Abstract
Childhood socioeconomic status (SES) is associated with cognitive achievement throughout life. How does SES relate to brain development, and what are the mechanisms by which SES might exert its influence? We review studies in which behavioral, electrophysio-logical and neuroimaging methods have been used to characterize SES disparities in neurocognitive function. These studies indicate that SES is an important predictor of neurocognitive performance, particularly of language and executive function, and that SES differences are found in neural processing even when performance levels are equal. Implications for basic cognitive neuroscience and for understanding and ameliorating the problems related to childhood poverty are discussed.
Why study the neuroscience of socioeconomic status?
What is socioeconomic status (SES), and why would a cognitive neuroscientist have anything to say about it? Volumes have been written about the first question, but for present purposes we will simply say that virtually all societies have better off and less well off citizens, and that differences in material wealth tend to be accompanied by noneconomic characteristics such as social prestige and education [1–6] (Box 1). SES refers to this compound of material wealth and noneconomic characteristics such as social prestige and education. SES is invariably correlated with predictable differences in life stress and neighborhood quality, in addition to less predictable differences in physical health, mental health and cognitive ability [1–15] (Box 1). The relevance of SES to cognitive neuroscience lies in its surprisingly strong relationship to cognitive ability as measured by IQ and school achievement beginning in early childhood. Which neurocognitive systems are implicated in these SES gradients, and what causes the gradients? These are questions for cognitive neuroscience.
A small but growing literature has addressed these questions using a variety of research methods. The picture that emerges, of substantial SES disparities in particular neurocognitive systems, has implications for the basic science of cognitive neuroscience and also for real-world solutions to SES-related problems.
Neurocognitive performance
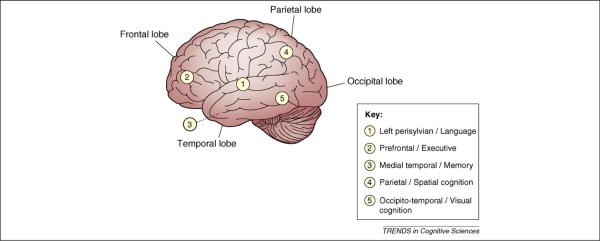
Although IQ tests reflect the function of the brain, they are relatively uninformative concerning the specific neurocognitive systems responsible for performance differences. Recent research has, therefore, incorporated behavioral tests that support more specific inferences. For purposes of relating task performance to underlying systems, we propose the following simple parse of brain function into five relatively independent neurocognitive systems defined anatomically based on studies of patients with lesions and functionally based on activation in brain regions in healthy subjects while performing a specific cognitive task (see Figure 1 and Refs [16–18] for the rationale): (1) the Left perisylvian/Language system; (2) the Prefrontal/Executive system, which can be further decomposed into the Lateral prefrontal/Working memory system, the Anterior cingu-late/Cognitive control system and the Ventromedial pre-frontal/Reward processing system; (3) the Medial temporal/Memory system; (4) the Parietal/Spatial cognition system and (5) the Occipitotemporal/Visual cognition system. These systems can be assessed behaviorally by tasks that tax the function of interest and place a minimal burden on the others.
Figure 1.
Heuristic illustrating a lateral view of the localization of five basic neurocognitive systems, defined anatomically based on the cognitive performance of patients with lesions in specific regions and activation in brain regions during specific cognitive tasks in healthy subjects (for further description of the rationale see Refs [16–18]). The five systems are: (1) the `Left perisylvian/Language' system, a complex, distributed system predominantly located in the temporal and frontal areas of the left hemisphere that surround the Sylvian fissure, which encompasses semantic, syntactic and phonological aspects of language; (2) the `Prefrontal/Executive' system, including the Lateral prefrontal/Working memory system that enables us to hold information `on line' to maintain it over an interval and manipulate it, the Anterior cingulate/Cognitive control system that is required when we must resist the most routine or easily available response in favor of a more task-appropriate response and the Ventromedial prefrontal/Reward processing system, which is responsible for regulating our responses in the face of rewarding stimuli; (3) the `Medial temporal/Memory' system (towards the interior of the brain from the visible surface of the temporal lobe depicted here), responsible for one-trial learning, the ability to retain a representation of a stimulus after a single exposure; (4) the `Parietal/Spatial cognition' system, underlying our ability to mentally represent and manipulate the spatial relations among objects and (5) the `Occipitotemporal/Visual cognition' system, responsible for pattern recognition and visual mental imagery, translating image format visual representations into more abstract representations of object shape and identity, and reciprocally translating visual memory knowledge into image format representations.
Language ability differs sharply as a function of SES. For example, in one classic study, the average vocabulary size of 3-year-old children from professional families was more than twice as large as for those on welfare [19]. SES gradients have been observed in vocabulary, phonological awareness and syntax at many different stages of development, providing clear behavioral evidence for Left Perisylvian/Language system disparities (see Ref. [20] for a review).
Several recent studies have also reported SES disparities in Prefrontal/Executive function [21–33]. For example, Lipina et al. [27] reported that infants from lower SES families are, on average, less advanced in the working memory and inhibitory control abilities needed to pass the `A not B' test [34]. In a study of 6 year-olds using Posner's Attention Network Task [35], Mezzacappa found pronounced SES disparities in the `executive attention' measure [29]. Studies of adults with neuropsychological tests converge on the same conclusion, showing SES disparities in tests of executive function [30,31].
What is the `profile' of SES disparities across different neurocognitive systems? Our group has addressed this question using task batteries designed to assess multiple neurocognitive systems within the same children. Across three samples of different ages, studied with a variety of tasks designed to tap the five systems named earlier, certain consistencies emerge. With kindergarteners, we found that middle-SES children performed better than their low-SES counterparts, particularly on tests of the Left perisylvian/Language system and the Prefrontal/Executive system; the other neurocognitive systems tested did not differ significantly between low and middle SES children [16]. With first graders, using a larger sample of continuously varying SES, we attempted to replicate and extend these findings. We also strengthened the validity and sensitivity of the Medial temporal/Memory system tasks by adding a filled delay interval and we subdivided our tests of Prefrontal/Executive function into relatively selective tests of three subsystems. As before, the Left perisylvian/Language system showed a highly significant relationship to SES. In addition, with the added delay interval, the Medial temporal/Memory system also showed an SES gradient, as did the Parietal/Spatial cognition system (cf. [36]) and the executive functions of Lateral prefrontal/Working memory and Anterior cingulate/Cognitive control (Figure 2) [17]. In a third study, with older children in middle school, a similar pattern was observed: SES disparities in language, memory and working memory, with borderline significant disparities in cognitive control and spatial cognition [18].
Figure 2.
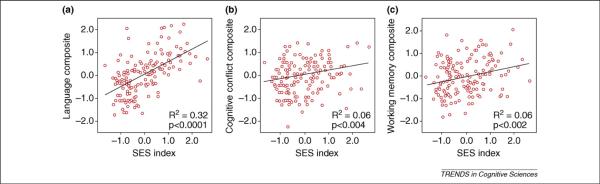
In first-graders, SES accounts for variance in neurocognitive composite measures of (a) `language' performance on vocabulary and phonological processing tasks; (b) `cognitive control' measures of the ability to inhibit a prepotent response and (c) `working memory', based on tasks assessing working memory of spatial location and figural stimuli. SES accounts for statistically more variance in the language composite than in all other composites, which do not statistically differ from each other. Figure adapted, with permission, from Ref. [18].
In summary, different neurocognitive systems are not uniformly affected by SES. On the basis of our three studies, the effects of poverty were disproportionate for certain neurocognitive systems, including language and executive function, in agreement with several of the single-system studies described earlier. Memory was associated with SES in the two studies with delayed recognition, consistent with most [32] (see Ref. [37] for a review) but not all [28] research on SES and memory.
Electrophysiological measures
To investigate SES disparities in brain development more directly, several research groups have recently turned to electrophysiological measures of neurocognitive processing. Baseline electroencephalographic (EEG) activity has been used to assess overall differences in resting brain function, and two studies have found differences in the pattern of EEG as a function of SES. In a study of Mexican preschool children, Otero and colleagues [38] found evidence consistent with a maturational lag in prefrontal cortex, and a study by Tomarken and colleagues [39] found a relative left-frontal hypoactivity in lower-SES adolescents. Both of these findings are consistent with the behavioral findings just reviewed on language and executive function.
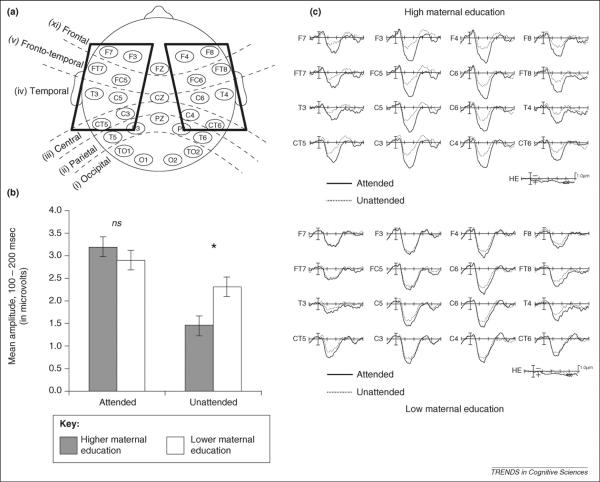
Recently, investigators have examined prefrontal-dependent functions such as selective attention and recency judgments as a function of SES [40–44] using event-related potentials (ERPs). These studies provide convergent evidence for the effect of poverty on executive function development and selective attention in particular. In a study of children between the age of 3 and 8 years, Stevens and colleagues [40] examined the effects of maternal education level on a selective auditory attention task. Children were presented two narrative stories simultaneously, one in each ear, and were cued to attend to one of the stories while ERPs to probe stimuli were recorded. Although the children remembered the stories equally well, they exhibited a different pattern of neural responses 100–200 milliseconds after auditory probes, an epoch in which there is a broad positive component indicative of attention (Figure 3). There were no SES differences in the ERP response in the attended channel, but low-SES children exhibited a higher amplitude response to the probes in the unattended channel, indicative of difficulty suppressing distracting stimuli early in the processing stream. The reduced effects of selective attention were observed electrophysiologically despite similar behavioral performance between the low and middle SES children. Such subtle attentional differences could influence language development, which requires selective attention to verbal stimuli [40], particularly in low-SES environments with a higher prevalence of noise and distracting environmental stimuli [11].
Figure 3.
Event-related potentials (ERPs) in response to auditory probes in attended and unattended stimuli during a selective spatial auditory attention task. Children age 3–8 years were presented with two simultaneous narrative stories, one in each ear, along with a visual cue directing attention to one ear. (a) Electrode configuration for ERP recording. The 16 electrodes included in analysis are enclosed in boxes. (b) Mean amplitude response (in μV) to probe stimuli in the attended and unattended channel, separately for children in the higher and lower maternal education groups; children with lower maternal education exhibited a higher amplitude response to the probes in the unattended channel, indicative of difficulty suppressing distracting stimuli early in the processing stream. (c) Grand average evoked potentials for attended and unattended stimuli in children in the higher maternal education group (upper panel) and lower maternal education group (lower panel). Figure adapted, with permission, from Ref [40].
D'Anguilli and colleagues [41,42] have found similar SES attentional differences in ERPs modulated by non-spatial auditory attention. In their task, low-SES children display reduced ERP evidence of selective attention, despite equivalent accuracy and reaction time. In older adults, higher SES subjects have a larger long-duration frontal negativity during prefrontal-dependent recency as compared to recognition judgments, indicating that they are able to recruit additional neural resources to compensate for the adverse effects of aging [43]. A recent study used the ERPs evoked by novel distracter stimuli to assess prefrontally mediated attentional processing in two groups of children, of low and middle SES [44]. Although the groups were not balanced for ethnicity, which makes the study difficult to interpret (Box 2), the results were consistent with higher SES children recruiting prefrontal attentional mechanisms to a greater degree than low SES children.
In summary, the ERP literature indicates that, even when performance differences do not emerge between lower and higher SES individuals, there are differences in the degree to which specific neural systems are recruited during cognitive processing. These differences are broadly consistent with the executive function performance differences reviewed in the previous section.
Neuroimaging
For purposes of localizing differences in cognitive ability to specific, anatomically defined neural systems, magnetic resonance imaging (MRI) provides far more direct and accurate information than the most carefully chosen behavioral tasks or the densest array of scalp electrodes. Unfortunately, there are few studies of SES using functional or structural neuroimaging. Two studies have so far examined SES disparities in cognitive function with functional MRI (fMRI) in normal children and one has examined emotion perception in normal adults.
Our group investigated the modulation of brain-behavior relationships in reading by SES. We found that 6–9-year-old children with below average reading ability showed different relationships between activation in the left fusiform gyrus (an area essential for visual word recognition) and phonological awareness (PA, a key language ability for learning to read) depending on their SES: there was a strong positive relationship between PA and left fusiform activity in lower SES children, whereas there was no relationship at higher SES levels [45]. This is consistent with the buffering of PA differences by the relatively enriched literacy environment in which higher SES children learn to read. In an fMRI study of 5 year-olds, Raizada and colleagues [46] found that SES is positively correlated with the degree of hemispheric specialization in the left inferior frontal gyrus (LIFG) while judging whether words and non-words rhyme, even when controlling for measures of cognitive and language ability, which is interpreted as indicating either a deficit or delay in normative specialization of language function in the left hemisphere. Although not about cognition per se, a third fMRI study exemplifying the impact of SES on brain activity found differences in amygdala activity in response to angry faces, with higher activity bilaterally in adults with lower childhood SES [47].
To our knowledge, only a small number of studies have investigated structural differences in the brain associated with SES. One study examined asymmetries in temporal and parietal brain areas in children, motivated by the association between asymmetry and language ability, and failed to find SES differences [48], whereas another looked specifically at the LIFG, an area important for both language and executive function, and found a borderline significant trend towards smaller volumes in lower SES children [46]. In a study focused on stress-related brain regions in adults, SES was positively related to the size of the perigenual anterior cingulate cortex [49].
Brain imaging studies of SES are thus few in number, and address a scattered set of questions. The emergence of a coherent imaging literature on SES and the brain awaits additional research. However, the current studies do tell us that SES influences brain function, modulating brain responses to stimuli as diverse as letter strings, spoken words and emotional faces. These results confirm the reality of SES disparities in neurocognitive function inferred from the earlier behavioral studies, and provide more direct evidence of the involvement of prefrontal cortex in the observed SES disparities.
Manipulations of social status
The vast majority of cognitive neuroscience laboratories conduct research with participants of middle SES. The restricted range of SES in easily accessible subject populations is undoubtedly partly responsible for the neglect of SES as a variable in human brain development and function. Some researchers who are interested in the effects of social hierarchy on neurocognitive function have found a way to address the issue with middle SES subjects: they have manipulated subjects' social status and power relations within an experimental session, which mimics the element of SES associated with relative prestige and status within a hierarchy [5]. Lower power in such experiments has been associated with executive function deficits, such as difficulty ignoring distracting information and focusing attention on task demands [50], and deficits in working memory, inhibition and planning [51]. A recent fMRI study examined the neural response to stable and unstable hierarchies created in an interactive economic game, and found widespread effects of hierarchy in cognitive and affective brain regions. Distinct activity patterns in the dorsolateral prefrontal cortex and the ventral striatum were observed when viewing an image of someone of higher status for both stable and unstable hierarchies, whereas in unstable hierarchies this elicited activity in regions such as the amygdala, medial prefrontal cortex, posterior cingulate and thalamus, regions involved in emotional processing and social cognition [52].
Despite the experimental control of such approaches and the convergent evidence they provide for SES differences in executive function and affective processing, it is not yet clear how these findings relate to real-world SES. Several possibilities exist. First, it could be that many SES effects are contextually primed, that is, emerge temporarily when social status is made salient – such as when visiting a university research facility staffed by higher SES professionals. Second, it is possible that routine reminders of one's lower social status sensitize or habituate those of lower SES to circumstances that call attention to hierarchy and power. Third, it is possible that such routine reminders engender habitual patterns of brain activity and cognition that become trait-like features of brain structure and function. Discriminating among these possibilities will be an important task for future research (Box 3).
Mechanisms
What is the cause of SES differences in brain function? Is it contextual priming? Is it social causation, reflecting the influence of SES on brain development? Alternatively, is it social selection, in which abilities inherited from parents lead to lower SES [9]? Current research on SES and brain development is not designed to answer this question. However, research on SES and IQ is relevant and supports a substantial role of SES and its correlated experience as causal factors [1,7–12,53,54].
Slightly less than half of the SES-related IQ variability in adopted children is attributable to the SES of the adoptive family rather than the biological [53]. This might underestimate environmental influences because the effects of prenatal and early postnatal environment are included in the estimates of genetic influence. Additional evidence comes from studies of when poverty was experienced in a child's life. Early poverty is a better predictor of later cognitive achievement than poverty in middle- or late-childhood [10], an effect that is difficult to explain by genetics. SES modifies the heritability of IQ, such that in the highest SES families, genes account for most of the variance in IQ because environmental influences are in effect `at ceiling' in this group, whereas in the lowest SES families, variance in IQ is overwhelmingly dominated by environmental influences because these are in effect the limiting factor in this group [54]. In addition, a growing body of research indicates that cognitive performance is modified by epigenetic mechanisms, indicating that experience has a strong influence on gene expression and resultant phenotypic cognitive traits [55]. Lastly, considerable evidence of brain plasticity in response to experience throughout development [56–58] indicates that SES influences on brain development are plausible.
Differences in the quality and quantity of schooling is one plausible mechanism that has been proposed. However, many of the SES differences summarized in this article are present in young children with little or no experience of school [16,18,19,25–27,29,33,38,40,46], so differences in formal education cannot, on their own, account for all of the variance in cognition and brain development attributable to SES. The situation is analogous to that of SES disparities in health, which are only partly explained by differential access to medical services and for which other psychosocial mechanisms are important causal factors (e.g. see Refs [1,6]).
The search for mechanisms must be informed by basic knowledge of human brain development. This is a prolonged process in which different areas and circuits reach maturity at different ages, with important consequences for the development of individual cognitive functions and with many regions, such as prefrontal gray matter and white matter tracts, undergoing considerable and often non-linear change throughout adolescence and beyond [59–65]. The finding of SES differences in executive function and language is broadly consistent with this literature because the long developmental trajectory of prefrontal regions might be expected to render them particularly susceptible to environmental influence. In addition, the development of language systems, although less drawn out, requires exquisite sensitivity to the complex environmental input of natural language, and so by similar logic might show prominent SES effects. However, there is no logical necessity for SES effects to express themselves primarily in systems undergoing the most extended or experientially dependent development. The current research reviewed here does not provide the longitudinal evidence necessary to determine how SES influences normative trajectories of brain development or to test the plausibility of mediating pathways present at times of heightened developmental sensitivity in affected regions. These are important issues for future research (Box 3).
Candidate causal pathways from environmental differences to differences in brain development include lead exposure, cognitive stimulation, nutrition, parenting styles and transient or chronic hierarchy effects [1,7,9, 11,12,14,66,67]. One particularly promising area for investigation is the effect of chronic stress. Lower-SES is associated with higher levels of stress [11,68,69] in addition to changes in the function of physiological stress response systems [22,28,70] in children and adults. Changes in such systems are likely candidates to mediate SES effects as they impact both cognitive performance and brain regions, such as the prefrontal cortex and hippocampus, in which there are SES differences [71,72]. Mechanistic pathways can be tested by measuring the different candidate factors in children of lower and higher SES and determining if the SES disparities are mediated by the measures of interest.
We recently found that SES disparities in the executive functions of attention, planning and verbal working memory were mediated by aspects of children's home environment and maternal sensitivity (D.A.H et al., unpublished). Other studies have relied on a less direct approach, examining the influence of environmental factors on neurocognitive abilities among low-SES children. One such study of executive function found that variance in poverty-related variables in addition to residential risk factors predicted executive control [73]. There is specificity in the factors that influence different neurocognitive system outcomes: we have found that measures of parental nurturance in early childhood uniquely predicted memory function at middle school age whereas measures of cognitive stimulation predicted later language function [74]. Research on the causal factors underlying SES disparities is in its early stage and is an important area for future research.
Current knowledge and future research directions
Although abundant research has documented the influence of SES on cognitive ability as measured by IQ tests and school achievement, we have only the most preliminary understanding of the specific neurocognitive effects of SES. Research to date indicates that SES disparities are most robust in language and executive functions, and perhaps also declarative memory, although they are not restricted to these functions, and much remains to be learned about the specific aspects of these functions affected. Therefore, a first recommendation for future research is to strengthen and generalize our understanding of the SES disparities in neurocognitive function (Box 3). As this research area develops, it will be important to bring the same precision and clarity to the definition and measurement of SES (Box 1) as we currently strive to do with the definition and measurement of brain function.
We hope that cognitive neuroscientists who are not themselves primarily interested in SES will also begin to assess and report the SES of their research subjects. This will ensure that SES can be controlled for as a confounding variable when appropriate and might help resolve discrepancies between studies employing different populations. It will also provide a database from which meta-analyses can draw to address the questions raised here more comprehensively. Finally, as cognitive neuroscience is increasingly applied in educational, marketing and forensic contexts, it will become more important to understand socioeconomic variability in brain function [75].
The currently available research also indicates that the environments and experiences of childhood in different socioeconomic strata are at least in part responsible for different neurocognitive outcomes for these children. To the extent that the effects of childhood SES decrease people's ability to succeed through education and skilled jobs, a better mechanistic understanding of these processes has the potential to reduce poverty and to prevent or ameliorate its burden. Economists have recently engaged the problem of the relationship between human capital and SES and argued persuasively that a societal investment in reducing the impact of childhood poverty on cognitive ability is far more efficient than programs designed to reverse its effects later in life [13]. However, the large-sample longitudinal designs that are most appropriate for addressing these questions also provide challenges for cognitive neuroscientists (Box 2).
Unlike many of the phenomena studied in cognitive neuroscience, SES does not lend itself to the kind of experimental manipulation needed to identify causal mechanisms. Randomized intervention studies, however, offer researchers experimental control over hypothesized mechanisms and are thus an important research strategy that is also socially valuable. Interventions can be targeted at any point along the pathway from SES to neurocognitive development, and neurocognitive outcome measures can be added to interventions already in place or in development. One recent study found improved language function in poor children whose families received additional income and education [76]. Interventions can also target the development of specific neurocognitive systems directly, for example with computerized games that train executive abilities [77]. One particularly successful example of an executive function training intervention is the `Tools of the Mind' program, in which low SES preschool children practiced thinking aloud, planning pretend games and other activities involving executive function, and developed dramatically improved performance on laboratory tests of cognitive control [78]. As more is learned about the relationship between SES and brain development, other neurocognitive targets for intervention will be suggested.
Although the cognitive neuroscience of SES has the potential to enable more appropriately targeted, and hence more effective, programs to protect and foster the neurocognitive development of low SES children, it can also be misused or misunderstood as a rationalization of the status quo or `blaming the victim'. This has precedent in social science research on SES, in which characteristic differences between individuals of higher and lower SES have been used by some to argue that low SES individuals are intrinsically less deserving or less valuable members of society. The biological nature of the differences documented by cognitive neuroscience can make these differences seem all the more `essential' and immutable. However, as already reviewed, abundant evidence from developmental neuroscience contradicts the fallacy that brain development follows a fixed, innate program and suggests specific causal pathways by which socioeconomic deprivation can affect brain function.Thus, there is little evidence to suggest differences are essential or immutable.
By studying SES within the framework of cognitive neuroscience, we have the potential to address societal problems and to broaden our understanding of the human brain. The more completely and explicitly SES disparities in cognitive functions can be characterized, the better our ability to test hypotheses concerning the causal mechanisms giving rise to them and the more rationally we can design programs for prevention and remediation. In addition, the data already at hand indicate that the normal human brain is considerably more variable in its organization and function than is reflected in the vast majority of the cognitive neuroscience literature. For the basic science of human brain function, and especially for the understanding of brain development and plasticity, socioeconomic variation is a key phenomenon in cognitive neuroscience.
Box 1. What is SES?
SES is a multidimensional construct that includes measures of economic resources in addition to social factors such as power, prestige and hierarchical social status [1–6]. In fact, multiple family, psychosocial and neighborhood experiences and characteristics that influence development negatively, systematically vary with SES [1,3,6,11]. Measurement of SES is thus complex and controversial, and the most common indicators are income, education and occupation, or some combination thereof [2,3,5]. Although these measures are correlated, there are enough discrepancies that they should not be used interchangeably as they reflect related but different components of SES [2,5]. SES operates at the individual, household or neighborhood level, and different factors that comprise SES influence developmental outcomes in divergent ways [2,5]. Children and adolescents have not yet been able to establish their own individual SES, and thus their status is best measured by the SES of their parents or caregivers, which can affect developmental outcomes independent of their achieved SES later in life. Another approach to SES measurement is subjective social status (SSS), which measures the appraisal of one's status relative to others. SSS is an individual's integration of the various factors that comprise SES and include constructs, such as the prestige of one's university, which traditional measures do not capture [4]. As research into SES, brain development and cognition progress, it will become necessary to disentangle the specific effects of each component and measure of SES on brain development. Resources concerning the theory and practice of SES measurement can be found at the MacArthur Research Network on Socioeconomic Status and Health website (http://www.macses.ucsf.edu/).
Box 2. Methodological challenges.
As research on SES and brain development proceeds, there are multiple methodological challenges that the field must confront, including:
Internal versus external validity
One tactic to strengthen inferences concerning SES effects is to exclude subjects with disorder or a family history of disorder (e.g. see Ref. [32]). The cost of this practice is external validity: because prevalence rates are higher in lower-SES populations (e.g. see Refs [1,6,7,14]), exclusion might eliminate the variability we are interested in explaining. Both exclusionary practices and more liberal inclusionary criteria have utility and should be carefully employed depending on the research question.
Control variables
SES-related variables can either be confounds or mediators of the observed effect, and it will be important to separate a priori which variables are confounders to control for and which are theoretically interesting mediators.
Correlated mediators
Many of the probable mediators underlying SES differences in brain development (i.e. stress, nutrition, family environment, etc.) are highly correlated (e.g. see Ref. [11], and thus research programs should aim to measure multiple mediators to separate the unique effects of each mechanism.
Separating SES effects from race and ethnicity effects
There is no reason to assume that the effects of SES are uniform across all racial and ethnic groups, and race, ethnicity and SES might operate independently or in concert [2,5,6]. Consequently, future research designs should focus on isolating specific SES effects and contextualizing findings in terms of race and ethnicity.
Sensitivity to non-linearity
Brain development is not linear [59–65], and Type II errors in cross-sectional designs are possible if there are SES effects on the timing of developmental trajectories that are not considered.
Designs promoting causal inferences
Investigators can take advantage of research design and analysis strategies such as repeated, time-lagged measurements, structural equation modeling and propensity scores to strengthen causal inferences [6]. This is particularly important when measuring adult outcomes, in which early- and late-SES effects must be isolated.
Performance differences
When aiming to identify group differences in neural processing, it is necessary to consider performance differences on the task at hand (e.g. see Ref. [79]). As there are SES disparities in neurocognitive performance, future studies must discriminate between SES differences in brain activation related to performance per se and SES differences in brain–behavior relationships that are independent of performance differences.
Box 3. Future directions.
Continued basic research on the main effects of SES on neurocognitive performance and brain development is warranted, as is research into the mechanisms underlying these relationships. Early research indicates the utility of a broader approach that also includes the following approaches:
Elaboration of the relationship using a developmental approach
Once initial main effects are established, several questions follow: is the SES–brain development relationship linear? When do disparities emerge, and do they represent a developmental delay or do they persist into adulthood? How do these differences alter our understanding of basic human brain development?
Context sensitivity
Given the effect of priming power and hierarchy [50–52], what is the effect of such manipulations across SES levels? Do SES differences remain when manipulating the experimental environment?
More than main effects
SES differences might not be detected unless SES is examined as a moderator of brain-behavior relationships.
Affect–Cognition interplay
Thus far, most research has concerned basic cognitive processes. However, baseline EEG, structural MRI and fMRI studies indicate differences in affective processing that should be explored in greater depth.
Deficits and adaptations
Differences in neural processing that lead to performance deficits in laboratory tasks could, in certain contexts, be useful adaptations. Future research should, in interpretation and design, expressly consider the context of performance and processing strategies and investigate possible strengths and weaknesses.
Resilience
Despite growing up in poverty, many individual children will not exhibit deficits or differences in performance or neural processing, and thus offer an opportunity to examine the protective factors that promote resilience.
Genetically informative approaches
As the field develops, the tools of behavioral genetics will be useful to help identify environmental effects and gene–environment interactions that are promising areas for intervention (for a methodological overview see Ref. [80]).
Interventions
Intervention studies are practically and ethically important to conduct to reduce the burden on low-SES communities and bring experimental rigor to the testing of mechanistic hypotheses. Any novel targets for intervention are tentative, although interventions on SES itself and the training of specific cognitive functions directly seem promising. Neurocognitive measures should also be used in evaluating the effects of interventions currently in progress or in development in addition to the effect of natural experiments influencing SES levels.
Acknowledgements
The writing of this article was supported by NIH grants R01-HD043078, R01-HD055689, R01-DA14129 and R01 DA0189913, as well as ONR grant N000140710034. The authors thank Kim Noble for her tremendous contributions to much of the research reviewed here and David Kraemer for assistance with figure preparation.
References
- 1.Adler NE, et al. Socioeconomic status and health: the challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Braveman PA, et al. Socioeconomic status in health research: one size does not fit all. J. Am. Med. Assoc. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 3.Duncan GJ, Magnuson KA. Off with Hollingshead: socioeconomic resources, parenting, and child development. In: Bornstein MH, Bradley RH, editors. Socioeconomic Status, Parenting, and Child Development. Lawrence Erlbaum Associates; 2003. pp. 83–106. [Google Scholar]
- 4.Goodman E, et al. Adolescents' perceptions of social status: development and evaluation of a new indicator. Pediatrics. 2001;108:e31–e38. doi: 10.1542/peds.108.2.e31. [DOI] [PubMed] [Google Scholar]
- 5.Krieger N, et al. Measuring social class in U.S. public health research: concepts, methodologies, and guidelines. Annu. Rev. Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 6.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu. Rev. Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 7.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 8.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7:55–71. [PubMed] [Google Scholar]
- 9.Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annu. Rev. Psychol. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- 10.Duncan GJ, et al. How much does childhood poverty affect the life chances of children? Am. Sociol. Rev. 1998;63:406–423. [Google Scholar]
- 11.Evans GW. The environment of childhood poverty. Am. Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 12.Guo G, Mullan-Harris K. The mechanisms mediating the effects of poverty on children's intellectual development. Demography. 2000;37:431–447. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 13.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- 14.McLoyd VC. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 15.Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev. Educ. Res. 2005;75:417–453. [Google Scholar]
- 16.Noble KG, et al. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 17.Noble KG, et al. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 18.Farah MJ, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 19.Hart B, Risely TR. Meaningful Differences in the Everyday Experiences of American Children. Brookes Publishing; 1995. [Google Scholar]
- 20.Whitehurst GJ. Language processes in context: language learning in children reared in poverty. In: Adamson LB, Romski MA, editors. Research on Communication and Language Disorders: Contribution to Theories of Language Development. Brookes; 1997. pp. 233–266. [Google Scholar]
- 21.Ardila A, et al. The influence of the parents' educational level on the development of executive functions. Dev. Neuropsychol. 2005;28:539–560. doi: 10.1207/s15326942dn2801_5. [DOI] [PubMed] [Google Scholar]
- 22.Evans GW, English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 23.Evans GW, Rosenbaum J. Self-regulation and the income-achievement gap. Early Child. Res. Q. 2008;23:504–514. [Google Scholar]
- 24.Howse RB, et al. Motivation and self-regulation as predictors of achievement in economically disadvantaged young children. J. Exp. Educ. 2003;71:151–174. [Google Scholar]
- 25.Hughes C, Ensor R. Executive function and theory of mind in 2 year olds: a family affair? Dev. Neuropsychol. 2005;28:645–668. doi: 10.1207/s15326942dn2802_5. [DOI] [PubMed] [Google Scholar]
- 26.Lipina SJ, et al. Poverty and executive performance in preschool pupils from Buenos Aires city (República Argentina) Interdisciplinaria. 2004;21:153–193. [Google Scholar]
- 27.Lipina SJ, et al. Performance on the A-not-B task of Argentinean infants from unsatisfied and satisfied basic needs homes. Int. J. Psychol. 2005;39:49–60. [Google Scholar]
- 28.Lupien SJ, et al. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 29.Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004;75:1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- 30.Singh-Manoux A, et al. Socioeconomic position across the lifecourse: how does it relate to cognitive function in mid-life? Ann. Epidemiol. 2005;15:572–578. doi: 10.1016/j.annepidem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Turrell G, et al. Socioeconomic position across the lifecourse and cognitive function in late middle age. J. Gerontol. Series B. 2002;57:S43–S51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- 32.Waber DP, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J. Int. Neuropsychol. Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 33.Wiebe SA, et al. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Dev. Psychol. 2008;44:575–587. doi: 10.1037/0012-1649.44.2.575. [DOI] [PubMed] [Google Scholar]
- 34.Diamond A. The development and neural bases of memory functions as indexed by the AB and delayed response tasks in human infants and infant monkeys. Ann. N. Y. Acad. Sci. 1990;608:267–317. doi: 10.1111/j.1749-6632.1990.tb48900.x. [DOI] [PubMed] [Google Scholar]
- 35.Rueda MR, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Levine SC, et al. Socioeconomic status modifies the sex difference in spatial skill. Psychol. Sci. 2005;16:841–845. doi: 10.1111/j.1467-9280.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 37.Hermann D, Guadagno MA. Memory performance and socioeconomic status. Appl. Cogn. Psychol. 1997;11:113–120. [Google Scholar]
- 38.Otero GA. Poverty, cultural disadvantage and brain development: a study of pre-school children in Mexico. Electroencephalogr. Clin. Neurophysiol. 1997;102:512–516. doi: 10.1016/s0013-4694(97)95213-9. [DOI] [PubMed] [Google Scholar]
- 39.Tomarken AJ, et al. Resting frontal brain activity: linkages to maternal depression and socio-economic status among adolescents. Biol. Psychol. 2004;67:77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Stevens C, et al. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Dev. Sci. doi: 10.1111/j.1467-7687.2009.00807.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Angiulli A, et al. Children's event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22:293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- 42.D'Angiulli A, et al. Towards a cognitive science of social inequality: children's attention-related ERPs and salivary cortisol vary with their socioeconomic status. Cogn. Sci. in press. [Google Scholar]
- 43.Czernochowski D, et al. Use it or lose it? SES mitigates age-related decline in a recency/recognition task. Neurobiol. Aging. 2008;29:945–958. doi: 10.1016/j.neurobiolaging.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishiyama MM, et al. Socioeconomic disparities affect prefrontal function in children. J. Cogn. Neurosci. doi: 10.1162/jocn.2009.21101. (in press) doi:10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- 45.Noble KG, et al. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 46.Raizada RDS, et al. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gianaros PJ, et al. Potential neural embedding of parental social standing. Soc. Cogn. Affect. Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckert MA, et al. Planar asymmetry tips the phonological playground and environment raises the bar. Child Dev. 2001;72:988. doi: 10.1111/1467-8624.00330. [DOI] [PubMed] [Google Scholar]
- 49.Gianaros PJ, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc. Cogn. Affect. Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guinote A. Power affects basic cognition: increased attentional inhibition and flexibility. J. Exp. Soc. Psychol. 2007;43:685–697. [Google Scholar]
- 51.Smith PK, et al. Lacking power impairs executive functions. Psychol. Sci. 2008;19:441–447. doi: 10.1111/j.1467-9280.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- 52.Zink CF, et al. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capron C, Duyme M. Assessment of effects of socioeconomic status on IQ in a full cross-fostering study. Nature. 1989;340:552–554. [Google Scholar]
- 54.Turkheimer E, et al. Socioeconomic status modifies heritability of IQ in young children. Psychol. Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- 55.Gräff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav. Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Pascual-Leone A, et al. The plastic human brain cortex. Annu. Rev. Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 58.Greenough WT, et al. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- 59.Lebel C, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 60.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagy Z, et al. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 63.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 64.Toga AW, et al. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casey BJ, et al. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 66.Bellinger D, et al. Longitudinal analyses of prenatal and postnatal lead exposure and early cognitive development. N. Engl. J. Med. 1987;316:1037–1043. doi: 10.1056/NEJM198704233161701. [DOI] [PubMed] [Google Scholar]
- 67.Surkan PJ, et al. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodman E, et al. Social disadvantage and adolescent stress. J. Adolesc. Health. 2005;37:484–492. doi: 10.1016/j.jadohealth.2004.11.126. [DOI] [PubMed] [Google Scholar]
- 69.Lantz PM, et al. Stress, life events, and socioeconomic disparities in health: results from the Americans' changing lives study. J. Health Soc. Behav. 2005;46:274–288. doi: 10.1177/002214650504600305. [DOI] [PubMed] [Google Scholar]
- 70.Cohen S, et al. Socioeconomic status is associated with stress hormones. Psychosom. Med. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 71.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 72.Lupien SJ, et al. Beyond the stress concept: allostatic load–a developmental biological and cognitive perspective. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Vol 2: Developmental Neuroscience. 2nd edn John Wiley & Sons; 2006. pp. 578–628. [Google Scholar]
- 73.Li-Grining CP. Effortful control among low-Income preschoolers in three cities: stability, change, and individual differences. Dev. Psychol. 2007;43:208–221. doi: 10.1037/0012-1649.43.1.208. [DOI] [PubMed] [Google Scholar]
- 74.Farah MJ, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev. Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 75.Farah MJ. Neuroethics: the practical and the philosophical. Trends Cogn. Sci. 2005;9:34–40. doi: 10.1016/j.tics.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Fernald LCH, et al. Role of cash in conditional cash transfer programmes for child health, growth, and development: an analysis of Mexico's Oportunidades. Lancet. 2008;371:828–837. doi: 10.1016/S0140-6736(08)60382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rueda MR, et al. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diamond A, et al. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fair DA, et al. A comparison of analysis of variance and correlation methods for investigating cognitive development with functional magnetic resonance imaging. Dev. Neuropsychol. 2006;30:531–546. doi: 10.1207/s15326942dn3001_2. [DOI] [PubMed] [Google Scholar]
- 80.Moffitt TE. The new look of behavioral genetics in developmental psychopathology: gene–environment interplay in antisocial behaviors. Psychol. Bull. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]