Abstract
Adiponectin is an adipocyte-derived collectin that acts on a wide range of tissues including liver, brain, heart, and vascular endothelium. To date, little is known about the actions of adiponectin in the lung. Herein, we demonstrate that adiponectin is present in lung lining fluid and that adiponectin deficiency leads to increases in proinflammatory mediators and an emphysema-like phenotype in the mouse lung. Alveolar macrophages from adiponectin-deficient mice spontaneously display increased production of tumor necrosis factor-α (TNF-α) and matrix metalloproteinase (MMP-12) activity. Consistent with these observations, we found that pretreatment of alveolar macrophages with adiponectin leads to TNF-α and MMP-12 suppression. Together, our findings show that adiponectin leads to macrophage suppression in the lung and suggest that adiponectin-deficient states may contribute to the pathogenesis of inflammatory lung conditions such as emphysema.
Keywords: lung, matrix metalloproteinases, collectin
RESEARCH ON THE OBESITY EPIDEMIC has led to important discoveries regarding the functional role of adipose tissue. Adipose tissue is now considered an important secretory organ that regulates a wide range of biological processes (33, 53, 54). Collectively, factors released from adipose tissue are called adipokines; to date, many such molecules have been identified (33, 53). Deficiency in select adipokines leads to varied metabolic derangements and susceptibility to disease, including diabetes, hypertension, and coronary artery disease (20, 22, 24).
Adipose tissue is present throughout the body; however, the relationship between adipose tissue and the lung is poorly understood. Epidemiological data support a relationship between these tissues (4, 5, 10). In these studies, extreme weight loss or gain has been associated with the development of emphysema and asthma, respectively. Further experimental studies have shown that leptin, a molecule derived from adipocytes, regulates surfactant production in type II pneumocytes and protects the lung from acute injury (2, 14).
Adiponectin is a 30-kDa molecule secreted from adipocytes and found at high levels in plasma (16, 27, 35, 43). This factor was initially described as an insulin-sensitizing and an anti-atherogenic agent, but recent findings demonstrate it has potent anti-inflammatory activity (1, 16, 29, 50, 57). Adiponectin's anti-inflammatory actions are mediated, in part, by downregulation of innate immune responses (30, 39). Consistent with this notion, adiponectin receptors are expressed abundantly on macrophages and monocytes. Activation of adiponectin receptors leads to transcriptional downregulation of NF-κB target genes in these cells (7, 32, 50, 57). Adiponectin also limits the response of immune cells to activation by proinflammatory stimuli such as LPS (49). It is currently believed that the anti-inflammatory properties of adiponectin mediate its cardiac and vascular protective effects (39–41).
To date, little is known about the role of adiponectin in the lung; however, recent findings suggest an important role for this protein in lung biological processes. For example, adiponectin receptors are expressed in the murine lung at key developmental time points, and exogenous administration of adiponectin protects adult mice from antigen-induced asthma-like bronchial hyperresponsiveness (42, 60). Of interest, cigarette smoke has been shown to result in a dose-dependent decline in human serum adiponectin levels (48).
In this study, we set out to further investigate the functional role of adiponectin in the lung using mice that are adiponectin deficient by target gene disruption. Our findings show that adiponectin is present at high concentrations in bronchoalveolar lavage (BAL) fluid of wild-type mice and that complete or partial deficiency leads to structure changes in the lung characteristic of an emphysema-like phenotype. Proinflammatory cytokines and matrix metalloproteinases (MMPs) are increased in the lung of adiponectin−/− mice, and in vitro studies demonstrate that adiponectin acts to suppress alveolar macrophage activation and the release of MMPs. Together, our findings indicate that adiponectin plays an important role in lung immune cell homeostasis and suggest that adiponectindeficient states may contribute to the pathogenesis of inflammatory lung conditions.
METHODS
Mice
Adiponectin−/− mice were generated by targeted gene disruption as previously described (28). Age-matched wild-type C57/BL6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used for generating heterozygotes(+/−). Mice were maintained in a 12-h light, 12-h dark schedule and given food and water ad libitum. Body mass was recorded in all time points and is shown in Supplemental Fig. 1 (supplemental data for this article is available online at the AJP-Lung web site.) Euthanasia was performed by isoflurane anesthesia followed by cervical dislocation. All animal studies were conducted according to protocols approved by the National Institutes of Health and the Boston University Institutional Animal Care and Use Committee.
Lung fixation
Lungs from 6-day-old, 18-day-old, and 3-mo-old mice were analyzed. Fixation was performed using a blunt 22-gauge needle inserted into the trachea, and lungs were inflated at a set pressure of 25 cm of water using 4% paraformaldehyde. Tissue was dissected en bloc from the thoracic cavity and fixed overnight at 4°C. The following morning, each lobe was separated, and a 5-mm-thick slice was cut from the left lung and the right upper lobe. Horizontal slices were taken from the left lung, and the right lung was sliced vertically. Each slice, as well as the remaining lung tissue, was dehydrated in graded alcohols and embedded in paraffin blocks. Horizontal and vertical slices were oriented in a similar fashion in paraffin wax. Five-micrometer sections were cut from paraffin blocks, placed on charged slides, and stained with hematoxylin and eosin.
Morphometry and volume measurements
Alveolar size was estimated by using an automated computer-assisted method and by mean linear intercept (MLI) calculations. Automated measurements of air space diameter were performed per published algorithm (31) while MLI was calculated using methods modified from published protocols (15, 31, 51, 52). In brief, lung images were captured using a Zeiss digital camera with a ×20 objective. Alveolar walls from the images were segmented using the watershed transform, and the equivalent diameter of air spaces was calculated as the diameter of a circle of the same area as that of the air space. While the segmentation is fully automatic, large airway and vascular structures were excluded manually before analysis of the diameters. For MLI, the image analysis was performed using Zeiss Axiovision software. MLI was calculated by placing a grid containing five horizontal and five vertical lines (the length of each line was 500 μm) in areas devoid of large airways and muscular blood vessels. The number of alveolar intercepts across each line was counted, and the total length of line was divided by the average number of intercepts. Two images were taken of five random sections from the right and left lung. Images and grid placement were performed in blinded fashion. For each time point, five to six animals were analyzed in each group. Comparisons were made between similar (same lobe) and dissimilar (different lobes) regions of the lung. Results were expressed as means plus SE (n = number of mice per group). Increases in MLI signify an increase in the average distance between alveolar walls, whereas increases in equivalent diameter represent alveolar air space enlargement characteristic of emphysema. The latter, being independent of shape, has been shown to be a more robust index of air space size (31). Lung volumes were estimated by volume displacement measurements per published protocols (45, 59).
Respiratory mechanics
Tissue elastance was measured in anesthetized and tracheostomized mice that were mechanically ventilated (Flexivent; SCIREQ, Montreal, Canada) in the supine position. Lung volume changes were measured when the positive end-expiratory pressure was increased from 5 to 25 cmH2O, and respiratory system elastance was obtained as 20 cmH2O normalized by the corresponding volume increase. These measurements were obtained only at 3 mo of age.
BAL
Lung lining fluid was obtained by BAL using standard techniques (47). Briefly, the trachea was cannulated with a 22-gauge needle and sutured in place. Lungs were lavaged with 1 ml of ice-cold saline plus EDTA (0.5 mM). For cytospins, BAL fluid (BALF) was first centrifuged at 300 g for 5 min at 4°C. Pellets were resuspended in 300 μl of PBS containing 0.5% fetal bovine serum and cytospun onto charged slides. Differential cell counts were performed after staining with Diff-Quik. Two-hundred cells were counted on each slide, and two slides were analyzed from each animal. Differential cell counts were compared between age-matched wild-type and adiponectin−/− mice (n = 5, each group).
Urea assay
The absolute concentration of proteins in airway lining fluid was determined by using the ratio of urea concentration in BALF to that in serum. For these studies, serum and BAL urea concentrations were measured by using a modified enzymatic assay (Urea Assay Kit, Bioassay System) as previously reported (6, 55).
Real-time reverse transcriptase-PCR
Quantitative expression was performed for MMP-2, MMP-9, MMP-12, and adiponectin transcripts. All reactions were performed with 1 μg of starting total RNA. Values were calculated based on the ΔΔCT method. For MMP genes, total RNA was isolated from wild-type and adiponectin−/− lungs at 6 days, 18 days, and 3 mo of age (n = 5, each group). Adiponectin was measured in 3-mo-old control lung (n = 2), and white adipose tissue was obtained from the inguinal fat pad (n = 3). 18S or β-actin was used to control for total mRNA in starting material.
Qualitative PCR
RNA was isolated from BAL cells obtained from wild-type and adiponectin−/− mice by using RNeasy mini kit (Qiagen, Valencia, CA). cDNA was generated from RNA extracts by using a reverse transcription kit (Promega, Madison, WI). PCR was performed using the following primers: adiponectin receptor 1 (APN-R1) primers 5′-GAATCTTGACGATGCTGAGAC-3′ and 5′-GAAGGTTGGACACACCATAGA-3′, adiponectin receptor 2 (APN-R2) primers 5′-ATCCCTCACGATGTGCTACC-3′ and 5′-CACTGAGAGACGATAATGGCTG-3′, β-actin primers 5′-GCTCGTTGCCAATAGTGATG-3′ and 5′-AAGAGAGGTATCCTGACCCT-3′. Cycling conditions were 94°C for 1 min, 51°C for 1 min, and 72°C for 1 min, 35 cycles for APN-R1; 94°C for 1 min, 52°C for 1 min, and 72°C for 1 min, 35 cycles for APN-R2, and 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, 35 cycles for β-actin.
ELISA
TNF-α measurements were performed on lung lysate supernatants and supernatants of cultured cells. In cultured cells, basal level of secretion was determined by measuring TNF-α concentration in cell supernatant after culture for 18 h in serum-free media (SFM). The influence of adiponectin on basal levels of TNF-α was determined by culturing cells overnight in the presence or absence of adiponectin (30 μg/ml). To determine adiponectin's anti-inflammatory affects, cells pretreated with adiponectin or vehicle were washed and then incubated with SFM containing 100 ng/ml LPS (Escherichia coli, Sigma, St. Louis, MO) for 6 h. TNF-α concentration was measured in supernatant fractions. All groups were performed in triplicate. ELISA for TNF-α was performed using a commercially available kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Serum and BAL adiponectin levels were determined with adiponectin ELISA kits (Otsuka Pharmaceutical, London, England).
Immunocytochemistry
Alveolar macrophages were cytospun onto charged slides, fixed with acetic acid/methanol (1:3) for 5 min, and washed in PBS. Immunostaining for MMP-12 was done using an immunofluorescent detection method. Before staining, autofluorescence was quenched with sodium borohydride. Fluorescent staining was carried out using a polyclonal rabbit anti-murine MMP-12 (Santa Cruz Biotech, Santa Cruz, CA) at dilution of 1:500. Secondary staining was performed using a FITC-conjugated goat anti-rabbit antibody (Santa Cruz Biotech) at dilution of 1:100. In parallel, isotype staining was performed using rabbit serum for the primary antibody. Upon completion of staining, slides were washed in PBS and counterstained using the nuclear dye 4′,6′-diamidino-2-phenylindole dihydrochloride (Sigma-Aldrich, St. Louis, MO). The percentage of positive cells was determined by averaging values obtained from two independent investigators who counted 100 cells at ×400 magnification.
Recombinant mouse adiponectin
Mouse adiponectin was prepared from E. coli as described previously (41).
Fluorometric assay
For these studies, cells were prepared as previously described above (ELISA). MMP-12 activity was measured using the Enzolyte 490 MMP-12 Assay Kit (Anaspec, San Jose, CA) per company protocol. MMP-12 activity leads to peptide cleavage and the release of EDANS (5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid) molecule from the DabcylPlus quenching agent. Assays were performed in triplicate in black 96-well plates according to company protocol. Solutions were incubated in the dark for 30 min. End point reading was performed using a fluorimeter set at excitation 340 ± 20 nm and emission 490 ± 30 nm. Substrate solution alone was used to calculate background fluorescence, and this value was subtracted from all samples.
Statistical analysis
Data are presented as means ± SE. For MLI and equivalent diameter, statistical analysis was performed by twoway ANOVA and Tukey-Kramer post hoc test of the means. A two-tailed unpaired Student's t-test was used for real-time PCR and in vitro assays. For all studies, a value of P < 0.05 was accepted as statistically significant.
RESULTS
Adiponectin deficiency leads to development of emphysema-like phenotype
To investigate the functional role of endogenous adiponectin in the lung, histological examination of wild-type and adiponectin−/− mice was performed. While published reports indicate that adiponectin−/− mice do not display a phenotype under nonstressed conditions, an obvious phenotype was observed in lungs of 3-mo-old mice (1, 39–41, 49). Most notably, dilated air spaces were seen in the distal lung architecture (Fig. 1A). To quantify structural differences, MLI and equivalent diameters were compared in wild-type and adiponectin−/− mice. Consistent with an emphysema phenotype, MLI and equivalent diameter were significantly increased in adiponectin−/− mice (P < 0.0001, Fig. 1B and Supplemental Fig. 2). Furthermore, the coefficient of variation of equivalent diameters were significantly larger in the adiponectin−/− mice than in control mice with values of 56% and 49%, respectively, independent of age (P < 0.003). In support of these findings, decreased tissue elastance and increased lung displacement volume were also observed in adiponectin−/− mice (Fig. 1, C and D).
Fig. 1.
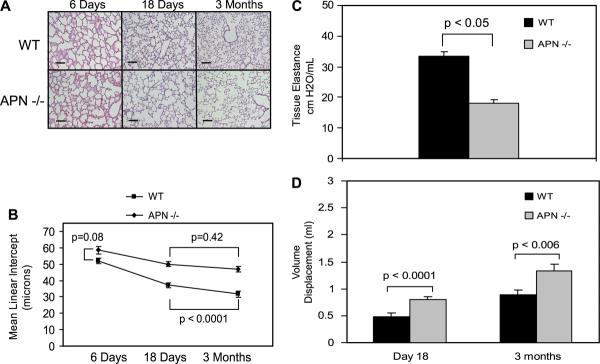
Emphysema-like phenotype develops in adiponectin−/− mice. A: histological examination of wild-type (WT) and adiponectin−/− (APN−/−) lungs at postnatal day 6, 18, and 3 mo of age (n = 5 in all groups except at 3 mo where n = 6). Wild-type and adiponectin−/− lungs are indistinguishable at postnatal day 6. Dilated air spaces consistent with emphysema-like phenotype are evident by postnatal day 18; however, findings are not as pronounced as those seen at 3 mo. Solid black line represents 100 μm. B: consistent with an emphysema phenotype, mean linear intercept (MLI) values are increased in adiponectin−/− lungs at 18 days and 3 mo of age (P < 0.0001). MLI measurements are not statistically different between wild-type and adiponectin−/− lungs 6 days after birth (P = 0.08). C: lung elastance is significantly decreased in adiponectin−/− lungs at 3 mo. D: lung volume, measured by volume displacement, is significantly increased in adiponectin−/− mice at 18 days and 3 mo of age.
To determine the relative onset of structural changes, histological and morphological comparison of aged-matched wild-type and adiponectin−/− lungs was performed. Results show that wild-type and adiponectin−/− lungs were indistinguishable 6 days after birth. In contrast, dilated air spaces were evident in the postnatal day 18 adiponectin−/− lung; however, findings were less pronounced than those observed at 3 mo. In wild-type mice, the decrease in MLI and equivalent diameters is a result of alveolarization (Fig. 1B and Supplemental Fig. 2); however, decreases in alveolar diameter were not observed in adiponectin−/− mice from 18 days to 3 mo of age. Together, these findings indicate that the emphysema-like phenotype in adiponectin−/− mice develops after birth but begins during the period of postnatal lung growth and development.
Adiponectin is present in BALF
Dilated air spaces in adiponectin−/− mice led us investigate whether adiponectin is present in lung lining fluid. To test this, adiponectin protein concentration was measured in lavage fluid by ELISA. To control for dilutional effects of saline, BAL values were adjusted based on BALF-to-serum urea ratios. In BALF, adiponectin was detected at a concentration of 2.7 ± 1.4 μg/ml, a value that was one-eighth the level of adiponectin in serum (Table 1). As expected, adiponectin was not detected in serum or BALF of adiponectin−/− mice.
Table 1.
Adiponectin is present in bronchoalveolar lavage fluid
| BAL, μg/ml | Serum, μg/ml | |
|---|---|---|
| Wild type | 2.70±1.36 | 18.46±5.91 |
| Adiponectin+/− | 0.839±0.23 | 8.5±0.99 |
| Adiponectin−/− | N. D. | N. D. |
Adiponectin protein concentrations were measured in serum and bronchoalveolar lavage fluid (BALF) by ELISA. Protein concentrations in BALF were one-eighth of serum values (n = 5). Similar lung-to-serum ratios were observed in heterozygous mice (n = 5), although serum and BALF concentrations were significantly lower in these mice. As expected, adiponectin was not detected in BALF or serum of adiponectin−/− mice (n = 2). The numbers reported represent the means and SE. N.D., not detected.
To determine whether adiponectin is locally produced in lung, adiponectin mRNA levels were measured by quantitative RT-PCR. White adipose tissue from the inguinal fat pad served as a positive control. As shown in Supplemental Fig. 3, negligible quantities of adiponectin mRNA are present in lung. In contrast, adiponectin mRNA was abundantly expressed in adipose tissue.
Relative deficiency of adiponectin leads to an emphysema-like phenotype
Hypoadiponectinemia, not adiponectin deficiency, is common in the obese population. To examine the influence of hypoadiponectinemia, adiponectin levels in lungs from adiponectin+/− mice gene were analyzed. Consistent with a hypoadiponectin state, serum and BALF adiponectin concentrations were reduced by greater than 50% in these mice (Table 1). Interestingly, we found that relative deficiency in adiponectin was also associated with development of the emphysema-like phenotype (Fig. 2). Histological and morphological analysis revealed dilated air spaces and increased lung displacement volumes. These results suggest a role for adiponectin in maintaining the structural integrity of the lung.
Fig. 2.
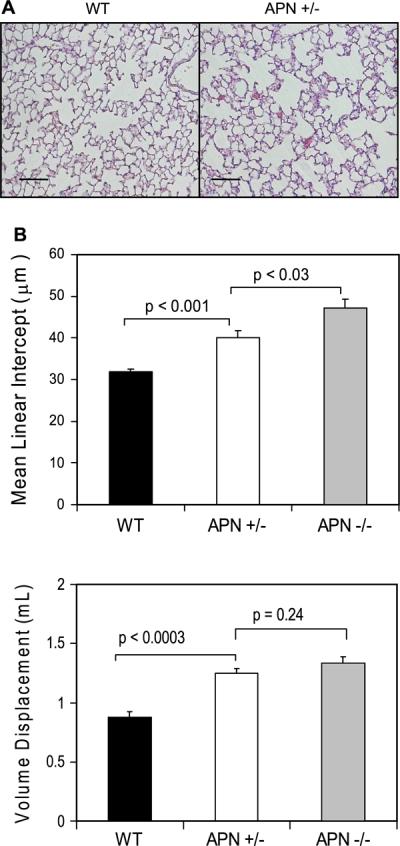
Three-month-old mice with a single functional adiponectin gene also develop histological and morphological evidence of emphysema. A: dilated air spaces were evident histologically in adiponectin+/− mice. Solid black line represents 100 μm. B: MLI and lung volumes were increased in adiponectin+/− mice.
MMPs are increased in the lungs of adiponectin-deficient mice
Emphysema is characterized by increases in proinflammatory cytokines (e.g., TNF-α) and MMP activity in the lung (38). In view of these findings, we measured TNF-α expression in lung lysate supernatants from wild-type and adiponectin−/− mice. Consistent with an inflamed state, TNF-α level was increased fourfold in adiponectin−/− lungs (Fig. 3).
Fig. 3.
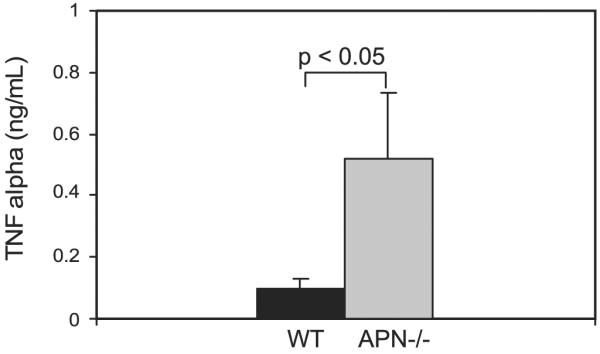
TNF-α levels are increased in adiponectin−/− lungs. By ELISA, a 4-fold increase in TNF-α levels is detected in the lung digest supernatant from 3-mo-old adiponectin−/− mice (n = 5, each group). The numbers reported represent the means and the SE.
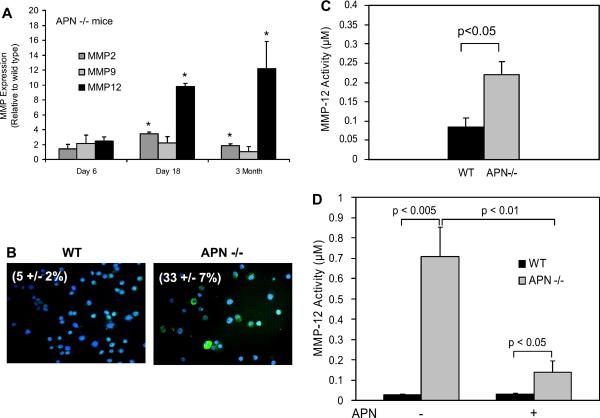
To investigate lung MMP gene expression, quantitative RT-PCR was performed on total lung RNA extracted from age-matched wild-type and adiponectin−/− mice. Figure 4A demonstrates that MMP-2 and MMP-12, but not MMP-9, expression was significantly increased in lungs of 18-day-old and 3-mo-old adiponectin−/− mice. MMP levels were not significantly increased in lungs of newborn (day 6) adiponectin−/− mice. Of interest, levels of the macrophage-specific MMP-12 transcript were markedly increased in adiponectin−/− mice. This finding was corroborated by MMP-12 immunostaining of alveolar macrophages in cytospins (Fig. 4B). We further confirmed these findings by assessing MMP-12 activity in supernatant fractions of alveolar macrophages from wild-type and adiponectin−/− mice that were maintained for 18 h in SFM (Fig. 4C). Notably, macrophages from adiponectin−/− mice produced MMP-12-related activity that was 2.5-fold greater than wild-type cells. These results suggest that adiponectin may exert a suppressive effect on the general state of alveolar macrophage inflammatory activity.
Fig. 4.
Matrix metalloproteinases are increased in lungs of adiponectin−/− mice. A: quantitative PCR performed for select MMPs (−2, −9, and −12) showed that MMP-2 and MMP-12 are significantly increased (*P < 0.05) in lungs of day 18 and 3-mo-old adiponectin−/− mice (n = 4). MMP levels in wild-type and adiponectin−/− mice are similar at postnatal day 6. B: MMP-12 protein expression was assessed by immunostaining alveolar macrophages that were cytospun onto glass slides. Increased number of cells expressing MMP-12 is found in alveolar macrophages from 3-mo-old adiponectin−/− mice (33 ± 7% vs. 5 ± 2% in age-matched wild-type). Counts were performed by 2 independent observers (n = 3, each group). C: MMP-12 activity is 2.5-fold greater in media (18-h incubation) of alveolar macrophages from adiponectin−/− mice (n = 3, each group). D: pretreatment with adiponectin (30 μg/ml) for 12 h decreased baseline MMP-12 activity in alveolar macrophages from adiponectin−/− mice (n = 3). MMP-12 activity was measured 6 h after removal of adiponectin. The numbers reported represent the means and the SE.
Adiponectin suppresses alveolar macrophage activation
To confirm that differential adiponectin responsiveness was not due to the absence of adiponectin receptors, RT-PCR analysis demonstrated that isolated alveolar macrophages express both adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2) (data not shown). To investigate whether adiponectin inhibits macrophage activation, cells from wild-type and adiponectin−/− mice were cultured overnight in the presence or absence of adiponectin. The state of macrophage activation was determined by measuring MMP-12 activity in media 6 h after removal of adiponectin. As shown in Fig. 4D, pretreatment with adiponectin resulted in fivefold reduction in MMP-12 activity in adiponectin−/− cells. MMP-12 activity was low in media of wild-type cells at baseline and after pretreatment with adiponectin.
To examine whether endogenous adiponectin suppresses macrophage production of other proinflammatory cytokines, we compared the relative accumulation of TNF-α in media from cultured wild-type and adiponectin−/− BALF cells. We found that TNF-α levels were 80-fold higher in media from adiponectin−/− cells (Fig. 5, left bars).
Fig. 5.
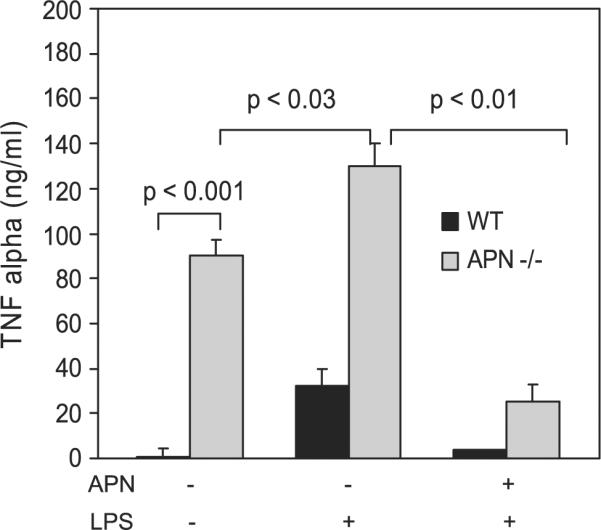
Adiponectin inhibits LPS-mediated release of the proinflammatory mediator TNF-α by alveolar macrophages. Alveolar macrophages were isolated from the bronchoalveolar lavage fluid of wild-type and adiponectin−/− mice and cultured in serum-free conditions in the presence or absence of adiponectin (30 μg/ml) and LPS (100 ng/ml). Baseline TNF-α levels were 80-fold higher in supernatant of cells obtained from adiponectin−/− mice. Pretreatment with adiponectin blocked LPS-induced TNF-α production in wild-type and adiponectin−/− cells (n = 3). The numbers reported represent the means and the SE.
To extend these findings, we investigated the ability of adiponectin to suppress responses to proinflammatory stimuli. For this, wild-type and adiponectin−/− BALF cells were cultured overnight in the presence or absence of adiponectin and then exposed to a lipopolysaccharide (100 ng/ml) challenge for 6 h. Pretreatment with adiponectin blocked LPS-induced TNF-α production and reduced levels by 3.7- and 5.7-fold in wild-type and adiponectin−/− cells, respectively (Fig. 5, middle and right bars).
DISCUSSION
We demonstrated that adiponectin, an adipocyte-derived collectin, was present in BALF at a concentration that was approximately one-eighth of serum levels. Since negligible quantities of adiponectin mRNA were detected in the lung, we propose that adiponectin primarily originates from extrapulmonary sources. However, physiologically relevant levels of adiponectin may be produced by select lung cell types, including the adipocytes that surround central airways and blood vessels in the lung. Based on high serum concentrations, it is likely that the majority of adiponectin enters the alveolar space from the pulmonary circulation. This is similar to the supply of α1-antitrypsin, which is primarily produced in the liver but found at much higher concentrations in blood (26, 44).
The main finding of this study is that adiponectin deficiency leads to immune cell activation and an emphysema-like phenotype. Importantly, dilated air spaces and increased MMP levels were not evident in newborn adiponectin−/− mice. These findings suggest that the development of emphysema in adiponectin−/− mice results, in part, from postnatal inflammatory cell activation. This is further supported by the observation that adiponectin suppresses the proinflammatory effects of LPS, which is ubiquitous in both mouse facility and natural environments.
It is noteworthy that MMP levels were found to be increased as early as 18 days postnatal in lungs of adiponectin−/− mice. This early postnatal period is characterized by rapid lung growth and extensive distal alveolarization. MMP levels are tightly regulated during this time point (12). We speculate that dysregulated MMP activity during this time point contributes to the emphysema-like phenotype of adiponectin−/− mice. It is also possible that adiponectin has a direct functional role in the remodeling events associated with postnatal alveolarization. This speculation will need to be addressed by further study.
It has been suggested that increases in apoptotic cell death contribute to the emphysema phenotype (11). In this context, it is important to note that adiponectin deficiency leads to an impaired clearance of apoptotic cell debris (49). However, in our studies, we found that the number of TUNEL-positive cells did not differ between wild-type and adiponectin−/− lungs at 6 days, 18 days, and 3 mo of age (Supplemental data 4). We also recognize that adiponectin may have important anti-inflammatory actions on other cells in the lung. For example, recent reports indicate that adiponectin protects endothelial cells from vascular injury.
Our findings show that adiponectin inhibits alveolar macrophage activation. Alveolar macrophages are the predominant inflammatory cell type in lung lining fluid of wild-type and adiponectin-deficient mice (Supplemental Fig. 5). These cells act as the first line of defense to particulate and infectious environmental insults, and toxic environmental exposures such as cigarette smoke lead to macrophage activation and release of proinflammatory mediators (e.g., TNF-α, MMP-12) (25, 38). Other investigators found that increased TNF-α and MMP-12 activity is directly linked to the pathogenesis of emphysema (8, 9, 15). Of interest, recent findings indicate that cigarette smoke leads to a dose-dependent decline in serum adiponectin levels (48).
The increase in MMP-2 may also play a role in the development of the physiological phenotype described herein. MMP-2 is known to cleave fibrillar collagen (13). In view of this, during alveolarization the most important stress-bearing element of the parenchyma may become weak and prone to mechanical failure. Ultimately, this may lead to rupture of alveolar walls and the subsequent coalescence of nearby alveoli, thus contributing to the increased terminal air space size and the reduced lung elasticity (23, 46).
Adiponectin is structurally and functionally related to the collectin family of proteins (49). Our findings indicate that adiponectin acts like other lung collectin proteins to maintain alveolar macrophages in a “quiescent state,” protecting the lung from dysregulated macrophage activation and subsequent tissue destruction (49). Surfactant protein D (SP-D) is a collectin family member that similarly suppresses macrophage activation. Interestingly, SP-D-deficient mice also display an emphysema phenotype, but in contrast to adiponectin−/− mice, this phenotype develops later in the postnatal period and is associated with peribronchiolar infiltrates and macrophage lipid accumulation (3, 56, 58). Together, these findings suggest that different collectin family members have overlapping, but distinct, activities in the lung.
We also reported that partial adiponectin deficiency leads to the development of an intermediate emphysema-like pheno-type (Fig. 2). Because adiponectin levels are known to be lower in obese humans, these findings suggest that obesity might be a risk factor for development of dysregulated immune cell activation and emphysema (16). Epidemiological studies have yet to link obesity to emphysema; however, observational and experimental studies support such a relationship. For example, dilated air spaces and diminished gas-exchange surface have been reported in rats made obese from high-calorie diets, and low lung diffusion capacity has been described in obesity subjects (17–19, 34). Furthermore, emphysema has also been reported in humans after extreme weight loss, and in rats, hamsters, and mice after severe calorie restriction (10, 21, 36, 37). Together, these findings suggest that adipocyte dysfunction resulting from extreme weight loss or gain may lead to the development of abnormal lung phenotypes.
In conclusion, we showed that a key signaling axis exists between adipose tissue and the lung. Our data demonstrate that the adipocyte-derived collectin adiponectin regulates lung homeostatic mechanisms by limiting local inflammation and by suppressing alveolar macrophage activation. These findings provide new insight into lung macrophage biology and suggest that adiponectin-deficient states may contribute to the development of inflammatory diseases of the lung.
Supplementary Material
Acknowledgments
GRANTS This work was supported by National Institutes of Health Grants K08-HL-077138, K08-HL-071607, AG-15052, and HL-59215.
REFERENCES
- 1.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 2.Bergen HT, Cherlet TC, Manuel P, Scott JE. Identification of leptin receptors in lung and isolated fetal type II cells. Am J Respir Cell Mol Biol. 2002;27:71–77. doi: 10.1165/ajrcmb.27.1.4540. [DOI] [PubMed] [Google Scholar]
- 3.Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, Hawgood S. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA. 1998;95:11869–11874. doi: 10.1073/pnas.95.20.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130:890–895. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol. 2002;155:191–197. doi: 10.1093/aje/155.3.191. [DOI] [PubMed] [Google Scholar]
- 6.Chinard FP. Quantitative assessment of epithelial lining fluid in the lung. Am J Physiol Lung Cell Mol Physiol. 1992;263:L617–L618. doi: 10.1152/ajplung.1992.263.6.L617. [DOI] [PubMed] [Google Scholar]
- 7.Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 8.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166:849–854. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- 9.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2004;170:492–498. doi: 10.1164/rccm.200404-511OC. [DOI] [PubMed] [Google Scholar]
- 10.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med. 2004;170:748–752. doi: 10.1164/rccm.200405-651OC. [DOI] [PubMed] [Google Scholar]
- 11.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7:53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueders MM, Foidart JM, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533:133–144. doi: 10.1016/j.ejphar.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 14.Gultekin FA, Kerem M, Tatlicioglu E, Aricioglu A, Unsal C, Bukan N. Leptin treatment ameliorates acute lung injury in rats with cerulein-induced acute pancreatitis. World J Gastroenterol. 2007;13:2932–2938. doi: 10.3748/wjg.v13.i21.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 17.Inselma LS, Milanese A, Deurloo A. Effect of obesity on pulmonary function in children. Pediatr Pulmonol. 1993;16:130–137. doi: 10.1002/ppul.1950160209. [DOI] [PubMed] [Google Scholar]
- 18.Inselman LS, Padilla-Burgos LB, Teichberg S, Spencer H. Alveolar enlargement in obesity-induced hyperplastic lung growth. J Appl Physiol. 1988;65:2291–2296. doi: 10.1152/jappl.1988.65.5.2291. [DOI] [PubMed] [Google Scholar]
- 19.Inselman LS, Wapnir RA, Spencer H. Obesity-induced hyperplastic lung growth. Am Rev Respir Dis. 1987;135:613–616. doi: 10.1164/arrd.1987.135.3.613. [DOI] [PubMed] [Google Scholar]
- 20.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlinsky JB, Goldstein RH, Ojserkis B, Snider GL. Lung mechanics and connective tissue levels in starvation-induced emphysema in hamsters. Am J Physiol Regul Integr Comp Physiol. 1986;251:R282–R288. doi: 10.1152/ajpregu.1986.251.2.R282. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 23.Kononov S, Brewer K, Sakai H, Cavalcante FS, Sabayanagam CR, Ingenito EP, Suki B. Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am J Respir Crit Care Med. 2001;164:1920–1926. doi: 10.1164/ajrccm.164.10.2101083. [DOI] [PubMed] [Google Scholar]
- 24.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 26.Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z. alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 27.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 28.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of air space enlargement in emphysema. J Appl Physiol. 2006;100:186–193. doi: 10.1152/japplphysiol.00424.2005. [DOI] [PubMed] [Google Scholar]
- 32.Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases TNF-alpha expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated IL-10 production. J Biol Chem. 2007;282:21695–21703. doi: 10.1074/jbc.M701419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajala MW, Scherer PE. Minireview: the adipocyte-at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 34.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 35.Ruan H, Lodish HF. Regulation of insulin sensitivity by adipose tissue-derived hormones and inflammatory cytokines. Curr Opin Lipidol. 2004;15:297–302. doi: 10.1097/00041433-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Sahebjami H, Vassallo CL, Wirman JA. Lung mechanics and ultra-structure in prolonged starvation. Am Rev Respir Dis. 1978;117:77–83. doi: 10.1164/arrd.1978.117.1.77. [DOI] [PubMed] [Google Scholar]
- 37.Sahebjami H, Wirman JA. Emphysema-like changes in the lungs of starved rats. Am Rev Respir Dis. 1981;124:619–624. doi: 10.1164/arrd.1981.124.5.619. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S29–S32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- 39.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 40.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, Ouchi N, Walsh K. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res. 2007;74:471–479. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocar-dial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 43.Spiegelman BM, Choy L, Hotamisligil GS, Graves RA, Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J Biol Chem. 1993;268:6823–6826. [PubMed] [Google Scholar]
- 44.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365:2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 45.Suga T, Kurabayashi M, Sando Y, Ohyama Y, Maeno T, Maeno Y, Aizawa H, Matsumura Y, Kuwaki T, Kuro O, Nabeshima Y, Nagai R. Disruption of the klotho gene causes pulmonary emphysema in mice. Defect in maintenance of pulmonary integrity during postnatal life. Am J Respir Cell Mol Biol. 2000;22:26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- 46.Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168:516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- 47.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 48.Takefuji S, Yatsuya H, Tamakoshi K, Otsuka R, Wada K, Matsushita K, Sugiura K, Hotta Y, Mitsuhashi H, Oiso Y, Toyoshima H. Smoking status and adiponectin in healthy Japanese men and women. Prev Med. 2007;45:471–475. doi: 10.1016/j.ypmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNFalpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–G1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurlbeck WM. Internal surface area of normal and emphysematous lungs. Aspen Emphysema Conf. 1967;10:379–393. [PubMed] [Google Scholar]
- 52.Thurlbeck WM. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis. 1967;95:765–773. doi: 10.1164/arrd.1967.95.5.765. [DOI] [PubMed] [Google Scholar]
- 53.Trayhurn P. Adipocyte biology. Obes Rev. 2007;8(Suppl 1):41–44. doi: 10.1111/j.1467-789X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 54.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 55.Von WP, Joseph K, Muller B, Franck WM. Bronchoalveolar lavage. Quantitation of intraalveolar fluid? Am Rev Respir Dis. 1993;147:148–152. doi: 10.1164/ajrccm/147.1.148. [DOI] [PubMed] [Google Scholar]
- 56.Wert SE, Yoshida M, LeVine AM, Ikegami M, Jones T, Ross GF, Fisher JH, Korfhagen TR, Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci USA. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, Hanazawa S, Yamashita Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6826. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Ikegami M, Crouch EC, Korfhagen TR, Whitsett JA. Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure. J Biol Chem. 2001;276:19214–19219. doi: 10.1074/jbc.M010191200. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Sun X, Jin L, Stringfield T, Lin L, Chen Y. Expression profiles of adiponectin receptors in mouse embryos. Gene Expr Patterns. 2005;5:711–715. doi: 10.1016/j.modgep.2005.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.