ocln (Fig. 3B,G), cgn (Fig. 3F,H), and ZO-1 (Fig. 3D) immunostained the cell boundaries of the most suprabasal cells. On the other hand, end point RT-PCR showed that 7-day post-confluent cell cultures expressed the mRNAs encoding cldns-1, -2 and -4 (Fig. 3K), and the mRNAs encoding ocln, cgn and ZO-1 (Fig. 3L,M); whereas cldns 3 and -7 were not detected (Fig. 3K). In addition, Western blot studies revealed that cldn-1 and -4, besides cgn, were found in the 7-day post-confluent epithelia (Fig. 3N).
Fig. 3. TJ components detected in cultured RCE1(5T5) corneal epithelial cells.
This was performed either by: (A–J) immunostaining, (K–M) end point RT-PCR, or (N) Western blot. (A) We used α-catenin as an indicator of the presence of adhesion complexes in the cultured epithelia. (B,G) Ocln, (D) ZO-1, (E) cldn-1, (C,I) cldn-4, and (F,H) cgn were easily located in cell borders of one day post-confluent cultures. (G–I) are transverse sections showing that TJs (arrows) were located at the cell boundaries of suprabasal cells. In (G), dashed line indicates the boundaries between a suprabasal cell and the basal cell layer; in (H–J) the dashed line indicate the basal side of the epithelium. Nuclei were stained either with propidium iodide or TO-PRO®-3. In 7-day confluent epithelia we found (K) the expression of cldn-1, -2, -4, but not of cldn-3 and -7; (L) cgn, ocln, and (M) ZO-1. Primers for cldn-3 and -7 were verified in PCR reactions using mouse kidney cDNA (K, m-kidney) that led to amplification products of the expected size and sequence. PR-P0 was used as an internal marker that does not change during the differentiation process. The antibodies were only appropriate to immunodetect cldn-1 and -4 and cgn (N); lanes 2 and 3 correspond to the loading control for the experiment. PCR reaction in the presence (+) or the absence of cDNA (−) (negative control). Results correspond to six duplicated experiments. A–D: Bar = 20 μm; E,F: Bar = 40 μm; G: Bar = 8 μm, and H–J: Bar = 25 μm.
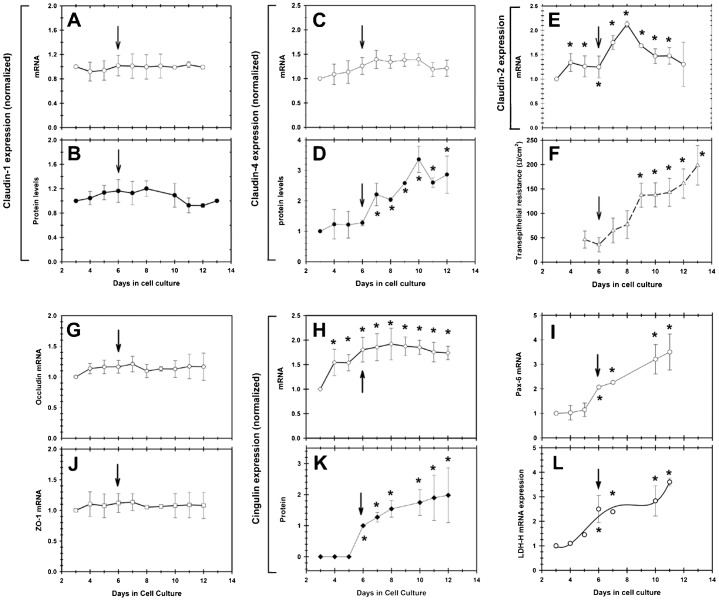
Above, we demonstrated the presence of functional TJ in the mature 3–5 layered epithelial sheet formed by RCE1(5T5) cells. Since some of our preliminary results suggested that TJ components were expressed at early times in culture when cells have not constituted an epithelial tissue (not shown), we determined the time of expression of cldns -1, -2 and -4, ocln, ZO-1 and cgn along the growth and differentiation of cultured RCE1(5T5) cells. We also correlated their change both with the expression of Pax-6 mRNA as the earliest marker of differentiation (García-Villegas et al., 2009), and with the change in TER as a marker of the assembly of functional TJ.
As depicted in Fig. 4, cldn-1 mRNA and protein levels were stable all along the experiment, without showing a significant change (Fig. 4A,B). A similar behavior was observed for ocln and ZO-1 mRNAs, which did not have significant variations (Fig. 4G,J). In contrast, although cldn-2 and -4 mRNAs and proteins were also detected from the beginning of the growth in cell culture (Fig. 4C–E), they underwent increases that began on the 6th day in cell culture, when cells reached confluence and began to constitute a 3–5 layers stratified epithelium (Fig. 4C–E). At first, the increase of cldn-2 and -4 had a similar time course to the expression of the early differentiation markers Pax-6 (Fig. 4I) and LDH-H (Fig. 4L), showing a close correlation with the increase on TER, which began one day after (Fig. 4F). However, while cldn-4 mRNA expression slightly increased during growth and differentiation of RCE1(5T5) cells (Fig. 4C), cldn-4 protein steadily augmented at the time of confluence in cell culture (6th day after plating), reaching amounts 3.5-fold higher to those found in 3-day growing cell cultures (Fig. 4D). On the other hand, cldn-2 mRNA levels increased 2-fold after cells became confluent and began to stratify (Fig. 4E); but, 2 days later its expression decreased to levels comparable to those found in growing cultures (Fig. 4E).
Fig. 4. Semi-quantitative RT-PCR analysis of the expression of mRNAs encoding TJ components during growth and differentiation of RCE1(5T5 cells).
(A) cldn-1, (C) cldn-4, (E) cldn-2, (G) ocln, (J) ZO-1 and cgn (H). When Western blot was possible, we determined protein levels of (B) cldn-1, (D) cldn-4, and (K) cgn. The results were correlated with (F) the change in TER, (I) the expression of the transcription factor Pax-6, which is the earliest corneal differentiation marker, and (L) the expression of LDH-H mRNA, which precedes for 2.5 days the expression of terminal differentiation marker K3 keratin. Arrows show the 6th day in cell culture, in which cells reached confluence and began the differentiation process as demonstrated by the rise in Pax-6 expression. Results correspond to six duplicated experiments. Asterisks indicate significant changes (P≤0.005).
The most dramatic changes were observed for cgn expression. While its mRNA showed an early increase reaching levels 2-fold higher to those found at third day in cell culture (Fig. 4H), protein appeared only in the cell extracts starting at the 6th day in cell culture, to augment up to 2-fold in the epithelial sheets (Fig. 4K) (12th day in cell culture and 6th day after confluence). Together, the results suggest that essential components of the TJ such as cldn-1, -2, -4, ocln and ZO-1 are present in the cells even at early times of cultivation, Interestingly, cldn-4 and cgn proteins showed changes parallel to the expression of terminal differentiation and the development of a 3–5 layered stratified epithelium, followed by the raise on TER which began 24 hours later (Fig. 4F,I,L), suggesting that TJ assembly is dependent on differentiation-linked changes that deserve a closer examination.
Corneal epithelial TJ permeability is modulated by EGF and retinoic acid
It was demonstrated that corneal epithelial barrier is disrupted by agents such as TNFα (Kimura et al., 2008), IL-1β (Kimura et al., 2009), 1–4 µg/ml LPS (Yi et al., 2000) among others. In addition, growth factors such as EGF may either derange TJ components as shown in A431 cells (Van Itallie et al., 1995) and thyroid epithelium (Nilsson and Ericson, 1995), or protect TJ integrity as seen in MDCK (Singh et al., 2007) and Caco-2 cells (Aggarwal et al., 2011) depending on the physiological condition of cells. Therefore, we assayed the action of the most effective concentration of EGF on epithelial cells (Cha et al., 1996; Barrandon and Green, 1987), on epithelial barrier function. The RCE1(5T5) cells were grown in medium without 10 ng/ml EGF; then, one day after confluence (7th day in culture) they were supplemented with medium containing EGF. Parallel cultures were maintained with medium without EGF (control). Seven days after beginning treatment with EGF (14th day in culture), cultures were either fixed and immunostained with a cldn-1 specific antibody, or used to measure trans-epithelial resistance (TER). As shown (Fig. 5), cells stimulated with 10 ng/ml EGF showed a well-defined and typical cldn-1 immunolocalization pattern at cell–cell borders, with some discontinuities along cell surroundings (Fig. 5B). In contrast, cells nonstimulated with the growth factor also showed strong cldn-1 immunostaining at cell–cell borders with an interrupted punctuate pattern; however, they showed less defined cell–cell contacts than those cells treated with EGF (Fig. 5A). The differences between both culture conditions suggested that RCE1(5T5) cell cultures stimulated with EGF had a lower permeability than those epithelia grown without EGF. Such assumption was confirmed after TER evaluation on epithelia grown under these culture conditions, which revealed 1.6-fold higher (160±30 Ω/cm2) TER values in EGF-stimulated epithelia than in epithelia grown without EGF (95±23 Ω/cm2) (Fig. 5C). Since the difference in epithelial permeability induced by EGF was not associated to significant changes in the expression of cldn-1, -2, -4, ocln, ZO-1 and cgn (not shown), we suggest that they may be related with modifications in TJ assembly in response to stimulation of EGFR signaling pathway.
Fig. 5. Epidermal growth factor modulates the permeability of the corneal epithelial TJ.
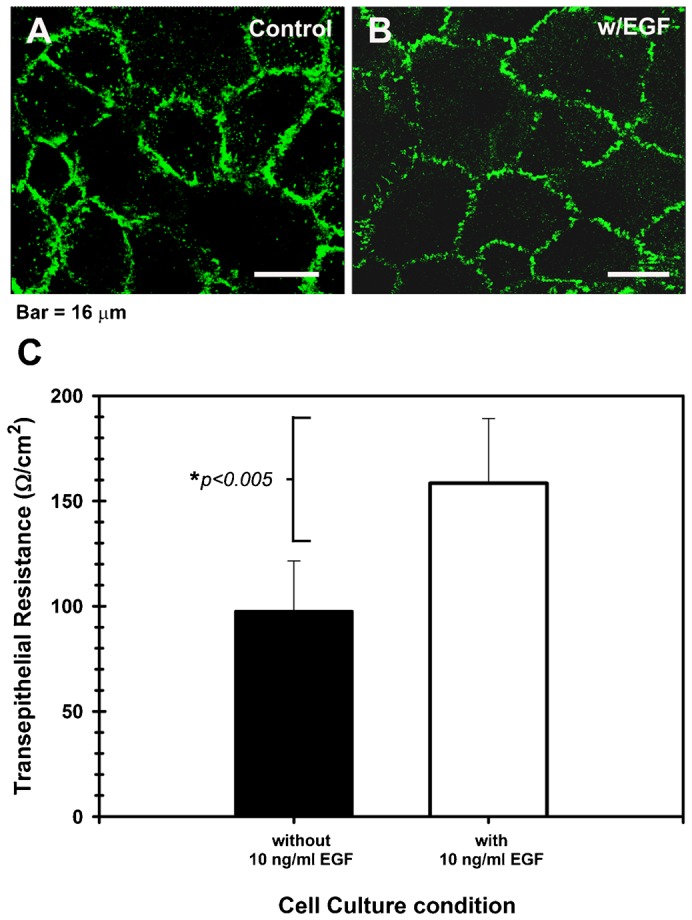
(A) Shows that the cldn-1 immunolocalization pattern is strong with punctuated discontinuities in cell cultures maintained in basal medium without EGF, while (B) cells cultured in the presence of 10 ng/ml EGF had a well-defined cell–cell contacts and a typical cldn-1 immunolocalization, with some discontinuities. The highest expression of cldn-1 correlated with (C) high TER values in the EGF-supplemented cultures which showed the tightest epithelial barrier. Effect of EGF was significant (P≤0.005) for 5 duplicated experiments. A,B scale bar = 16 μm.
Vitamin A and its analogs, mainly retinoic acid (RA), modulate corneal epithelial differentiation and proliferation in a concentration-dependent manner, both in vivo and in vitro (Kim et al., 2012; Kruse and Tseng, 1994; Stonecipher et al., 1988; Tseng et al., 1984). In vivo, vitamin A deficiency leads to keratinization of the corneal and conjunctival epithelia (Tseng et al., 1984), while application or treatment with RA at 1 µM concentrations or higher results in an aberrant phenotype similar to that observed in conjunctival epithelium (Kim et al., 2012).
Considering that retinoids may modulate TJ in other epithelia (Rong and Liu, 2011; Hatakeyama et al., 2010; Telgenhoff et al., 2008), we tested the effect of different concentrations of all-trans retinoic acid (RA) on the development of TER along the growth and differentiation of RCE1(5T5) cells. Cells were plated as described (see Materials and Methods) and after the third day in culture, medium was changed to medium containing 0.1, 1.0 or 10 µM RA; control cultures were supplemented with medium without retinoid. All cultures were maintained up to the 14th day after plating; TER was determined every day for each culture condition. As shown in Fig. 6, the increase in TER observed in control cultures was completely blocked even at late times in culture, when cells were grown in the presence of 1 or 10 µM RA (Fig. 6E). In contrast, treatment with 0.1 µM RA partially blocked the change in TER, which increased beginning at the 11th day in culture to reach values 50% lower than those observed in control cultures (Fig. 6E). In addition, when cultures grown in the presence of RA were immunostained to study cldn-1 localization, we observed that retinoid treatment modified cldn-1 distribution along cell boundaries in a concentration dependent-manner (Fig. 6A–D). While control cultures showed the typical interrupted immunostaining at the cell boundaries, characteristic of the corneal epithelium and corneal endothelium (Fig. 6A), cells treated with RA had a weak, discontinuous, punctuated pattern of cldn-1 (Fig. 6B–D), with the highest discontinuity of TJ complexes in those cultures grown with 10 µM RA (Fig. 6D).
Fig. 6. TJ assembly is disrupted by retinoic acid (RA).
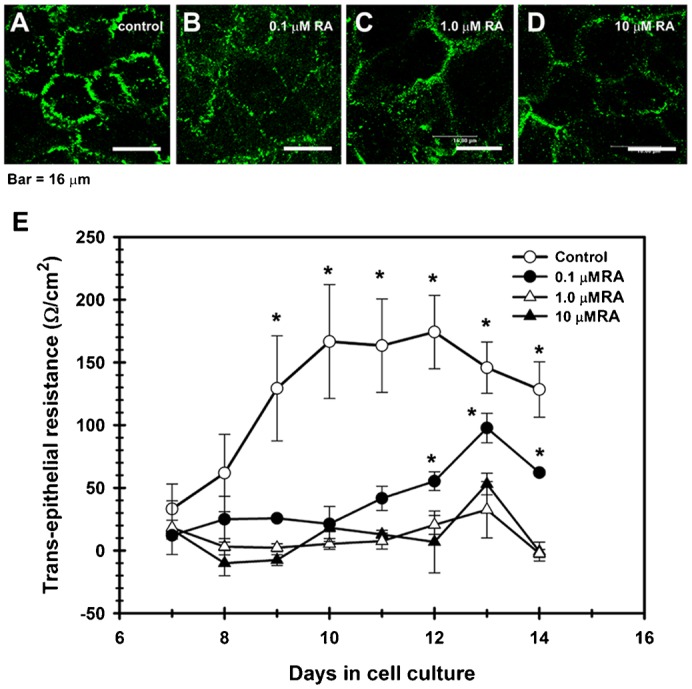
In contrast with control cultures (A), corneal epithelial cells grown in the presence of RA, had a weak, discontinuous, punctuated pattern of cldn-1 at cell boundaries, which decreased in a concentration-dependent manner; and with cldn immunolocalization in cell cytoplasm in the RA-treated cells (B–D). (E) This change correlated with a partial (0.1 µM RA) or a complete blockage of the increase of TER observed in control cultures which were not treated with RA. (P≤0.05) for 5 duplicated experiments. A–D: scale bar = 16 μm.
Next, we determined whether RA disrupts corneal epithelial barrier when it is added to stratified epithelia, and if this effect is reversible once the retinoid is removed from the culture medium. To carry out this experiment, one day post-confluent 3–5 cell layered stratified epithelia, grown under standard conditions, were changed to medium containing 0.1, 1.0 or 10 µM RA. After a 48-hour incubation with the retinoid, when TER reached its lowest values, cultures were rinsed and changed back to medium without the retinoid. Beginning at the time when the retinoid was added, TER was determined every day. As seen in Fig. 7, treatment with any of the tested RA concentrations rapidly decreased TER to values about 60% lower than those found in control cultures maintained in medium without the retinoid. Subsequent to RA elimination from the culture medium, TER slowly recovered to normal values, with a delay proportional to RA concentration. The slowest recovery rate was observed in those cultures treated with 10 µM RA (Fig. 7). These results show that treatment of stratified epithelia with retinoids leads to a disruption of the epithelial barrier, an effect that is reversible after removal of the retinoids.
Fig. 7. Reversibility of disruption of epithelial barrier by RA.
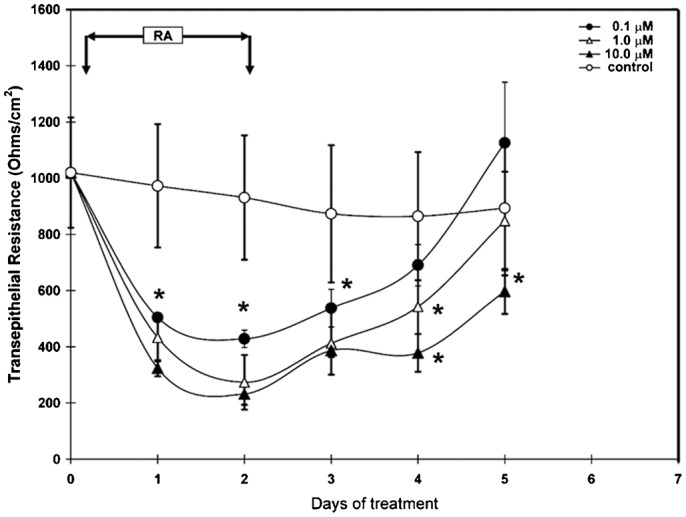
RCE1(5T5) corneal epithelial cells were grown as described (see Materials and Methods). One day after confluence, 3–5 layered epithelia were incubated with medium containing 0.1, 1.0 or 10 µM RA for 48 hours (arrows). Then, cells were rinsed thrice with PBS and changed to basal medium. TER was determined every day for each cell culture condition beginning at the day in which cells were changed to medium containing the retinoid. As shown, RA disrupted epithelial barrier in a reversible manner, with the highest effect and the slower recovery in cell cultures treated with 10 µM RA. Results correspond to three duplicate experiments; asterisks indicate significant differences (P≤0.05).
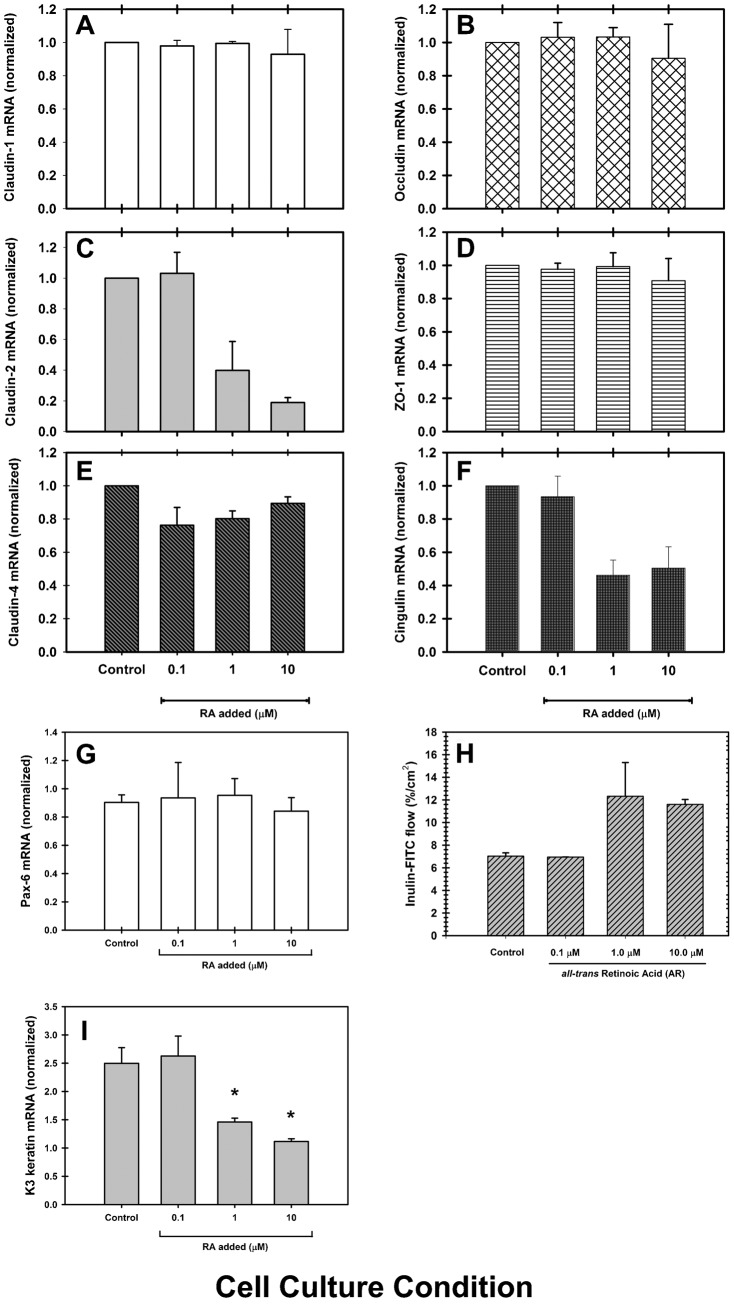
As shown, treatment with RA promoted changes in cldn distribution and immunostaining. These changes could be explained either by an alteration in TJ assembly, or through a decrease in the expression of TJ components. Therefore, we analyzed the expression of cldn-1, -2, -4, ocln, ZO-1 and cgn in cell cultures treated with RA. One day post-confluent RCE1(5T5) cultures were supplemented with medium containing 0.1, 1.0 or 10 µM RA for 48 hours; an incubation time that, as shown above, decreases the epithelial barrier strength to 60% of TER values found in nontreated epithelia. Control cultures were maintained with medium w/o the retinoid. Then, cultures were extracted to study TJ component expression by semi-quantitative RT-PCR. Also, we quantified the expression of Pax-6 and K3 keratin as differentiation markers, to verify the modulatory effect of RA on epithelial cell differentiation. As expected, treatment with RA led to a decrease on the expression of K3 keratin, which is a late differentiation marker linked to the expression of terminal phenotype; but did not have an effect on the expression of Pax-6 which seems to regulate corneal epithelial cell commitment (Fig. 8G,I). Results demonstrated that cldn-2 and cgn mRNA levels were markedly decreased by the 48-hour treatment with RA; cldn-2 expression had the higher sensitivity to RA treatment showing a 60% and 80% reduction in cultures maintained with 1 and 10 µM RA, respectively (Fig. 8C). Similarly, cgn was reduced by RA concentrations of 1 and 10 µM to levels 40–50% of those found in control cultures (Fig. 8F). Surprisingly, cldn-4 expression showed a slight decrease in cells maintained in the presence of RA only at RA concentrations of 0.1 and 1.0 µM (Fig. 8E). In contrast, cldn-1, ocln and ZO-1 did not show a significant change in their expression (Fig. 8A,B,D). These results could suggest that TJ assembly is modulated by RA, in part through a decrease in the levels of mRNAs encoding their components. However, since epithelial barrier disruption could be explained by a decrease in the number of cell layers promoted by RA treatment, we studied the epithelial structure of cultures incubated for 48 hours with 10 µM RA, as above. As demonstrated (supplementary material Fig. S1), RA did not affect the number of epithelial cell layers (supplementary material Fig. S1A,B); instead, it promoted an increase in the size of intercellular spaces and a reduction in the number of desmosomes (supplementary material Fig. S1C–F). Such an effect may also explain the disruption of the epithelial barrier.
Fig. 8. The disruption of corneal epithelial barrier is in part explained by a decrease in the expression of cldn-2, cldn-4 and cgn.
In RCE1(5T5) epithelial sheets treated with 0.1, 1.0 or 10 µM RA for 48 hours, (F) cgn and (C) cldn-2 showed high susceptibility to RA treatment, while cldn-1 (A), ocln (B) and ZO-1 (D) were not affected. Surprisingly, cldn-4 (E) showed a slight decrease in cultures treated with 0.1 and 1.0 µM, but not with 10 µM RA. At the same time (H), treatment with 1.0 or 10 µM RA for 48 hours induced a 2-fold increase in the paracellular flux of inulin-FITC. (I) Changes in cgn and cldn-2 expression induced by RA were similar to the decrease on the expression of Keratin K3, a late differentiation marker, linked to the expression of corneal terminal phenotype. (G) Given that the expression of the transcription factor Pax-6 did not undergo a significant change after treatment with RA, the results establish a tight link between corneal phenotype expression and TJ assembly. RA seems to modulate phenotype expression of corneal epithelial cells and TJ assembly, whereas Pax-6 seems to regulate corneal epithelial cell commitment. Asterisks indicate statistically significant changes (P≤0.001).
We further confirmed the disruption of the epithelial barrier after incubation with retinoids, measuring paracellular flux by the use of inulin-FITC as previously described (Aggarwal et al., 2011; Singh et al., 2007). One day post-confluent epithelia grown into transwell inserts were supplemented with medium containing 0.1, 1.0 or 10 µM RA and 500 µg/ml inulin-FITC. After a 48-hour incubation, fluorescence was determined both in the basal and apical compartments, and compared with controls which were not treated with retinoid. As expected, those cultures which were treated with 1.0 or 10 µM RA had a higher permeability than control cultures or those epithelia which were incubated with 0.1 µM RA (Fig. 8H), demonstrating that retinoids increased paracellular flux through the cultured epithelia.
Discussion
TJ assembly, development and physiology have been extensively studied in simple epithelia; such analysis has allowed to understand part of the mechanisms that regulate them. TJ assembly seems to depend on cell–cell contact, given that confluence leads to development of TER (Cereijido et al., 1978; Meza et al., 1980; Martínez-Palomo et al., 1980). Results obtained mainly in simple epithelia, indicate the existence of mechanisms which regulate TJ organization during cell proliferation, migration and response to different cytokines and hormones. From these studies, it was concluded that TJs are regulated by Ca2+ levels (Griepp et al., 1983; Martínez-Palomo et al., 1980), cytokines and growth factors (Capaldo and Nusrat, 2009); and that they may be assembled from internalized or preexisting junctional components (Stamatovic et al., 2009; Andreeva et al., 2006; Bruewer et al., 2005), even in the absence of protein synthesis (Martínez-Palomo et al., 1980).
In contrast, studies about assembly and development of the TJ complex in stratified epithelia are not extensive. Evidence suggests that TJ assembly in stratified epithelia, such as epidermis, is regulated by the same basic principles described in simple epithelia; however, TJ components show a differential expression apparently related with other functions such as cell proliferation and differentiation besides their major role in the establishment of the epithelial barrier (Kirschner et al., 2012; O'Neill and Garrod, 2011). Thus far, our understanding of TJ regulation and its relation with cell differentiation is limited. Since corneal epithelium shows their TJ complexes in cell layers composed by terminally differentiated cells (Ban et al., 2003b; Yoshida et al., 2009), it is possible that their assembly could be coordinated or dependent on the expression of cell differentiation. Controlled cell permeabilization experiments which show that the loss of the most upper layer of the corneal surface elicits the synthesis and assembly of TJ components by underlying cells (Wolosin, 1988; Wang et al., 1993), together with data showing that exists a gradual expression of TJ components along the different layers of the corneal epithelium (Sugrue and Zieske, 1997), strongly support the existence of a strict mechanism that subordinates TJ development to the expression of terminal differentiation.
To further explore these links, we studied the expression of TJ components during proliferation and differentiation of the RCE1(5T5) corneal epithelial cell line. Morphological, molecular and physiological analysis showed that the epithelial sheet formed by RCE1(5T5) cells possessed TJs similar to those found in vivo. In these complexes, we detected the presence of cldn-1, -2, -4, cgn, ZO-1 and ocln. Although we did not carry out a specific search for other TJ components, we do not discard the presence of cldn-9, -14 and -15, which were detected in human and rat corneal epithelium (Yi et al., 2000; Ban et al., 2003a; Ban et al., 2003b; Elkouby-Naor and Ben-Yosef, 2010). Considering that most rabbit cldn sequences have not been reported, end point RT-PCR experiments were carried out by the use of primers designed to detect human or rat cldns (Table 1). Since primers used to amplify cldn-3 and -7 lead to detection of the corresponding PCR products in mouse kidney, the lack of detection of cldn-3 and -7 in our rabbit corneal epithelial cell line might be due to mispairing of the corresponding oligonucleotides. Nevertheless, species–specific differences cannot be overruled.
Table 1. Primer sequences used in semi-quantitative RT-PCR to assay the expression of tight junction components.
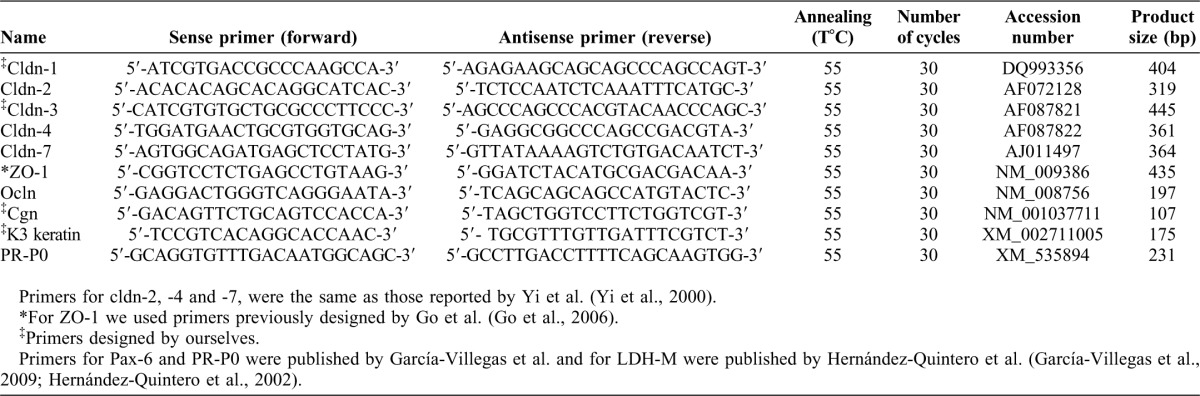
These junctional complexes were entirely functional, as demonstrated by the paracellular impermeability to tracers such as biotin or ruthenium red, as well as by the measurement of TER, which showed that RCE1(5T5) epithelia have a moderate barrier activity similar to that reported for primary and SV40-transformed human corneal epithelial cells (Yi et al., 2000); and contrasting with high barrier strength epithelia such as MDCK cells which show TER values in the 4000–6000 Ω/cm2 range (Anderson, 2001), or human epidermal keratinocytes which have a low to moderate barrier (Yuki et al., 2007).
When we studied the change on the expression of cldn-1, -2, -4, ZO-1, ocln and cgn during growth and differentiation of the RCE1(5T5) cells, to establish if TJ expression and assembly were coordinated with the differentiation process, we found that TJ components were differentially regulated during the development of the 3–5 layered epithelia. So, cldn-1, ocln and ZO-1 mRNAs and proteins, essential for TJ assembly and critical determinants of barrier formation (Anderson and Van Itallie, 2009; Fanning and Anderson, 2009), were present in cell cultures as early as the third day after plating when growing colonies have not constituted an epithelium and there were not clear evidences about TJ assembly (not shown). Later, as early as 7 day after plating, cultures showed the increase in TER and cldn-1 immunostaining at cell borders suggesting that TJ were assembled.
Surprisingly, cldn-2 and cgn were highly sensitive to attainment of cell confluence. Cgn mRNA and protein levels augmented once cells reached confluence and began to differentiate showing an expression pattern similar to the rise in TER and the expression of Pax-6. In this case, cgn protein was undetectable during the exponential growth phase; once cells reached confluence cgn augmented suggesting that its accumulation could be crucial for the assembly, and perhaps maintenance, of TJ. On the other hand, since cldn-2 is a protein forming pores in the TJ (Amasheh et al., 2002), the initial 2-fold increase at 2 days after confluence and the later decrease to basal levels may indicate that cldn-2 could be essential to turn on the assembly of TJ; afterwards its levels decreased possibly to stabilize epithelial barrier function. Unusually, cldn-4 protein increased up to 4-fold at the 4th day after confluence, while its mRNA levels remained stable, suggesting a complex regulation that should be given at different levels. Together, our results suggest that the expression and assembly of TJ components represent early events of the differentiation process. Nevertheless, our experiments did not reveal the events involved in the regulation of TJ assembly during differentiation. Further exploration should be oriented to establish which proteins could be involved. Possible candidates for such task could be ZO-2, which in concert with ZO-1, adducins and symplekin, regulate TJ organization (Chang et al., 2012; Fanning et al., 2012; Naydenov and Ivanov, 2010).
We also showed that effectors such as EGF and RA modulate the expression and assembly of TJ. While EGF prompted an augment on epithelial barrier strength, RA disrupted TJ in a concentration-dependent manner. The effect of RA seems to depend on the sensitivity and origin of the epithelial cell type tested. Although some authors have reported that RA increases epithelial barrier in cell types such as retinal pigmentary (Rong and Liu, 2011), intestinal (Osanai et al., 2007; Baltes et al., 2004) or mammary epithelia (Marshall et al., 2009), a major finding in this work is the reversible modulation of epithelial barrier integrity exerted by RA. It was reported that RA modulates corneal epithelial differentiation and proliferation in a concentration-dependent manner, both in vivo and in vitro (Kim et al., 2012; Kruse and Tseng, 1994; Stonecipher et al., 1988; Tseng et al., 1984); likewise RA inhibited the increase of TER when RCE1(5T5) cells grew in its presence. Interestingly, its addition to confluent epithelia disrupted the epithelial barrier until retinoid was removed from media. Such effect was partly associated to i) a marked decrease on cldn-2 and cgn expression; and ii) to a change in the assembly of TJ complexes as suggested by the alterations in cldn-1 immunostaining.
So, the results suggest that RA disrupts corneal epithelial barrier in part by decreasing the expression of some TJ components. However, the mechanism involved is still unknown. Since the expression of cldn-2, cgn and the differentiation-linked K3 keratin were reduced in the same extent, whereas cldn-1, cldn-4, ocln and ZO-1 did not undergo significant changes, it is possible that TJ integrity depends on the expression of another component(s) regulated by the differentiation state of cells. In view of the decrease in K3 keratin expression, without having a reduction in Pax-6 expression which seems to play an important role as a master gene in corneal epithelial differentiation (García-Villegas et al., 2009), the results provide support to the tight coordination between TJ assembly and the expression of terminal phenotype. In such sense, it is tempting to speculate the existence of common regulatory elements between the genes encoding differentiation markers and the genes encoding TJ components. Such possibility is supported by experimental data showing that cldn-1 (Dufresne and Cyr, 2007), cldn-19 (Luk et al., 2004), cldn-3 and other TJ components (Sze et al., 2008), are regulated by Sp1 and KLF4 transcription factors, which also seem to control corneal epithelial-specific differentiation (Swamynathan et al., 2007; Nakamura et al., 2005; Adhikary et al., 2005; Banks et al., 1999; Chen et al., 1997).
Conversely, TJ assembly could be dependent on cytoskeletal network and desmosomal organization, which may be altered by RA as previously shown (Kubilus et al., 1981; Kopan et al., 1987; Debal et al., 1997; Wanner et al., 1999; Hatakeyama et al., 2004). Supporting this alternative, examination of the cultured epithelia treated with 10 µM RA showed that epithelial barrier disruption was associated to increased intercellular spaces and a reduced desmosome number (supplementary material Fig. S1), suggesting that RA treatment also disrupted the epithelial barrier through the promotion of a looser epithelial structure.
In conclusion, we demonstrate that TJ components show differential responses to stimuli such as RA depending on the epithelial cell type. Whereas in corneal epithelial cells RA modifies the expression of cldn-2 and -4 without affecting cldn-1, in oral epithelia (Hatakeyama et al., 2010) and in human keratinocytes (Telgenhoff et al., 2008) the retinoids regulate cldn-1, -2 and -4 in a completely different manner. A fact that might be related with particular biological roles carried out by each epithelial cell type. Analysis of the response to retinoids in models such as corneal epithelial primary or RCE1(5T5) cells, should lead us to understand the tissue-specific regulation of TJ.
Materials and Methods
Materials
Fetal bovine serum (FBS), Eagle's medium modified by Dulbecco-Vögt (DMEM), the Ham-F12 nutrient mixture, the Trizol reagent and TO-PRO®-3 Iodide were from Invitrogen Life Technologies, Inc. (Gaithersburg, MD). The antibodies anti-claudin-1 (working dilution 1:200) (Cat no. 71-7800), anti-claudin-4 (working dilution 1:200) (cat. no. 32-400), anti-cingulin (working dilution 1:500) (cat. no. 36-4401), anti-human occludin-FITC (working dilution 1:50) (cat. no. 33-1511), anti-ZO-1 (working dilution 1:100) (cat. no. 61-7300), anti-mouse IgG-biotinylated (working dilution 1:1000) (cat. no. B2763) and anti-rabbit IgG biotinylated (working dilution 1:1000) (cat. no. B2770) were purchased from Zymed (San Francisco, CA). The mouse mAb raised against actin (working dilution 1:500) was kindly provided by Dr José Manuel Hernández (Department of Cell Biology, CINVESTAV-IPN). The Vectashield fluorescence mounting medium with propidium iodide, Streptavidin-FITC, Streptavidin-Texas Red and Streptavidin HRP (working dilution 1:1000 for the three conjugates) were from Vector Laboratories (Burlingame, CA.). All other reagents used were analytical grade.
Cell culture
The RCE1(5T5) rabbit corneal epithelial cell line was obtained earlier (Castro-Muñozledo, 1994). Cells were seeded and cultured in the presence of mitomycin C-treated 3T3 cells, as previously (Gómez-Flores et al., 2011). When necessary, primary corneal epithelial cells were obtained, plated and maintained as previously (Gómez-Flores et al., 2011). For experiments with retinoic acid (RA), RA was prepared in ethanol and added as indicated; the final concentration of ethanol in the culture medium never exceeded 0.1% (v/v). In this case, medium was changed under subdued light conditions. In all experiments, media were changed every 3 days; the cultures were maintained in a 10% CO2 and 90% air humidified atmosphere. All experiments were repeated at least four times in duplicate culture dishes or inserts.
Immunofluorescence staining
Cells were grown on 18×18-mm glass coverslips as described above. At different times in cell culture, they were fixed with 3.5% (w/v) para-formaldehyde in PBS, permeabilized with 0.05% (v/v) Triton X-100 in PBS, and stained with the indicated antibodies. F-actin staining was done by incubating the permeabilized cell cultures with rhodamine-phalloidin for 30 minutes before rinsing and mounting. Confocal analysis was carried out with a Leica high-speed confocal/multiphoton system (Model TCS SP2; Leica Microsystems, Wetzlar, Germany); both xyz and xzy serial optical sections 0.1–0.4 µm thick were taken. To prevent interference from the fluorescent probes, images of the same optical section were taken as separate channels between frames, and they were analyzed with LCS Leica Confocal Software TM v2.0 (Leica Microsystems). Nuclei were counterstained either with propidium iodide (red) or TO-PRO®-3 Iodide (blue).
RNA extraction, RT-PCR, and sequencing
Total RNA from cell cultures was isolated with Trizol reagent and stored at −70°C as an ethanol precipitate until further use. For the end point PCR assays, reverse transcription was carried out using 4.0 µg total RNA. Primers were designed according the published sequences for the mouse and human mRNAs, respectively (Table 1). Identity of amplicons was established by sequencing of the amplification products. Briefly, the amplification products were cloned into the pCR2.1 cloning vector using the TOPO®TA cloning kit (Invitrogen, Gaithersburg, MD) and then sequenced with both T7 and SP6 sequencing primers. Sequencing was carried out using an ABI PRISM™ 310 DNA Sequencer (PerkinElmer) and the Big Dye Terminator kit v3.1 (PerkinElmer). Data were compared with the published gene sequences using the BLASTN 2.2 search program (Altschul et al., 1997).
Semi-quantitative RT-PCR
After 3T3 cell removal with 0.01% (w/v) EDTA in PBS, total RNA was isolated from cell cultures at the indicated times, and the relative levels of expression of the mRNAs encoding cldns -1, -2, -4, ocln, ZO-1 and cgn were determined by semi-quantitative RT-PCR (Marone et al., 2001), using the primers and amplification conditions shown on Table 1. Expression of Pax-6 mRNA was used as a terminal differentiation marker of the corneal cells (García-Villegas et al., 2009) (Table 1). We used the acidic ribosomal phosphoprotein P0 (PR-P0) as an internal control to normalize for sample to sample variations in total RNA amounts and for reaction efficiency (Marone et al., 2001). PR-P0 was chosen as internal control since its mRNA levels do not change during growth and differentiation of corneal epithelial cells; the specific primers were previously reported (García-Villegas et al., 2009) (Table 1). Densitometric analysis and quantitation of specific mRNA expression was carried out as previously (Gómez-Flores et al., 2011). Mean and standard deviation of all experiments were calculated after normalization to PR-P0.
Transmission electron microscopy
For electron microscopy, epithelia were grown on plastic tissue culture dishes, as above. Samples were fixed for 60 minutes with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, and post-fixed for 60 minutes with 1% (w/v) osmium tetroxide in the same buffer. After dehydration with increasing concentrations of ethanol and propylene oxide, samples were embedded in epoxy resins and polymerized to 60°C for 24 hours. Thin sections (60 nm) were stained with uranyl acetate and lead citrate, and examined in a JEOL-JEM-1011 electron microscope. When ruthenium red (Mr = 786.35) was used as a tracer to localize TJ, cells were incubated with 0.2 mg/ml ruthenium red dissolved in 2.5% (v/v) glutaraldehyde- 0.1 M sodium cacodylate buffer pH 7.4 for 30 minutes. After repeated washes with cacodylate buffer, cultures were processed for electron microscopy studies as above.
Biotin tracer assay
The biotin tracer assay was carried out using the modified cell-surface biotinylation method described by Umeda et al. (Umeda et al., 2006). Briefly, the cultured cells were washed with 1 mM MgCl2+1 mM CaCl2 in PBS. Then, they were incubated with the same buffer containing 1 mg/ml EZ-Link sulfo-NHS-LC-biotin (Mr = 556.59) at room temperature; after 30 minutes, cells were washed with 1 mM MgCl2+1 mM CaCl2+100 mM glycine in PBS and fixed with methanol at −20°C for 3 minutes. Cells were then washed with PBS, blocked with 5% (w/v) BSA in PBS for 30 minutes, and incubated with anti-claudin-1 antibody (see Materials) for 2 hours. After rinsing with PBS, they were incubated for 30 minutes with Texas Red Streptavidin to detect biotin labeled proteins. Cldn-1 was immunodetected using the appropriate secondary antibody labeled with Alexa-Fluor 488.
Measurement of transepithelial electrical resistance (TER)
RCE1(5T5) cells were plated into Costar® 24-transwell, 4 µm polycarbonate cell culture inserts (Corning, Acton, MA). TER measurements were performed using an EVOM voltmeter (World Precision Instruments, Sarasota, FL, USA) according to the experimental protocol. In some experiments, TER was recorded every day beginning at the third day after plating; in others, TER was measured every day after cells reached confluence. TER was calculated by subtracting the resistance of a transwell insert plated with confluent 3T3 fibroblasts, and normalized by the area of the epithelium expressed in standard units of Ω/cm2.
Paracellular inulin flux measurement
The RCE(5T5) epithelia were plated into Costar® 24-transwell, 4 µm polycarbonate cell culture inserts. After cells reached confluence and organized into a 3–5 layered epithelium, the transwell inserts were incubated in the presence of 500 µg/ml FITC-inulin dissolved in medium containing or not RA, as indicated. Epithelia were incubated for 24 hours and media were collected form the upper and lower reservoirs. Medium containing 500 µg/ml FITC-inulin was used as a reference of the initial experimental conditions, and medium without FITC-inulin as blank. Fluorescence was measured (excitation at 485 nm and emission at 538 nm) using a Synergy HT Multi-Mode Microplate Reader (BioTEK Instruments, New Jersey, USA). The unidirectional flux of inulin into the apical compartment was calculated as a percentage of inulin administered into the upper compartment, per cm2 of surface area of the epithelial sheet.
Statistical analysis
All experiments were repeated at least four times in duplicate culture dishes or inserts. Statistical analysis was performed by one way ANOVA for multiple comparisons versus control groups, using Sigmaplot 11.0 (Systat Software Inc., Chicago, IL).
Supplementary Material
Acknowledgments
This work was supported in part by grants no. 101552 (to F.C.-M.) and 127557 (to J.V.) from the National Council of Science and Technology (CONACyT) of Mexico, and grant no. 138/2012 from the Institute of Science and Technology from Mexico City (ICyTDF) (to F.C.-M.). We are grateful to Biol. Lizbeth Salazar Villatoro for her technical assistance in preparation of electron microscopy material, to Ms Maria Elena Rojano for secretarial assistance, and Mrs Columba Guadarrama for her technical assistance in the preparation of all materials. M.T.O.-M. received a fellowship from CONACyT.
Footnotes
Competing interests: The authors have no competing interests to declare.
References
- Adhikary G., Crish J. F., Gopalakrishnan R., Bone F., Eckert R. L. (2005). Involucrin expression in the corneal epithelium: an essential role for Sp1 transcription factors. Invest. Ophthalmol. Vis. Sci. 46, 3109–3120 10.1167/iovs.05-0053 [DOI] [PubMed] [Google Scholar]
- Aggarwal S., Suzuki T., Taylor W. L., Bhargava A., Rao R. K. (2011). Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem. J. 433, 51–63 10.1042/BJ20100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho S., Lupo J., Coly P. A., Sabine A., Castellazzi M., Morand P., Sergeant A., Manet E., Boyer V., Gruffat H. (2009). Characterization of the ubinuclein protein as a new member of the nuclear and adhesion complex components (NACos). Biol. Cell 101, 319–334 10.1042/BC20080072 [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh S., Meiri N., Gitter A. H., Schöneberg T., Mankertz J., Schulzke J. D., Fromm M. (2002). Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115, 4969–4976 10.1242/jcs.00165 [DOI] [PubMed] [Google Scholar]
- Amasheh S., Fromm M., Günzel D. (2011). Claudins of intestine and nephron - a correlation of molecular tight junction structure and barrier function. Acta Physiol. (Oxf.) 201, 133–140 10.1111/j.1748-1716.2010.02148.x [DOI] [PubMed] [Google Scholar]
- Anderson J. M. (2001). Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 16, 126–130. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Van Itallie C. M. (2009). Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1, a002584 10.1101/cshperspect.a002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva A. Y., Piontek J., Blasig I. E., Utepbergenov D. I. (2006). Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int. J. Biochem. Cell Biol. 38, 222–233 10.1016/j.biocel.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Balda M. S., Matter K. (2008). Tight junctions at a glance. J. Cell Sci. 121, 3677–3682 10.1242/jcs.023887 [DOI] [PubMed] [Google Scholar]
- Balda M. S., Garrett M. D., Matter K. (2003). The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 160, 423–432 10.1083/jcb.200210020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes S., Nau H., Lampen A. (2004). All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev. Growth Differ. 46, 503–514 10.1111/j.1440-169x.2004.00765.x [DOI] [PubMed] [Google Scholar]
- Ban Y., Cooper L. J., Fullwood N. J., Nakamura T., Tsuzuki M., Koizumi N., Dota A., Mochida C., Kinoshita S. (2003a). Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp. Eye Res. 76, 735–743 10.1016/S0014-4835(03)00033-2 [DOI] [PubMed] [Google Scholar]
- Ban Y., Dota A., Cooper L. J., Fullwood N. J., Nakamura T., Tsuzuki M., Mochida C., Kinoshita S. (2003b). Tight junction-related protein expression and distribution in human corneal epithelium. Exp. Eye Res. 76, 663–669 10.1016/S0014-4835(03)00054-X [DOI] [PubMed] [Google Scholar]
- Banks E. B., Crish J. F., Eckert R. L. (1999). Transcription factor Sp1 activates involucrin promoter activity in non-epithelial cell types. Biochem. J. 337, 507–512 10.1042/0264-6021:3370507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y., Green H. (1987). Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-α and epidermal growth factor. Cell 50, 1131–1137 10.1016/0092-8674(87)90179-6 [DOI] [PubMed] [Google Scholar]
- Bruewer M., Utech M., Ivanov A. I., Hopkins A. M., Parkos C. A., Nusrat A. (2005). Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 19, 923–933 10.1096/fj.04-3260com [DOI] [PubMed] [Google Scholar]
- Buchert M., Darido C., Lagerqvist E., Sedello A., Cazevieille C., Buchholz F., Bourgaux J. F., Pannequin J., Joubert D., Hollande F. (2009). The symplekin/ZONAB complex inhibits intestinal cell differentiation by the repression of AML1/Runx1. Gastroenterology 137, 156–164, e1-e3 10.1053/j.gastro.2009.03.037 [DOI] [PubMed] [Google Scholar]
- Buzza M. S., Netzel-Arnett S., Shea-Donohue T., Zhao A., Lin C. Y., List K., Szabo R., Fasano A., Bugge T. H., Antalis T. M. (2010). Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc. Natl. Acad. Sci. USA 107, 4200–4205 10.1073/pnas.0903923107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C. T., Nusrat A. (2009). Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788, 864–871 10.1016/j.bbamem.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Muñozledo F. (1994). Development of a spontaneous permanent cell line of rabbit corneal epithelial cells that undergoes sequential stages of differentiation in cell culture. J. Cell Sci. 107, 2343–2351. [DOI] [PubMed] [Google Scholar]
- Castro-Muñozledo F. (2008). Corneal epithelial cell cultures as a tool for research, drug screening and testing. Exp. Eye Res. 86, 459–469 10.1016/j.exer.2007.11.017 [DOI] [PubMed] [Google Scholar]
- Cavey M., Lecuit T. (2009). Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1, a002998 10.1101/cshperspect.a002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. (1978). Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 77, 853–880 10.1083/jcb.77.3.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha D., O'Brien P., O'Toole E. A., Woodley D. T., Hudson L. G. (1996). Enhanced modulation of keratinocyte motility by transforming growth factor-α (TGF-α) relative to epidermal growth factor (EGF). J. Invest. Dermatol. 106, 590–597 10.1111/1523-1747.ep12345083 [DOI] [PubMed] [Google Scholar]
- Chang H., Zhang C., Cao Y. (2012). Expression and distribution of symplekin regulates the assembly and function of the epithelial tight junction. Histochem. Cell Biol. 137, 319–327 10.1007/s00418-011-0906-z [DOI] [PubMed] [Google Scholar]
- Chen T. T., Wu R. L., Castro-Munozledo F., Sun T. T. (1997). Regulation of K3 keratin gene transcription by Sp1 and AP-2 in differentiating rabbit corneal epithelial cells. Mol. Cell. Biol. 17, 3056–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H., Osanai M., Murata M., Kojima T., Sawada N. (2008). Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 1778, 588–600 10.1016/j.bbamem.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Debal V., Breillout F., Manfait M. (1997). Concomitant decrease of resistance and modifications of the cytoskeleton after all-trans retinoic acid and phorbol ester treatments in a navelbine-resistant bladder carcinoma cell line. Anticancer Res. 17, 1147–1154. [PubMed] [Google Scholar]
- Denker B. M., Nigam S. K. (1998). Molecular structure and assembly of the tight junction. Am. J. Physiol. 274, F1–F9. [DOI] [PubMed] [Google Scholar]
- Dufresne J., Cyr D. G. (2007). Activation of an SP binding site is crucial for the expression of claudin 1 in rat epididymal principal cells. Biol. Reprod. 76, 825–832 10.1095/biolreprod.106.057430 [DOI] [PubMed] [Google Scholar]
- Elkouby-Naor L., Ben-Yosef T. (2010). Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int. Rev. Cell Mol. Biol. 279, 1–32 10.1016/S1937-6448(10)79001-8 [DOI] [PubMed] [Google Scholar]
- Fanning A. S., Anderson J. M. (2009). Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 1165, 113–120 10.1111/j.1749-6632.2009.04440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A. S., Van Itallie C. M., Anderson J. M. (2012). Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 23, 577–590 10.1091/mbc.E11-09-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman G. J., Mullin J. M., Ryan M. P. (2005). Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 57, 883–917 10.1016/j.addr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Furuse M. (2010). Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2, a002907 10.1101/cshperspect.a002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Villegas R., Escamilla J., Sánchez-Guzmán E., Pastén A., Hernández-Quintero M., Gómez-Flores E., Castro-Muñozledo F. (2009). Pax-6 is expressed early in the differentiation of a corneal epithelial model system. J. Cell. Physiol. 220, 348–356 10.1002/jcp.21771 [DOI] [PubMed] [Google Scholar]
- Go M., Kojima T., Takano K., Murata M., Koizumi J., Kurose M., Kamekura R., Osanai M., Chiba H., Spray D. C. et al. (2006). Connexin 26 expression prevents down-regulation of barrier and fence functions of tight junctions by Na+/K+-ATPase inhibitor ouabain in human airway epithelial cell line Calu-3. Exp. Cell Res. 312, 3847–3856 10.1016/j.yexcr.2006.08.014 [DOI] [PubMed] [Google Scholar]
- Gómez-Flores E., Sánchez-Guzmán E., Castro-Muñozledo F. (2011). Asymmetrical cell division and differentiation are not dependent upon stratification in a corneal epithelial cell line. J. Cell. Physiol. 226, 700–709 10.1002/jcp.22380 [DOI] [PubMed] [Google Scholar]
- Griepp E. B., Dolan W. J., Robbins E. S., Sabatini D. D. (1983). Participation of plasma membrane proteins in the formation of tight junctions by cultured epithelial cells. J. Cell Biol. 96, 693–702 10.1083/jcb.96.3.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 10.1016/j.bbamem.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S., Hayashi S., Yoshida Y., Otsubo A., Yoshimoto K., Oikawa Y., Satoh M. (2004). Retinoic acid disintegrated desmosomes and hemidesmosomes in stratified oral keratinocytes. J. Oral Pathol. Med. 33, 622–628 10.1111/j.1600-0714.2004.00245.x [DOI] [PubMed] [Google Scholar]
- Hatakeyama S., Ishida K., Takeda Y. (2010). Changes in cell characteristics due to retinoic acid; specifically, a decrease in the expression of claudin-1 and increase in claudin-4 within tight junctions in stratified oral keratinocytes. J. Periodontal Res. 45, 207–215 10.1111/j.1600-0765.2009.01219.x [DOI] [PubMed] [Google Scholar]
- Hernández-Quintero M., García-Villegas R., Castro-Muñozledo F. (2002). Differentiation-dependent increases in lactate dehydrogenase activity and isoenzyme expression in rabbit corneal epithelial cells. Exp. Eye Res. 74, 71–82 10.1006/exer.2001.1110 [DOI] [PubMed] [Google Scholar]
- Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. (2005). Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 171, 939–945 10.1083/jcb.200510043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Nusrat A., Parkos C. A. (2005). Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays 27, 356–365 10.1002/bies.20203 [DOI] [PubMed] [Google Scholar]
- Jaramillo B. E., Ponce A., Moreno J., Betanzos A., Huerta M., Lopez-Bayghen E., Gonzalez-Mariscal L. (2004). Characterization of the tight junction protein ZO-2 localized at the nucleus of epithelial cells. Exp. Cell Res. 297, 247–258 10.1016/j.yexcr.2004.03.021 [DOI] [PubMed] [Google Scholar]
- Kavanagh E., Buchert M., Tsapara A., Choquet A., Balda M. S., Hollande F., Matter K. (2006). Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J. Cell Sci. 119, 5098–5105 10.1242/jcs.03297 [DOI] [PubMed] [Google Scholar]
- Kim S. W., Seo K. Y., Rhim T., Kim E. K. (2012). Effect of retinoic acid on epithelial differentiation and mucin expression in primary human corneal limbal epithelial cells. Curr. Eye Res. 37, 33–42 10.3109/02713683.2011.620728 [DOI] [PubMed] [Google Scholar]
- Kimura K., Teranishi S., Fukuda K., Kawamoto K., Nishida T. (2008). Delayed disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-α in a manner dependent on NF-κB. Invest. Ophthalmol. Vis. Sci. 49, 565–571 10.1167/iovs.07-0419 [DOI] [PubMed] [Google Scholar]
- Kimura K., Teranishi S., Nishida T. (2009). Interleukin-1β-induced disruption of barrier function in cultured human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 50, 597–603 10.1167/iovs.08-2606 [DOI] [PubMed] [Google Scholar]
- Kirschner N., Rosenthal R., Günzel D., Moll I., Brandner J. M. (2012). Tight junctions and differentiation – a chicken or the egg question? Exp. Dermatol. 21, 171–175 10.1111/j.1600-0625.2011.01431.x [DOI] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. (1987). Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J. Cell Biol. 105, 427–440 10.1083/jcb.105.1.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse F. E., Tseng S. C. (1994). Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest. Ophthalmol. Vis. Sci. 35, 2405–2420. [PubMed] [Google Scholar]
- Kubilus J., Rand R., Baden H. P. (1981). Effects of retinoic acid and other retinoids on the growth and differentiation of 3T3 supported human keratinocytes. In Vitro 17, 786–795 10.1007/BF02618445 [DOI] [PubMed] [Google Scholar]
- Luk J. M., Tong M. K., Mok B. W., Tam P. C., Yeung W. S., Lee K. F. (2004). Sp1 site is crucial for the mouse claudin-19 gene expression in the kidney cells. FEBS Lett. 578, 251–256 10.1016/j.febslet.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Marone M., Mozzetti S., De Ritis D., Pierelli L., Scambia G. (2001). Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol. Proced. Online 3, 19–25 10.1251/bpo20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. M., Pai V. P., Sartor M. A., Horseman N. D. (2009). In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res. 335, 383–395 10.1007/s00441-008-0719-0 [DOI] [PubMed] [Google Scholar]
- Martínez-Palomo A., Meza I., Beaty G., Cereijido M. (1980). Experimental modulation of occluding junctions in a cultured transporting epithelium. J. Cell Biol. 87, 736–745 10.1083/jcb.87.3.736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Balda M. S. (2007). Epithelial tight junctions, gene expression and nucleo-junctional interplay. J. Cell Sci. 120, 1505–1511 10.1242/jcs.005975 [DOI] [PubMed] [Google Scholar]
- McLaughlin B. J., Caldwell R. B., Sasaki Y., Wood T. O. (1985). Freeze-fracture quantitative comparison of rabbit corneal epithelial and endothelial membranes. Curr. Eye Res. 4, 951–962 10.3109/02713689509000002 [DOI] [PubMed] [Google Scholar]
- Meza I., Ibarra G., Sabanero M., Martínez-Palomo A., Cereijido M. (1980). Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J. Cell Biol. 87, 746–754 10.1083/jcb.87.3.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Ueda J., Sugar J., Yue B. Y. J. T. (2005). Developmentally regulated expression of Sp1 in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 46, 4092–4096 10.1167/iovs.05-0324 [DOI] [PubMed] [Google Scholar]
- Naydenov N. G., Ivanov A. I. (2010). Adducins regulate remodeling of apical junctions in human epithelial cells. Mol. Biol. Cell 21, 3506–3517 10.1091/mbc.E10-03-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Ericson L. E. (1995). Effects of epidermal growth factor and phorbol ester on thyroid epithelial integrity. Exp. Cell Res. 219, 626–639 10.1006/excr.1995.1273 [DOI] [PubMed] [Google Scholar]
- O'Neill C. A., Garrod D. (2011). Tight junction proteins and the epidermis. Exp. Dermatol. 20, 88–91 10.1111/j.1600-0625.2010.01206.x [DOI] [PubMed] [Google Scholar]
- Osanai M., Nishikiori N., Murata M., Chiba H., Kojima T., Sawada N. (2007). Cellular retinoic acid bioavailability determines epithelial integrity: role of retinoic acid receptor α agonists in colitis. Mol. Pharmacol. 71, 250–258 10.1124/mol.106.029579 [DOI] [PubMed] [Google Scholar]
- Paris L., Tonutti L., Vannini C., Bazzoni G. (2008). Structural organization of the tight junctions. Biochim. Biophys. Acta 1778, 646–659 10.1016/j.bbamem.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Peng L., Li Z. R., Green R. S., Holzman I. R., Lin J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck W. C., Edwards J. R., Lamar P. C., Smith C. S. (2006). Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol. In Vitro 20, 942–953 10.1016/j.tiv.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Pummi K., Malminen M., Aho H., Karvonen S. L., Peltonen J., Peltonen S. (2001). Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J. Invest. Dermatol. 117, 1050–1058 10.1046/j.0022-202x.2001.01493.x [DOI] [PubMed] [Google Scholar]
- Remue E., Meerschaert K., Oka T., Boucherie C., Vandekerckhove J., Sudol M., Gettemans J. (2010). TAZ interacts with zonula occludens-1 and -2 proteins in a PDZ-1 dependent manner. FEBS Lett. 584, 4175–4180 10.1016/j.febslet.2010.09.020 [DOI] [PubMed] [Google Scholar]
- Rong J., Liu S. (2011). Effect of all-trans retinoic acid on the barrier function in human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 407, 605–609 10.1016/j.bbrc.2011.03.080 [DOI] [PubMed] [Google Scholar]
- Sabath E., Denker B. M. (2006). Cell-cell interactions in the kidney: inducible expression of mutant G protein α-subunits in Madin-Darby canine kidney cells for studies of epithelial cell junction structure and function. Methods Mol. Biol. 341, 61–72 10.1385/1-59745-113-4:61 [DOI] [PubMed] [Google Scholar]
- Singh A. B., Sugimoto K., Dhawan P., Harris R. C. (2007). Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am. J. Physiol. Cell Physiol. 293, C1660–C1668 10.1152/ajpcell.00274.2007 [DOI] [PubMed] [Google Scholar]
- Stamatovic S. M., Keep R. F., Wang M. M., Jankovic I., Andjelkovic A. V. (2009). Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J. Biol. Chem. 284, 19053–19066 10.1074/jbc.M109.000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. R., Keon B. H. (1998). The tight junction: morphology to molecules. Annu. Rev. Cell Dev. Biol. 14, 89–109 10.1146/annurev.cellbio.14.1.89 [DOI] [PubMed] [Google Scholar]
- Stonecipher K. G., Jensen H. G., Rowsey J. J., Nordquist R. E. (1988). Topical application of all-trans-retinoic acid. A look at the cornea and limbus. Graefes Arch. Clin. Exp. Ophthalmol. 226, 371–376 10.1007/BF02172970 [DOI] [PubMed] [Google Scholar]
- Sugrue S. P., Zieske J. D. (1997). ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp. Eye Res. 64, 11–20 10.1006/exer.1996.0175 [DOI] [PubMed] [Google Scholar]
- Swamynathan S. K., Katz J. P., Kaestner K. H., Ashery-Padan R., Crawford M. A., Piatigorsky J. (2007). Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol. Cell. Biol. 27, 182–194 10.1128/MCB.00846-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze K. L., Lee W. M., Lui W. Y. (2008). Expression of CLMP, a novel tight junction protein, is mediated via the interaction of GATA with the Kruppel family proteins, KLF4 and Sp1, in mouse TM4 Sertoli cells. J. Cell. Physiol. 214, 334–344 10.1002/jcp.21201 [DOI] [PubMed] [Google Scholar]
- Tamariz E., Hernández-Quintero M., Sánchez-Guzman E., Argüello C., Castro-Muñozledo F. (2007). RCE1 corneal epithelial cell line: its variability on phenotype expression and differential response to growth factors. Arch. Med. Res. 38, 176–184 10.1016/j.arcmed.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Telgenhoff D., Ramsay S., Hilz S., Slusarewicz P., Shroot B. (2008). Claudin 2 mRNA and protein are present in human keratinocytes and may be regulated by all-trans-retinoic acid. Skin Pharmacol. Physiol. 21, 211–217 10.1159/000135637 [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Hatchell D., Tierney N., Huang A. J., Sun T. T. (1984). Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. J. Cell Biol. 99, 2279–2286 10.1083/jcb.99.6.2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Furuse M., Itoh M. (2001). Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- Turner J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M., Tsukita S. (2006). ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126, 741–754 10.1016/j.cell.2006.06.043 [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M. (2006). Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403–429 10.1146/annurev.physiol.68.040104.131404 [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Balda M. S., Anderson J. M. (1995). Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J. Cell Sci. 108, 1735–1742. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen M., Wolosin J. M. (1993). ZO-1 in corneal epithelium; stratal distribution and synthesis induction by outer cell removal. Exp. Eye Res. 57, 283–292 10.1006/exer.1993.1126 [DOI] [PubMed] [Google Scholar]
- Wanner R., Wolff B., Glowacki F., Kolde G., Wittig B. (1999). The loss of desmosomes after retinoic acid treatment results in an apparent inhibition of HaCaT keratinocyte differentiation. Arch. Dermatol. Res. 291, 346–353 10.1007/s004030050420 [DOI] [PubMed] [Google Scholar]
- Wolosin J. M. (1988). Regeneration of resistance and ion transport in rabbit corneal epithelium after induced surface cell exfoliation. J. Membr. Biol. 104, 45–55 10.1007/BF01871901 [DOI] [PubMed] [Google Scholar]
- Yi X., Wang Y., Yu F. S. (2000). Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest. Ophthalmol. Vis. Sci. 41, 4093–4100. [PubMed] [Google Scholar]
- Yoshida Y., Ban Y., Kinoshita S. (2009). Tight junction transmembrane protein claudin subtype expression and distribution in human corneal and conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 50, 2103–2108 10.1167/iovs.08-3046 [DOI] [PubMed] [Google Scholar]
- Yuki T., Haratake A., Koishikawa H., Morita K., Miyachi Y., Inoue S. (2007). Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp. Dermatol. 16, 324–330 10.1111/j.1600-0625.2006.00539.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.