Abstract
Purpose of review
Despite a strong correlation between obesity and insulin resistance, 25% of severely obese (BMI >40) individuals are insulin sensitive. In this review, we will examine the factors in adipose tissue that distinguish the two groups, as well as reasons for believing the insulin-sensitive group will be less disease prone.
Recent findings
Obesity has been linked to the metabolic syndrome with an increase in visceral (intra-abdominal) compared to subcutaneous fat. Recent studies in which adipose tissue of insulin-sensitive and insulin-resistant patients with severe obesity were compared indicate that the insulin-resistant group is also distinguished by increases in oxidative stress and decreases in AMP-activated protein kinase (AMPK) activity. In contrast, changes in the expression of genes for SIRT1, inflammatory cytokines, mitochondrial biogenesis and function, and the two α-isoforms of AMPK showed more depot variation. Studies of how these and other changes in adipose tissue respond to bariatric surgery are still in their infancy.
Summary
Available data suggest that increases in oxidative stress, decreases in AMPK activity and SIRT1 gene expression, depot-specific changes in inflammatory, mitochondrial and other genes distinguish adipose tissue of insulin resistant from insulin-sensitive individuals with severe obesity.
Keywords: AMP-activated protein kinase, inflammation, insulin resistance, oxidative stress, SIRT1
INTRODUCTION
Mildly and moderately obese individuals (BMI 30–40) are predisposed to disorders such as type 2 diabetes, atherosclerotic cardiovascular disease, essential hypertension, nonalcoholic fatty liver disease (NAFLD), certain cancers, and neurodegenerative disorders including Alzheimer’s disease [1▪]. It has long been appreciated that some equally obese individuals do not show these disease predilections. In general, such individuals are more insulin sensitive and have relatively less abdominal fat (both visceral and subcutaneous) than their disease-prone counterparts [2,3▪,4]. The pathogenetic events that distinguish these patients are incompletely understood, although such factors as ectopic lipid disposition and low-grade inflammation in adipose tissue of the insulin-resistant obese group have been implicated. Diet and exercise can decrease both insulin resistance and inflammation in such obese patients. For instance, in a randomized prospective trial in middle-aged individuals with type 2 diabetes and mild obesity, diet and exercise have been shown to diminish coronary heart disease mortality [5]. However, their effectiveness in large populations over extended periods of time has been limited. Likewise, the long-term efficacy of pharmacological interventions remains to be demonstrated.
The same limitations apply to patients with severe obesity (BMI >40), although they too can respond to diet and exercise [6]. In contrast, bariatric surgery has shown substantial and lasting benefits. For instance, the Roux-en-Y gastric bypass procedure has proven effective in roughly 80% of such individuals in achieving sustained weight loss. In addition, it reverses type 2 diabetes, hypertension, and other disorder in many of these patients. Collectively, it and other procedures have been shown to diminish mortality because of atherosclerotic cardiovascular disease, an effect primarily observed in individuals in the two highest quintiles of plasma insulin (i.e. those with insulin resistance) [7▪▪].
Interestingly, as is the case with their less obese counterparts [4], approximately 25% of severely obese individuals are insulin sensitive, as assessed by hyperinsulinemic–euglycemic clamps [8▪▪] or homeostasis model of assessment (HOMA) [9▪,10▪, 11,12▪▪]. Thus, a comparison of these patients and their insulin-resistant counterparts could provide useful information as to what distinguishes these two groups and potentially identify pathogenetic factors.
In this review, we will discuss the recent efforts to make such a comparison, based in large part on analyses of subcutaneous and visceral adipose tissue depots from the two patient groups taken at the time of bariatric surgery and in some instances afterward. The findings indicate that the insulin sensitive and resistant patients differ with respect to AMP-activated protein kinase (AMPK) activity and oxidative stress in all of their fat depots [12▪▪] and in the expression of genes related to inflammation, mitochondrial function, SIRT1/Nampt, and many others [8▪▪,10▪,12▪▪,13▪,14▪] in selected depots (Table 1). In addition, they suggest that differences in the abundance of lipid droplet proteins that regulate the storage and breakdown of triglycerides in the fat cell could play a key role [11].
Table 1.
Summary of reported key differences in proteins and genes in adipose tissue of obese insulin-resistant vs. obese insulin-sensitive patients
| Subcutaneous fat | Omental fat | |
|---|---|---|
| (a) Protein (Xu et al. [12▪▪]) | ||
| p-AMPK/AMPK | − | − |
| Nampt | 0 | − |
| Protein carbonylation | + | + |
| (b) Gene expression | ||
| Xu et al. [12▪▪] | ||
| CD4 | + | + |
| CD68 | 0 | + |
| MPO | 0 | + |
| CCL5 | 0 | + |
| p-Selectin | 0 | + |
| SIRT1 | 0 | 0 |
| Nampt | 0 | − |
| PGC1α | 0 | − |
| Angiotensinogen | + | + |
| AMPKα1 | − | 0 |
| AMPKα2 | + | 0 |
| Klöting et al. [8▪▪] | ||
| SIRT1 | − | − |
| IL-6 | + | + |
| IL-8 | 0 | + |
| Nampt | 0 | + |
| Hardy et al. [10▪] | ||
| CCL2, 3, 4, 8 | 0 | + |
| IL-8 | 0 | + |
| Goossens et al. [13▪] | ||
| PGC1α | − | ND |
| Gillum et al. [14▪] | ||
| SIRT1 | − | ND |
In study by Xu et al. [12▪▪], seven of the eight patients in the insulin-resistant group and three of eight in the insulin-sensitive group were diabetic (not insulin treated). Patients with diabetes or a family history of diabetes were excluded by Klöting et al. [8▪▪]. + and − indicate a factor in increased or decreased. 0, no change; ND, not determined.
OVERVIEW
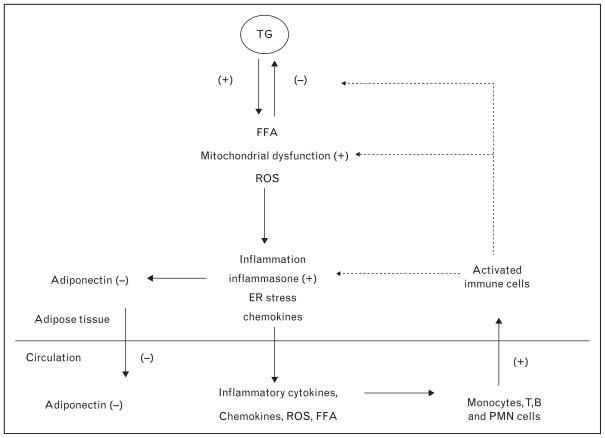
Figure 1 depicts the events that occur in adipose tissue of the 75% of severely obese people who are insulin resistant. Key abnormalities appear to be impaired triglyceride storage and increased lipolysis by lipid droplets, mitochondrial dysfunction, inflammation, and increases in oxidative and endoplasmic reticulum stress [15]. Many of these abnormalities could be related to increased synthesis and release of chemokines from the adipocytes or more likely adjacent vascular cells that attract monocytes (CD68), T (CD4) and B lymphocytes, and neutrophils (MPO) from circulating blood [16] (Table 1). The resultant increases in the release of free fatty acid (FFA), reactive oxygen species (ROS), and inflammatory cytokines and the decreased release of adiponectin from the adipocyte are thought to act on peripheral tissues to cause such disorders as type 2 diabetes, atherosclerosis, and NAFLD. Not shown in the diagram is that in subcutaneous abdominal fat, the indicated changes may also be associated with decreased capillarity [13▪] and impaired O2 consumption and increased synthesis of type VI collagen [13▪,17], all of which could limit adipose tissue expansion. The nature of the initiating event(s) and the factors responsible for the above-mentioned changes are incompletely understood. What is clear is that many of the depicted events do not occur or are less prominent in adipose tissue of severely obese people who are insulin sensitive. As will be discussed later, decreases in AMPK and probably SIRT1 activity are likely important pathogenetic factors.
FIGURE 1.
Pathophysiology of adipose tissue in an obese, insulin-resistant individual. Adipose tissue consists of the adipocyte and cells present in the stroma including those in the microvasculature, resident macrophages, and other inflammatory cells taken up from the circulation. It is assumed that mononuclear cells taken up are predominantly converted to type 1 macrophages that produce inflammatory cytokines. As discussed in the text, decreases in AMPK and SIRT1 activity, such as that found in the adipose tissue of insulin-resistant patients with massive obesity, very likely contribute to these events. An early occurrence is presumably a decrease in lipid droplet proteins that simultaneously diminish fatty acid deposition and increase free fatty acid (FFA) releases from the lipid droplet. This could account for observed increases of FFA and reactive oxygen species in the cytosol of the adipocytes; however, this remains to be proven.
FREE FATTY ACID AND LIPID DROPLET PROTEINS
An impaired ability to deposit triglycerides together with an increased release of FFA is one of the hallmarks of adipose tissue in severely obese people who are insulin resistant. Such abnormalities are not present in adipose tissue of equally obese people who are insulin sensitive. As reported by Puri et al. [11], these findings could be explained by differences in the abundance of three lipid droplet proteins, Cide A, perilipin, and FSP 27 (Cide C), all of which appear to regulate triglyceride storage and lipolysis in the lipid droplet so as to increase its size. They found that the expression of all three proteins was substantially greater in insulin sensitive than in insulin-resistant individuals with massive obesity. In addition, they observed that treatment of ob/ob mice which lack leptin with the thiazolidinedione or rosiglitazone increases Cide A, adiponectin, mitochondrial biogenesis, and lipid deposition in adipose tissue [11]. Others have found that such treatment activates AMPK in rodents in vivo [18] and that it does so at least by increasing the level of adiponectin, a known AMPK activator [19].
OXIDATIVE STRESS, INFLAMMATION, AND MITOCHONDRIA
Oxidative stress
Oxidative stress as reflected by lipid peroxidation, protein carbonylation, and DNA damage is increased in both adipose tissue and plasma of severely obese humans [20,21] and in particular those who are insulin resistant [12▪▪,22]. Studies in humans [21] suggest that adipose tissue is a major source of ROS in these patients. The relative contribution of increased NADPH oxidase activity, ROS generation by mitochondria, and decreased activity of antioxidant enzymes is uncertain. Elevated oxidative stress in adipose tissue is usually associated with insulin resistance and inflammation; however, the insulin resistance can occur in the absence of the latter. Thus, treatment of 3T3L1 adipocytes with glucocorticoids increases oxidative stress and causes insulin resistance even though it does not produce inflammation [23]. Such a situation presumably also occurs in humans with excess glucocorticoids (e.g. Cushing’s syndrome) in whom central obesity and insulin resistance are concurrent events [24]. Interestingly, AMPK activity is diminished in visceral adipose tissue of these patients [24], just as it is in the adipose tissue of severely obese insulin-resistant patients without Cushing’s syndrome [9▪,12▪▪]. The basis for the decreased AMPK activity in the setting of Cushing’s syndrome is not known. However, in light of the above-mentioned link between insulin resistance and oxidative stress in adipocytes [23], it is noteworthy that the lipid peroxide, 8-hydroxy nonenal (HNE), can bind to a specific lysine residue on the AMPK upstream kinase, LKB1, leading to its inhibition and secondarily that of AMPK [25].
Of further relevance to this discussion, studies in cultured 3T3L1 murine adipocytes have revealed that oxidative stress increases acutely when lipolysis is stimulated, and that this is associated with the activation of AMPK because of an increase in the AMP/ATP ratio caused by the energy demand of fatty acid re-esterification [26]. Interestingly, when AMPK activation was prevented in this setting, ROS release by the cell was markedly increased, raising the possibility that decreased AMPK activity could be a cause as well as a result of oxidative stress in the adipocyte. In keeping with this notion, a similar increase in ROS production has been observed in AMPK (−/−) endothelial cells incubated with palmitate [27].
Inflammation
Although inflammation may not always be necessary for the development of insulin resistance in adipose tissue (see above), insulin resistance in most obese individuals is typically preceded by increases in leukocyte infiltration and inflammatory cytokines [28]. The inflammation is also usually associated with increases in plasma FFA and oxidative stress and mitochondrial dysfunction in adipose tissue [29]. Another contributing factor could be an increase in circulating lipopolysaccharide (LPS), possibly related to alterations in the microbiome and the permeability of the gut, such as have been described in obese humans [29].
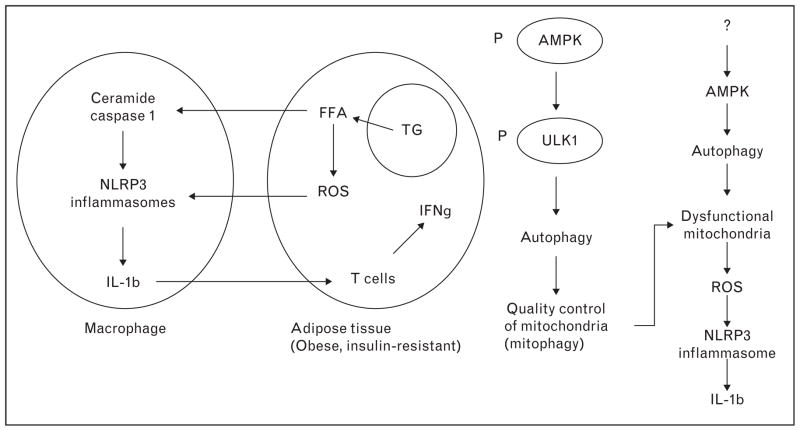
Recently, it has been proposed that inflammation in adipose and other tissues can be mediated by activation in macrophages of the inflammasome. An inflammasome is a multiprotein complex that mediates the cleavage and activation of caspase 1, which in turn leads to the maturation and release of the inflammatory cytokines IL-1β and IL-18. Cytosolic NOD like-receptors such as NLRP3, together with an adaptor protein, ASC, have been shown to mediate these events in response to many stimuli. Based predominantly on the studies in cultured cells and gene knockout animals, a hypothetical mechanism for inflammasome activation and action in adipose tissue is depicted in Fig. 2 [30,31▪,32▪▪]. Inflammasomes are induced in the macrophage in response to increases in such factors as fatty acids (palmitate), ROS, and potentially many others. To what extent their activation is initiated by fatty acids and ROS derived from adipocytes unable to store lipids in lipid droplets (see previous section) is unclear. As depicted in Fig. 2, autophagy removes damaged mitochondria and this could also diminish inflammasome formation and secondarily, the generation of inflammatory cytokines. The potential roles of AMPK and a closely related molecule SIRT1 in regulating inflammasome formation and other events that occur in adipose tissue in the setting of insulin resistance will be discussed in a later section. Suffice it to say, it has been proposed that decreased AMPK activity impairs the activity of the autophagosome, a key element in the autophagic process, and secondarily increases oxidative stress [30,32▪▪]. As already noted, research on the inflammasome has been performed predominantly in cultured cells and experimental animals. Recently, however, Vandanmagsar et al. [31▪] observed that in obese humans with type 2 diabetes, a 1-year diet and exercise intervention program decreased both insulin resistance and the expression of three elements of the inflammasome, NLRP3, ASC, and IL1β-mRNA in subcutaneous adipose tissue.
FIGURE 2.
Hypothetical mechanisms for the activation of inflammasomes in adipose tissue of obese insulin-resistant individuals (adapted with permission [30]). The identity of the factor(s) that decrease AMPK activity in cells in which inflammasomes are found is uncertain, although oxidative stress could be a factor. Not shown here is that activation of caspase 1 in the inflammasome complex cleaves and presumably inactivates SIRT1. ULK1, a regulator of the autophage lysosome is phosphorylated and activated by AMPK.
Mitochondrial dysfunction
Mitochondrial dysfunction can precipitate and be a consequence of oxidative stress. Because of their relative paucity in white adipose tissue, the role of mitochondria in regulating adipocyte function has traditionally received little attention. More recently, diminished mitochondrial mass and function, as well as abnormally low levels of mitochondrial DNA were found in adipose tissue of ob/ob and db/db mice and were reversed by treatment with thiazolidinediones [33,34]. In humans, the scenario appears to be more complex. Mitochondrial DNA levels are diminished in adipose tissue of obese patients with type 2 diabetes [35]; however, it has been suggested that this is because of the presence of the diabetes phenotype [36]. In our own work, we found a reduced expression of the mRNA for PGC1α, the master activator of mitochondrial biogenesis in omental fat of obese insulin-resistant patients compared to their insulin-sensitive counterparts [12▪▪]. A similar finding was reported in subcutaneous fat in another study where omental fat was not concurrently examined [13▪]. The integrity and function of mitochondrial DNA were not examined in either study.
AMPK AND SIRT1
AMPK has been implicated in regulating a variety of cellular functions including energy state, fuel metabolism, mitochondrial biogenesis, protein and ceramide synthesis, and cell growth and proliferation. In addition, its activation was initially shown to inhibit glucose, palmitate and TNFα-induced inflammation, insulin resistance, apoptosis, and oxidative stress in cultured human umbilical vein endothelial cells [27,37] and later in other cells, suggesting it plays an even broader role [1▪,38▪]. Conversely, decreased AMPK activity has been observed in rodents with metabolic syndrome associated disorders including ob/ob and db/db mice, fat-fed rodents of many types, and ZDF rats, all of which are obese and insulin resistant [1▪]. Furthermore, in the ZDF rat in which the primary defect is an absence of the leptin receptor, pharmacological AMPK activation has been shown to prevent the development of diabetes. In doing so, AMPK activation diminishes ectopic lipid deposition, inflammation, and apoptosis in pancreatic islets, strongly suggesting the decrease in its activity plays a key pathogenetic role [39].
As already noted, AMPK activity is diminished in omental, subcutaneous, and epiploic fat of severely obese humans who are insulin resistant compared to equally obese individuals who are insulin sensitive. Why such a decrease in AMPK activity occurs and whether it is the cause or the result of the increases in oxidative stress and inflammation, and mitochondrial dysfunction is unclear. In support of a causal role, AMPK activation has been shown to inhibit inflammasome formation in macrophages [30,32▪▪] as well as the conversion of monocytes to M1 macrophages [40]. In addition, as already noted, in various cell types, AMPK inhibits the adverse effects of such molecules as palmitate, TNFα, and LPS. On the other hand, both oxidative stress [25] and inflammation as well as fatty acids have been shown to diminish AMPK activity by their effects on protein phosphatases [38▪] in cultured cells and rodents in vivo. Thus, the question of which events are causal in adipose tissue must be considered unresolved. Perhaps adding to the conundrum in humans, decreases in AMPK activity and increases in oxidative stress appear to be present in multiple fat depots of insulin-resistant patients, whereas changes in the expression of genes for specific inflammatory cells and other molecules (e.g. PGC1α, various cytokines and chemokines, and adhesion molecules) are more depot specific [12▪▪].
The sirtuins are a family of histone protein deacetylases that have long been linked to the anti-aging effect of caloric restriction in rodents and other species [41]. More recently, it has become apparent that sirtuins can activate AMPK and vice versa and that these molecules have many actions and target molecules (e.g. PGC1α, FOXO, and p53) in common [1▪]. Thus, downregulation of SIRT1 in adipose tissue (like that of AMPK) has been shown to increase obesity, macrophage accumulation, and inflammation in rodent fat [14▪,42▪] and conversely, activation of both molecules has been shown to diminish inflammation in macrophages [40,43]. Likewise, decreased SIRT1 expression has been observed by several investigators in adipose tissue of obese humans who are insulin resistant (Table 1) and where studied, it was associated with increased macrophage infiltration [14▪]. Also of note, both SIRT1 and AMPK have been linked to inflammasome formation (presumably in macrophages). As already noted, AMPK has been reported to inhibit inflammasome formation by effects on mitophagy and oxidative stress [32▪▪]. In contrast, SIRT1 is cleaved and inactivated by caspase 1 of the NLRP3 inflammasome [42▪]. Finally, decreases in the activity of both AMPK and SIRT1 have been linked to the development of obesity and insulin resistance in a wide variety of rodent models [1▪,42▪,44]. In fat-fed obese rats, increases in AMPK activity and SIRT1 abundance have been observed after gastric bypass surgery [45].
BARIATRIC SURGERY
Our understanding of type 2 diabetes has been radically changed by the effects of bariatric surgery, as durable and full remission can now be achieved in a matter of days with the gastric bypass procedure in contrast to a similar correction, but one that may take months after the adjustable gastric banding operation. Bariatric surgery reverses type 2 diabetes and other disorders associated with the metabolic syndrome including hypertension, dyslipidemias, polycystic ovary syndrome (PCOS), and non-alcoholic steatosis hepatitis (NASH), as well as asthma and gastroesophageal reflux disease (GERD), even prior to significant weight loss. In addition, as already noted, it diminishes long-term mortality because of coronary heart disease [7▪▪] and the prevalence of solid tumors by over 70% within 5 years after surgery [46–48].
With respect to coronary heart disease, the greatest effect was observed in patients with higher insulin levels, suggesting that the major benefit likely occurred in the insulin-resistant population. However, in a preliminary study, we observed that recovery from diabetes (as measured by fasting glucose 3 months after surgery) correlated most closely with insulin sensitivity, that is, the more insulin-resistant patients had a less favorable outcome [49]. To our knowledge, other than these studies, the relation of the response to bariatric surgery to preoperative insulin resistance has not been systematically studied in humans.
In contrast to the reversal of diabetes, the increase in insulin sensitivity after bariatric surgery appears to occur much more slowly. Insulin sensitivity, measured with a frequently sampled intravenous glucose tolerance test (IVGTT), was not statistically changed 1 month [21] or 3 months [50] after gastric bypass surgery. In patients who were weight stable more than a year after surgery, insulin sensitivity was equal to that of normal lean individuals and greater than that of weight-matched obese patients [21,51]. In short, the resolution of diabetes occurs within a few days after surgery, in line with the rapid return of insulin levels to normal, whereas insulin resistance recovers far more slowly. As reviewed elsewhere, the latter could be related to the fact that inflammation and oxidative stress both in adipose tissue and systemically take many months to an excess of a year to dissipate [21,52].
CONCLUSION
Although the majority of severely obese individuals who undergo bariatric surgery are insulin resistant, 25% of such patients are insulin sensitive. The latter are characterized by higher levels of AMPK and lipid droplet protein expression in adipose tissue. They also have less oxidative stress in all of their fat depots and a decreased expression of inflammatory genes that is more depot-selective.
Bariatric surgery reverses metabolic syndrome associated disorders such as type 2 diabetes within days, but only decreases insulin resistance, inflammation, and oxidative stress more slowly (months) in parallel with weight loss. How AMPK and SIRT1 change has not been studied. Whether the insulin sensitive and resistant patients have different risks for cardiovascular disease preoperatively is not known, although recent data indicate that cardiovascular mortality over 20 years after surgery is higher in patients initially in the highest quintiles of plasma insulin (i.e. the insulin-resistant group).
KEY POINTS.
Most patients with severe obesity are insulin resistant; however, approximately 25% are insulin sensitive.
AMPK activity is diminished, and oxidative stress is increased in both subcutaneous abdominal and visceral fat of the insulin-resistant subgroup.
The expression of inflammatory and other genes also differs in adipose tissue of the insulin sensitive and resistant patients; however, in contrast to the alteration in AMPK and oxidative stress, the differences are depot specific.
Bariatric surgery readily reverses type 2 diabetes and other metabolic syndrome-associated disorders in these patients. In addition, it subsequently diminishes cardiovascular events and mortality.
Acknowledgments
This work was supported by the NIH grants 2R01DK019514-31 and 1R24DK094749-01. The authors thank Drs Caroline Apovian, Konstantin Kandror, Barbara Nikolajczyk, Vishwajeet Puri, and Orian Shirihai for their helpful comments and Ayesha Iyer, Christina Nielsen, and Nga Tong for assistance in preparing the manuscript.
Footnotes
Conflicts of interest
The authors have no conflict of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 92).
- 1▪.Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. This study reviews the interplay of SIRT1 and AMPK in regulating each other and additional molecules that affect cellular function and the predisposition to metabolic syndrome-associated diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 3▪.Samocha-Bonet D, Chisholm DJ, Tonks K, et al. Insulin-sensitive obesity in humans – a ‘favorable fat’ phenotype? Trends Endocrinol Metab. 2012;23:116–124. doi: 10.1016/j.tem.2011.12.005. A review of cross-sectional and longitudinal studies that compare cardiovascular disease, type 2 diabetes, and all-cause mortality in obese humans who are insulin sensitive and insulin resistant. [DOI] [PubMed] [Google Scholar]
- 4.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 5.Hu G, Tuomilehto J, Silventoinen K, et al. The effects of physical activity and body mass index on cardiovascular, cancer and all-cause mortality among 47 212 middle-aged Finnish men and women. Int J Obes (Lond) 2005;29:894–902. doi: 10.1038/sj.ijo.0802870. [DOI] [PubMed] [Google Scholar]
- 6.Bruun JM, Helge JW, Richelsen B, et al. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. Clinical study demonstrates that in severely obese patients, bariatric surgery can reduce cardiovascular mortality and incidence of cardiovascular events. Also showed greater treatment benefits occur in patients with initially higher insulin levels. [DOI] [PubMed] [Google Scholar]
- 8▪▪.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515. doi: 10.1152/ajpendo.00586.2009. The first demonstration that SIRT1 gene expression is diminished in omental and subcutaneous abdominal fat of insulin resistant compared with insulin-sensitive individuals with severe obesity. [DOI] [PubMed] [Google Scholar]
- 9▪.Gauthier MS, O’Brien EL, Bigornia S, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. Initial demonstration that decreased AMPK activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in severely obese humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪.Hardy OT, Perugini RA, Nicoloro SM, et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis. 2011;7:60–67. doi: 10.1016/j.soard.2010.05.013. Increased expression of genes for chemokines, chemokine receptors, and IL-8 in omental, but not subcutaneous adipose tissue of insulin resistant compared to insulin-sensitive obese humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri V, Ranjit S, Konda S, et al. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci USA. 2008;105:7833–7838. doi: 10.1073/pnas.0802063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Xu XJ, Gauthier MS, Hess DT, et al. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. The first study to characterize the differences between subcutaneous abdominal and two types of visceral fat (omental and epiploic) in insulin sensitive and resistant obese individuals. Results indicate that AMPK activity is diminished, and protein carbonylation is increased in all three depots of insulin-resistant patients, whereas the expression of inflammation and other genes varied between depots. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. A clear demonstration that subcutaneous abdominal adipose tissue blood flow and O2 consumption are diminished in severely obese individuals, and that this is accompanied by impaired capillarity and increased expression of inflammatory cell markers. [DOI] [PubMed] [Google Scholar]
- 14▪.Gillum MP, Kotas ME, Erion DM, et al. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235–3245. doi: 10.2337/db11-0616. Studies in rodents and humans that demonstrate decreased SIRT1 expression is associated with increased inflammation in adipose tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:639–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson EK, Gutierrez DA, Hasty AH. Adipose tissue recruitment of leukocytes. Curr Opin Lipidol. 2010;21:172–177. doi: 10.1097/MOL.0b013e3283393867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasarica M, Gowronska-Kozakm B, Burk D, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha AK, Aviluceaa PR, Yeb J-M, et al. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 19.Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 20.Frohnert BI, Sinaiko AR, Serrot FJ, et al. Adipocyte biology. Obesity. 2011;19:1735–1741. doi: 10.1038/oby.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gletsu-Miller N, Hansen JM, Jones DP, et al. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity. 2008;17:439–446. doi: 10.1038/oby.2008.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murri M, García-Fuentes E, García-Almeida JM, et al. Changes in oxidative stress and insulin resistance in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20:363–368. doi: 10.1007/s11695-009-0021-6. [DOI] [PubMed] [Google Scholar]
- 23.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a casual role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 24.Kola B, Christ-Crain M, Lolli F, et al. Changes in adenosine 5′-monophosphate-activated protein kinase as a mechanism of visceral obesity in Cushing’s syndrome. J Clin Endocrinol Metab. 2008;93:4969–4973. doi: 10.1210/jc.2008-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolinsky VW, Chan AY, Robillard FI, et al. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier MS, Miyoshi H, Souza SC, et al. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte. J Biol Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J-E, Song SE, Kim Y-W, et al. Adiponectin inhibits palmitate-induced apoptosis through suppression of reactive oxygen species in endothelial cells: involvement of cAMP/protein kinase A and AMP-activated protein kinase. J Endocrinol. 2010;207:35–44. doi: 10.1677/JOE-10-0093. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker RG, Hayden MS, Ghosh S. NF-kB inflammation metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi AMK, Nakahira K. Dampening insulin signaling by an NLRP3 ‘meta-flammasome’. Nat Immunol. 2011;12:379–380. doi: 10.1038/ni.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Vandanmagsar B, Youm YH, Ravussin A, et al. The NALP3/NLRP3 inflammasome instigates obesity-induced autoinflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. The first evidence that weight loss induced by caloric restriction and exercise in obese humans leads to enhanced insulin sensitivity, decreased inflammation, and reduced NLRP3 expression in subcutaneous adipose tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪▪.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. The demonstration that palmitate induces activation of the NLRP3-ASC inflammasome by a pathway that involves oxidative stress and the AMPK–ULK1a autophagy signaling cascade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choo HJ, Kim JH, Kwon OB, et al. Mitochondria are impaired in the adipocytes of type 2 diabetic. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 34.Rong JX, Qiu Y, Hansen MK, et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 35.Bogacka I, Xie H, Bray GA, et al. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 36.Dahlman I, Forsgren M, Sjogren A, et al. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes. 2006;55:1792–1799. doi: 10.2337/db05-1421. [DOI] [PubMed] [Google Scholar]
- 37.Cacicedo JM, Yagihashi N, Keaney JF, et al. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 38▪.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. A recent review of various links between AMPK and inflammation and their physiological relevance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X, McCorkle S, Wang M, et al. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 40.Sag D, Carling D, Stout RD, et al. AMP-activated protein kinase promotes macrophage polarization to anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Chalkiadaki A, Guarente L. High fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. A demonstration that a high-fat diet causes cleavage of SIRT1 by caspase 1, the effector protein of the NLRP3 lysosome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Z, Kahn BB, Shi H, et al. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 45.Peng Y, Rideout DA, Rakita SS, et al. Does LKB1 mediate activation of hepatic AMP-protein kinase (AMPK) and sirtuin1 (SIRT1) after Roux-en-Y gastric bypass on obese rats? J Gastrointest Surg. 2009;14:221–228. doi: 10.1007/s11605-009-1102-5. [DOI] [PubMed] [Google Scholar]
- 46.Adams TD, Hunt SC. Cancer and obesity: effect of bariatric surgery. World J Surg. 2009;33:2028–2033. doi: 10.1007/s00268-009-0169-1. [DOI] [PubMed] [Google Scholar]
- 47.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;57:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 48.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and healthcare use in morbidly obese patients. Ann Surg. 2004;240:416–424. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reed MA, Pories W, Chapman W. Insulin sensitivity and not insulin secretion is associated with resolution of type 2 diabetes following gastric bypass surgery. Diabetes. 2010;59:A479. [Google Scholar]
- 50.Reed MA, Pories WJ, Chapman W, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab. 2011;96:2525–2531. doi: 10.1210/jc.2011-0165. [DOI] [PubMed] [Google Scholar]
- 51.Bikman BT, Zheng D, Pories WJ, et al. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab. 2008;93:4656–4663. doi: 10.1210/jc.2008-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Illán-Gömez F, Gonzálvez-Ortega M, Orea-Soler I, et al. Obesity and inflammation: change in adiponectin, c-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–955. doi: 10.1007/s11695-012-0643-y. [DOI] [PubMed] [Google Scholar]