Abstract
Purpose
There is growing evidence implicating that neutrophil gelatinase–associated lipocalin (NGAL) plays a role in the development and progression of cancers. However, the effect of NGAL in colorectal carcinoma (CRC) has not been clearly elucidated. In this study, we investigated the role of NGAL in the tumorigenesis and progression of CRC and evaluated the clinical value of NGAL expression.
Experimental Design
We examined NGAL expression in 526 colorectal tissue samples, including 53 sets of matched specimens (histologically normal mucosa, adenomas, and carcinomas) using immunohistochemical analysis. In CRCs, correlations between NGAL expression and clinicopathologic parameters were analyzed, and survival analysis was conducted. The role of NGAL was further tested using mouse xenograft models.
Results
NGAL expression was elevated during the colorectal adenoma–carcinoma sequence both among the 526 cases (rs = 0.66, P < 0.001) and in the 53 sets of matched specimens (rs = 0.60, P < 0.001). In CRCs, NGAL expression was associated with cancer stage (P = 0.041) and tumor recurrence in stage II patients (P = 0.037). Survival analysis revealed that NGAL expression was an independent prognostic factor for overall survival (HR = 1.84, P = 0.004) and for disease-free survival of stage II patients (HR = 5.88, P = 0.021). In mouse models, the xenografts in cecum and spleen were heavier and more numerous in the group injected with NGAL-overexpressing CRC cells (P < 0.05).
Conclusions
NGAL overexpression may promote the tumorigenesis and progression of CRC. Detecting NGAL expression in tumor tissues may be useful for evaluating prognosis of patients with CRC.
Introduction
Colorectal cancer (CRC) is the third-most common cancer in men (accounting for 10.0% of the total) and the second-most common cancer in women (accounting for 9.4% of the total) worldwide (1). Moreover, CRC is the fourth-most common cause of cancer deaths, accounting for 8% of all cancer-related deaths (1). Most CRCs undergo neoplastic progression through the adenoma–carcinoma sequence accompanied by an accumulation of successive genetic alterations (2-4). Despite extensive research, many key factors and mechanisms underlying the colorectal adenoma-carcinoma sequence and the progression of CRC are still unknown. Even with improvements in surgical and therapeutic methods, especially molecularly targeted therapy, the 5-year survival rate still ranges from 96% for patients with stage I CRC to 5% for patients with stage IV CRC (5). Therefore, characterizing novel molecular events involved in the development and progression of CRC would help elucidate its mechanisms and identify potential therapeutic targets.
Neutrophil gelatinase–associated lipocalin (NGAL), a member of the lipocalin superfamily, was first isolated as a 25-kDa glycoprotein covalently bound with matrix metalloproteinase 9 (MMP9) in human neutrophils (6, 7). NGAL binds bacterial catecholate-type ferric siderophores and acts as a potent bacteriostatic agent by sequestering iron (8). Although initially found in neutrophils, NGAL was later found to be expressed in most types of cells (especially epithelial cells) and to actively participate in the diverse processes of growth, development, and differentiation of many tissues (9-13). Recently, deregulation of NGAL expression has also been reported to play a role in the development and progression of several cancers (14-20). However, the reported effects of NGAL in human tumors are often contradictory. For instance, NGAL was shown to have a protumoral effect in breast (14-16), stomach (17), and esophageal cancers (18). In contrast, some studies showed that NGAL exerted an antitumoral and antimetastatic effect in ovarian (19) and pancreatic cancers (20).
For CRC, the expression and role of NGAL during the adenoma-carcinoma sequence have not been defined. Moreover, while most studies have shown that NGAL overexpression promoted the progression of CRC (21-23), one study suggested that NGAL had an antimetastatic effect in colon cancer (24). Thus, the role of NGAL in the initiation and progression of CRC needs to be further clarified to determine whether NGAL is a valuable marker for CRC prognosis and/or a potential target for therapy.
The purpose of our current study was to explore the role of NGAL in the tumorigenesis and progression of CRC and evaluate the clinical value of NGAL determination in tissues and plasma. We examined the expression of NGAL in 526 colorectal tissue samples (including histologically normal mucosa, adenomas with low-grade dysplasia, adenomas with high-grade dysplasia, and carcinomas), especially in 53 sets of matched specimens, to evaluate the role of NGAL in tumorigenesis of CRC. We analyzed the association of NGAL expression with clinicopathologic parameters and conducted survival analysis to assess the effects of NGAL on the progression of CRC. We also used xenograft mouse models to test the role of NGAL in the development and progression of CRC. In addition, we evaluated the prognostic value of NGAL expression in CRC tissues and the feasibility of using NGAL level in plasma as a biomarker that reflects disease progression for CRC patients.
Materials and Methods
Clinical samples
We obtained 526 formalin-fixed, paraffin-embedded colorectal tissue samples (including 287 carcinomas, 105 adenomas with low-grade dysplasia, 40 adenomas with high-grade dysplasia, and 94 samples of histologically normal mucosa which were distant from the corresponding cancer or adenoma tissues more than 5 cm) from the Department of Pathology at Tianjin Medical University Cancer Institute and Hospital. All of the samples were from inpatients undergoing surgical operation from January 2000 to December 2004, and none of the patients had received any chemotherapy or radiotherapy before operation. All of the specimens and clinical data (see Results) were collected after we received Institutional Review Board approval from Tianjin Medical University Cancer Institute and Hospital. Among those 526 colorectal tissue samples, there were 53 matched samples (159 specimens, including carcinomas, adenomas and histologically normal mucosa) that had been from 1 operation for each patient.
We also obtained blood samples from 39 inpatients with CRC at the Tianjin Medical University Cancer Institute and Hospital between January 2007 and December 2009. Age (±5 years) frequency-matched controls with no evidence of cancer were recruited from donors who attended health screening in the Center of Health Examination in the same hospital during the same period. The study protocol was approved by the hospital’s Institutional Review Board. All participants gave informed consent for the use of their samples for research purposes. Whole blood (10 mL) was drawn by vein puncture and collected into heparinized tubes and centrifuged at 1,380 × g for 10 minutes. Plasma was aliquoted and stored at −80 °C until analyzed.
Tissue microarray construction and immunohistochemical analysis
Tissue microarrays (TMA) were constructed using a manual tissue microarray instrument (Beecher Instruments) equipped with a 2.0-mm punch needle. Each TMA was composed of 48 tissue samples (8 × 6 array), and 11 TMA blocks were prepared for the 526 colorectal tissue specimens. For each tissue sample, the typical area was selected on the basis of the appearance of original hematoxylin and eosin (H&E)-stained slides.
To carry out the immunohistochemical analysis, we used the streptavidin–biotin peroxidase (SP) method as described previously (25). Briefly, after antigen retrieval for 3 minutes in 0.1 mol/L citrate buffer (pH 6.0) and subsequent incubation in 10% normal goat serum for 15 minutes at room temperature, we incubated the TMA sections overnight at 4 °C with rat antihuman NGAL monoclonal antibody (25 μg/mL in PBS; R&D Systems). We then immunostained the TMA sections using the Histostain-SP Kit (Invitrogen) and revealed the signals using 3, 3′-diaminobenzidine as the substrate. For a positive control, we used a tissue section of colitis in which infiltrative neutrophils were positive for NGAL. We prepared a negative control by substituting PBS for the antibody.
The NGAL expression was analyzed only in histologically normal and neoplastic epithelial cells, not in stroma tissues. NGAL-positive samples were defined as those with brown staining in the cytoplasm. The results of NGAL immunohistochemical analysis were estimated according to the method described by Moniaux and colleagues (26) with some modifications. The staining intensity of NGAL was graded on a scale from 0 to 3 (0 for no staining, 1 for weak immunoreactivity, 2 for moderate immunoreactivity, and 3 for strong immunoreactivity) The percentage of immunoreactivity was scored on a scale from 0 to 3 (0 for no positive cells, 1 for <25% of cells positive, 2 for 25%–50% of cells positive, and 3 for >50% of cells positive). The staining intensity score and the percentage of immunoreactivity score were then multiplied to obtain a composite score (CS; 0, 1, 2, 3, 4, 6, or 9). Depending on the CS, NGAL expression was classified as negative (−; CS = 0), weakly positive (+; CS = 1 or 2), moderately positive (++; CS = 3 or 4), or intensely positive (+++; CS = 6 or 9). In analysis, NGAL high expression survival was defined as (+++) and (+++), whereas low expression was defined as (−) and (+).
Determination of plasma NGAL levels by sandwich ELISA
We determined the concentration of NGAL in the plasma using the Lipocalin-2/NGAL Human ELISA Kit (BioVendor R&D) according to the manufacturer’s instructions. Briefly, standards, quality controls, and samples were incubated on plates and precoated with the polyclonal antihuman NGAL antibody. Biotinylated polyclonal NGAL detection antibody was added and incubated for 1 hour. Finally, horseradish peroxidase (HRP)-conjugated streptavidin was added and results were obtained by measuring the absorbance at 450 nm in a microplate reader. The obtained data were expressed as ng/mL plasma.
Animal model experiments
In our previous study, we found that NGAL overexpression promoted the invasion of KM12C CRC cells in vitro (23). In this study, we further determined the role of NGAL in CRC in vivo. Male 8- to 12-week-old athymic nude mice were purchased from the Animal Production Area of the National Cancer Institute at Frederick and fed a commercial diet, given water ad libitum and subjected to a 12-hour light–dark cycle. Our experimental protocol was approved by the Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center.
The experimental animal model for cecal xenografts was established as previously described (27) with some modifications. Briefly, 3 groups of mice (n = 5 per group) received intraperitoneal anesthesia (0.2 mL sodium pentobarbital at 9 mg/mL), and the abdomen of each mouse was prepared for sterile surgery. A small midabdominal incision was made, and the cecum was exteriorized and supported on sterile gauze. NGAL-overexpressing KM12C (KM12C-NGAL; ref. 23), vehicle control (KM12C-Neo; ref. 23), and parental control (KM12C) CRC cells (5 × 105 cells in 50 μL of HBSS) were injected into the cecal wall of the nude mice using a 30-gauge needle. The cecum was reinternalized and the incision closed using 1 layer of wound clips. All mice were killed at 6 weeks after surgery because some mice were dying due to the tumor burden. After the body weight was recorded, the cecal tumors and livers were excised from each mouse, weighed, fixed in formalin, and embedded in paraffin. Then, the tissues were subjected to H&E and immunohistochemical staining.
An experimental animal model for liver metastasis of CRC cells was established as previously described (27) with some modifications. Briefly, we anesthetized 3 groups of mice (n = 7 per group) as stated previously and lifted the spleen onto sterile gauze just outside the wound created by a left-center subcostal incision. We then injected KM12C, KM12C-Neo, or KM12C-NGAL cells (2.5 × 105 cells in 50 μL of HBSS) into the spleen parenchyma. The spleen was returned to the abdominal cavity and the incision was closed in 1 layer with metal wound clips. At 4 months following the injection of the tumor cells, some mice were dying because of the tumor burden; therefore, all mice were killed and their body weight was recorded. We collected and processed spleen and liver tissues and subjected them to H&E staining.
Statistical analysis
Differences in NGAL immunohistochemical staining between groups were compared using χ2 or Fisher’s exact tests in human samples. The correlation between NGAL expression and colorectal adenoma–carcinoma sequence was evaluated by calculating the Spearman rank correlation coefficient. Moreover, mean ± SD of human plasma NGAL levels and percentages of xenograft weight relative to total body weight in animal models were calculated, and differences in the means were analyzed using 1-way ANOVA or Student’s t test. We also used the Kaplan–Meier method and the log-rank test in univariate survival analysis, and we used the Cox proportional hazards regression model in our multivariate analysis. We used SPSS version 16.0 (IBM) to conduct our statistical analysis. Two-tailed values of P < 0.05 were considered statistically significant.
Results
NGAL expression was elevated during the colorectal adenoma–carcinoma sequence
To examine the effects of NGAL expression during the initiation of CRC, we carried out immunohistochemical staining for NGAL using TMAs derived from 526 colorectal tissue specimens (see Materials and Methods). We observed significantly different NGAL expression during the colorectal adenoma-carcinoma sequence (P < 0.001; Table 1). In most histologically normal mucosa tissue samples, NGAL was absent (Fig. 1A and B and Table 1). Weak-to-moderate immunostaining for NGAL was detected mainly in the apocrine area in the adenomas with low-grade dysplasia (Fig. 1C and D). In the adenomas with high-grade dysplasia, NGAL showed a diffuse cytoplasmic expression pattern and was detected in more tumor cells than in adenomas with low-grade dysplasia (Fig. 1E and F). The most intense immunostaining for NGAL occurred in the carcinomas (Fig. 1G and H). Furthermore, a positive relationship existed between NGAL expression and the adenoma-carcinoma sequence (rs = 0.66, P < 0.001).
Table 1.
NGAL expression increased during the colorectal adenoma–carcinoma sequence
| Specimens | n | NGAL expression, n (%) |
χ 2 | P | |||
|---|---|---|---|---|---|---|---|
| (−) | (+) | (++) | (+++) | ||||
| All specimens | |||||||
| Histologically normal mucosa | 94 | 76 (80.9) | 16 (17.0) | 2 (2.1) | 0 (0) | 251.054 | <0.001 |
| Adenoma with low-grade dysplasia | 105 | 59 (56.2) | 24 (22.9) | 9 (8.6) | 13 (12.4) | ||
| Adenoma with high-grade dysplasia | 40 | 15 (37.5) | 9 (22.5) | 6 (15.0) | 10 (25.0) | ||
| Carcinoma | 287 | 17 (5.9) | 58 (20.2) | 77 (26.8) | 135 (47.0) | ||
| Matched specimensa | |||||||
| Histologically normal mucosa | 53 | 44 (83.0) | 8 (15.1) | 1 (1.9) | 0 (0) | 71.734 | <0.001 |
| Adenoma | 53 | 19 (35.8) | 15 (28.3) | 6 (11.3) | 13 (24.5) | ||
| Carcinoma | 53 | 8 (15.1) | 14 (26.4) | 5 (9.4) | 26 (49.1) | ||
Every set of normal mucosa, adenoma, and carcinoma specimens was from 1 surgery per patient.
Figure 1.
Immunohistochemical staining for NGAL (brown) in tissues representing colorectal adenoma–carcinoma sequence (SP method). NGAL was absent from histologically normal mucosa (A and B) and showed weak expression in apocrine area of adenoma with low-grade dysplasia (C and D), moderate diffuse cytoplasmic expression in adenoma with high-grade dysplasia (E and F), and intense expression in carcinoma (G and H).
Heterogeneity in baseline gene or protein expression existed among individuals; therefore, to gain a more definitive picture of NGAL expression in the adenoma–carcinoma sequence, we analyzed NGAL expression in the 53 sets of matched specimens (including histologically normal mucosa, adenomas, and carcinomas) obtained from 1 operation for each patient. Table 1 showed that NGAL expression levels increased consistently from normal mucosa to adenoma to carcinoma within individual patients. We also observed a positive correlation between NGAL expression and the adenoma–carcinoma sequence in these 53 sets of matched samples (rs = 0.60, P < 0.001).
Increased NGAL levels were associated with advanced stages and poor prognosis in CRC
Table 2 shows the relationships between NGAL expression and clinicopathologic parameters in the 287 CRC tissue samples. The expression of NGAL was significantly higher in advanced-stage carcinomas (stages III and IV) than in early-stage carcinomas (stages I and II). There was no significant correlation between NGAL expression and the patients’ gender, age, tumor location, tumor size, and tumor grade.
Table 2.
NGAL expression and clinicopathologic parameters for 287 patients with CRC
| NGAL expression, n (%) |
P | |||||
|---|---|---|---|---|---|---|
| n | (−) | (+) | (++) | (+++) | ||
| Gender | ||||||
| Men | 144 | 12 (8.3) | 24 (16.7) | 44 (30.6) | 64 (44.4) | 0.088 |
| Women | 143 | 5 (3.5) | 34 (23.8) | 33 (23.1) | 71 (49.7) | |
| Age, y | ||||||
| <59 | 130 | 7 (5.4) | 27 (20.8) | 32 (24.6) | 64 (49.2) | 0.842 |
| ≥59 | 157 | 10 (6.4) | 31 (19.7) | 45 (28.7) | 71 (45.2) | |
| Tumor locationa | ||||||
| Right side | 147 | 7 (4.8) | 27 (18.4) | 43 (29.3) | 70 (47.6) | 0.599 |
| Left side | 140 | 10 (7.1) | 31 (22.1) | 34 (24.3) | 65 (46.4) | |
| Tumor size, cm | ||||||
| <5 | 104 | 9 (8.7) | 19 (18.3) | 28 (26.9) | 48 (46.2) | 0.497 |
| ≥5 | 183 | 8 (4.4) | 39 (21.3) | 49 (26.8) | 87 (47.5) | |
| Tumor grade | ||||||
| I and II | 161 | 7 (4.3) | 33 (20.5) | 43 (26.7) | 78 (48.4) | 0.635 |
| III | 126 | 10 (7.9) | 25 (19.8) | 34 (27.0) | 57 (45.2) | |
| Cancer stage | ||||||
| I and II | 189 | 14 (7.4) | 44 (23.3) | 52 (27.5) | 79 (41.8) | 0.041 |
| III and IV | 98 | 3 (3.1) | 14 (14.3) | 25 (25.5) | 56 (57.1) | |
Right side comprises the cecum, ascending colon, hepatic flexure, and transverse colon; left side comprises the splenic flexture, descending and sigmoid colon, and rectum.
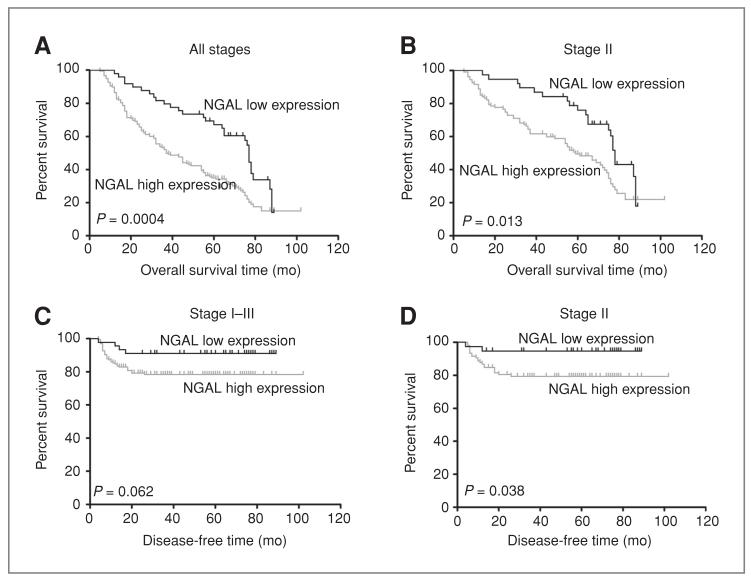
Survival analysis showed that among the 227 CRC cases with a follow-up period longer than 5 years, 65 patients (28.6%) were alive at the end of the follow-up period, with a median survival time of 70 months (range: 60-102 months), and 162 patients (71.4%) had died as a result of CRC. The univariate analysis showed that the patients with high NGAL expression had worse overall survival time than did those patients with low NGAL expression (P = 0.0004; Fig. 2A). Because there was a correlation between NGAL expression and stage, we conducted univariate analysis for NGAL in patients with stage II, III, or IV CRC, respectively (only 1 patient had stage I CRC and so was excluded from this analysis). Figure 2B showed that in patients with stage II CRC, those with high NGAL expression had a shorter overall survival time than did patients with low NGAL expression (P = 0.013). In patients with stages III and IV CRC, the overall survival time was not significantly different between patients with different NGAL expression (P = 0.244 and P = 0.185, respectively). The subsequent multivariate analysis revealed that high NGAL expression (HR = 1.84, P = 0.004), advanced cancer stage (HR = 2.78, P < 0.001), and no chemotherapy (HR = 2.06, P < 0.001) were independent indicators of poor prognosis for patients with CRC (Supplementary Table S1). For patients with stage II CRC, high NGAL expression (HR = 2.13, P = 0.004), tumors in the left side (HR = 1.74, P = 0.020), and no chemotherapy (HR = 2.17, P = 0.003) were adverse predictors for overall survival (Supplementary Table S2).
Figure 2.
Univariate overall survival (A and B) and disease-free survival (C and D) analysis for NGAL in patients with colorectal carcinoma. The patients with high NGAL expression had worse overall survival than those with low NGAL expression in the 227 CRC cases with the follow-up period longer than 5 years (A) and in the 145 patients with stage II CRC (B). For the 211 patients with stages I to III CRC, there was a trend that patients with high NGAL expression had worse disease-free survival time (C). For the stage II patients, the cases with high NGAL expression had worse disease-free survival than those with low NGAL expression (D).
In the follow-up period, 18.0% (38 of 211) CRC patients of stages I to III had local or distant recurrence (Table 3). More patients with high NGAL expression were found to relapse, compared with those with low NGAL expression (P = 0.073; Table 3). For CRC patients of stage II, there was a significant association between NGAL expression and recurrence (P = 0.037; Table 3). The survival analysis for patients with stages I to III CRC showed a trend that the disease-free time was shorter in patients with high NGAL expression (P = 0.062; Fig. 2C). For stage II CRC, patients with high NGAL expression had worse disease-free survival than those with low NGAL expression (P = 0.038; Fig. 2D). The multivariate analysis showed that high NGAL expression (HR = 5.88, P = 0.021) and no chemotherapy (HR = 4.98, P = 0.009) were independent indicators of poor disease-free survival for patients with stage II CRC (Supplementary Table S2).
Table 3.
Relationship between NGAL expression and local or distant recurrence in CRC patients of stages I to III
| NGAL expression |
n | Stage I–IIIa |
Stage II |
Stage III |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rec | No-rec | P | Rec | No-rec | P | Rec | No-rec | P | ||
| Low | 45 | 4 | 41 | 0.073 | 2 | 36 | 0.037 | 2 | 5 | 0.658 |
| High | 166 | 34 | 132 | 21 | 86 | 13 | 45 | |||
| Total | 211 | 38 | 173 | 23 | 122 | 15 | 50 | |||
Abbreviations: Rec, recurrence; No-rec, no recurrence.
There was only 1 patient of stage I, who had no recurrence.
Given that NGAL is a secreted protein, we considered that NGAL could be detected in body fluids and might be associated with disease status. We investigated NGAL plasma levels in healthy adults and patients in different CRC stages using ELISA. Although plasma NGAL levels seemed to be higher in CRC patients than in healthy subjects, there was no significant difference between the 2 groups (t = 0.94, P = 0.352). Likewise, NGAL in the plasma of patients with advanced-stage (stages III and IV) CRC was higher than in patients with early-stage (stages I and II) CRC (Supplementary Fig. S1A); the patients with metastasis had higher plasma NGAL levels than did patients without metastasis (Supplementary Fig. S1B). However, the differences were not statistically significant.
NGAL overexpression enhanced tumorigenesis and potentially increased liver metastasis of CRC cells in animal models
The aforementioned clinical correlation studies provided evidence that NGAL might be a protumorigenesis and prometastasis factor for CRC. Furthermore, NGAL overexpression was shown to promote cell invasion in KM12C CRC cells in our previous study (23). To test further the role of NGAL in CRC, we carried out mouse xenograft experiments using 2 established mouse model systems.
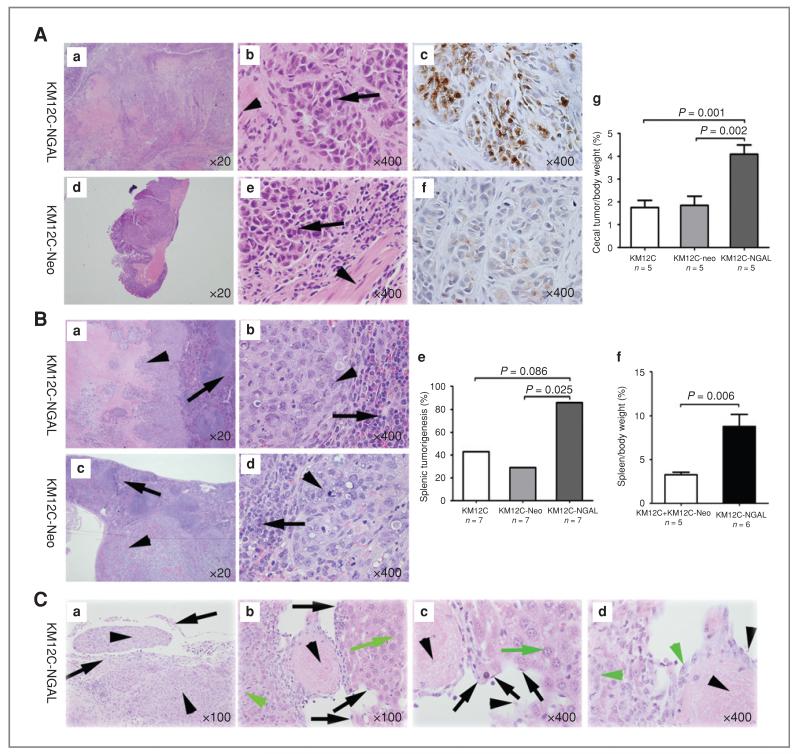
In the first in vivo experiment, we injected NGAL overexpressing KM12C (KM12C-NGAL), vehicle control (KM12C-Neo), or parental control (KM12C) cells into the cecum of nude mice. Tumors grew in the wall of the cecum in every injected mouse, with poorly differentiated cancer cells infiltrating into the muscularis of the cecum (Fig. 3A). Immunohistochemical staining for NGAL in xenografts showed that NGAL expression was higher in the tumors of the mice injected with KM12C-NGAL cells than in the mice injected with KM12C or KM12C-Neo cells (Fig. 3A). The percentage of cecal tumor/body weight in the mice injected with KM12C-NGAL cells was significantly higher than that in the control groups (Fig. 3A). However, no metastasis was found in any mouse in this experiment.
Figure 3.
NGAL overexpression enhanced tumorigenesis and potentially increased liver metastasis of CRC cells in animal models. A, xenografts in cecum after intracecal injection. KM12C-NGAL, KM12C-Neo, or KM12C cells were injected into the cecal wall of nude mice. Tumor cells of the xenografts (arrows) invaded into muscularis (arrowheads) of cecum (b and e). The immunohistochemical staining for NGAL was strong in xenografts of the mice injected with KM12C-NGAL cells (c), and weak in the xenografts of the mice injected with KM12C-Neo cells (f). The percentage of cecal tumor/body weight in the mice injected with KM12C-NGAL was higher than in the mice with KM12C or KM12C-Neo cells (g). B, xenografts in spleen after intrasplenic injection. KM12C-NGAL (a and b), KM12C-Neo (c and d), or KM12C cells were injected into the spleen of nude mice. Arrows denote splenic tissue, and arrowheads indicate tumor cells. The number of mice with splenic xenografts was larger in the group injected with KM12C-NGAL than in the group with KM12C-Neo cells (e). For the mice with splenic tumors, the percentage of spleen/body weight in the mice injected with KM12C-NGAL was higher than that in control groups (f). C, liver metastasis was observed in a nude mouse injected with KM12C-NGAL cells. a, CRC cells (arrowheads) invaded splenic capsule (arrows) in the mouse with liver metastasis; b–d, vascular invasion was found in liver metastasis. Black arrows denote vascular endothelial cells, black arrowheads indicate blood cells, green arrows denote hepatocytes, and green arrowheads indicate metastatic CRC cells.
To maximize our understanding of the effects of NGAL on metastasis, we next carried out intrasplenic injection experiment. As shown in Figure 3B, more tumor nodules were observed in the spleen of the mice injected with KM12C-NGAL (85.7%; 6 of 7) than in the mice injected with KM12C-Neo (28.6%; 2 of 7) or KM12C cells (42.9%; 3 of 7). For the mice with splenic tumors, the percentage of spleen/body weight was significantly higher in the group injected with the KM12C-NGAL cells than that in the control groups (Fig. 3B). We observed liver metastasis in 1 mouse (14.3%; 1 of 7) injected with KM12C-NGAL cells. In the mouse with liver metastasis, the CRC cells markedly destroyed the spleen and invaded splenic capsule, and there were massive liver metastases (15 nodules) and vascular invasion in the liver (Fig. 3C). However, there was no visible metastasis in the groups injected with KM12C or KM12C-Neo cells.
Discussion
NGAL has been shown to be overexpressed in inflammatory bowel diseases and colorectal neoplasms (28); however, there has not been a study on NGAL expression in the colorectal adenoma–carcinoma sequence with a sample size large enough to explore the effect of NGAL expression on tumorigenesis of CRC. In this study, our examination of NGAL immunohistochemistry staining on 526 colorectal tissue specimens revealed a positive correlation between NGAL expression and the colorectal adenoma–carcinoma sequence. We also observed the same relationship between NGAL expression and the adenoma–carcinoma sequence in 53 sets of matched colorectal specimens in which the influence of other factors (especially associated with heterogeneity among individuals) was reduced. This expression pattern suggests a role of NGAL in contributing to the tumorigenesis of CRC. In our succeeding animal model experiments, the xenografts in the cecum and spleen were heavier and more numerous in the group injected with NGAL-overexpressing CRC cells. Therefore, our data in the current study provide consistent evidence that NGAL overexpression plays an important role in colorectal tumorigenesis. Similarly, NGAL has been shown to be closely associated with the tumorigenesis of leukemia and breast cancers (16, 29-31).
The second key observation from our present study was the expression pattern of NGAL during the progression of CRC. We observed that NGAL expression was significantly higher in advanced-stage CRCs, which is consistent with the finding of Zhang and colleagues that NGAL mRNA upregulation correlated significantly with advanced stages of rectal cancer (21). Bousserouel and colleagues also found an increase of NGAL expression in advanced stages of CRC when they used a rat azoxymethane–induced colon carcinogenesis model to measure the gene expression profiles of biomarkers related to cancer progression (22). In addition, our study showed that CRCs with high NGAL expression had more potential to relapse locally or distantly.
In the animal model experiments of the current study, the influence of NGAL on metastasis was relatively weak within the time frame of the experiments. There was no visible metastasis in the mice injected with KM12C or KM12C-Neo cells; however, we observed massive liver metastasis (15 nodules) in 1 mouse (14.3%) injected with NGAL overexpressing CRC cells. In the mouse with liver metastasis, the overexpressing NGAL CRC cells markedly destroyed the spleen and liver, invaded into splenic capsule and into vessels in the liver. This result is consistent with our previous in vitro study showing that NGAL overexpression enhanced invasion of the tumor cells (23). NGAL was reported to covalently bind with MMP9 which plays an important role in the enzymatic digestion of the extracellular matrix and, therefore, in neoplastic invasion and metastasis (6, 7). By forming the NGAL–MMP9 complex, NGAL can protect MMP9 from proteolytic degradation and trigger an enhancement of the enzymatic activity of MMP9, which might explain enhanced tumor invasiveness and extension (32). The protumoral and prometastasis effect of NGAL by forming the NGAL–MMP9 complex has been shown in breast (14, 32), stomach (17), and esophageal cancers (33). In addition, we showed NGAL decreased E-cadherin–mediated cell–cell adhesion and increased cell motility and invasion through Rac1 in CRC cells in our previous study (23), which is another possible mechanism through which NGAL promotes tumorigenesis and progression of CRC. If we modify the in vivo experimental systems in future studies to allow for a longer observation time, we might detect a more potent prometastasis effect of NGAL overexpression. Our preliminary results from our mouse models, together with the large-scale human tissue analysis in the present study and the previously reported in vitro data, suggest that NGAL overexpression likely contributes to the progression of CRC.
Nevertheless, we point out that there are inconsistent and even contradictory reports on the role of NGAL in the development and progression of cancers. One study showed the suppression effect of NGAL on metastasis in colon cancer (24). Likewise, there were different results about the influence of NGAL on metastasis of breast cancer in animal model experiments. Yang and colleagues observed that NGAL overexpression increased both local invasion and lymph node metastasis by injecting MCF-7 cells with different NGAL expression levels into the inguinal mammary fat pads of female nude mice (15). Leng and colleagues found that the inhibition of NGAL impaired ErbB2-induced mammary tumor formation, and that tumor invasion and lymph node metastasis were significantly reduced in NGAL knockout mice injected with breast cancer cells (16). However, another study reported that the loss of NGAL did not significantly reduce lung metastasis of breast cancer cells in transgenic mice initiated by mammary tumor virus polyoma middle T antigen (31). NGAL likely interacts with different gene products in determining metastatic potential, which may explain seemingly different results about the role of NGAL in metastasis. NGAL was found to be strongly induced by NF-κB, an important factor involved in tumor growth and neoplastic development in both thyroid (34) and breast cancers (16, 35). Recently, NGAL was shown to promote breast cancer progression by inducing epithelial to mesenchymal transition (EMT) through the estrogen receptor-Slug axis (15). However, NGAL was shown to reduce cell adhesion and invasion [partly by suppressing focal adhesion kinase (FAK) activation] and to inhibit angiogenesis (partly by blocking VEGF production) in pancreatic cancer cells (20). In ovarian cancer cells, NGAL was reported to be markedly reduced after induction of EMT by epidermal growth factor (EGF) and was negatively correlated with the degree of cellular spread (19). Therefore, the effects of NGAL on the development and progression of tumors are likely tumor type–dependent and influenced by multiple mechanisms. More research is needed to further elucidate the function and mechanisms of NGAL in CRC and other cancers.
An exciting aspect of potential application of NGAL in cancer screening was shown by reports that NGAL concentration was elevated in the plasma/serum or urine of patients with breast cancer (14, 15, 36). We thus evaluated whether NGAL might also be a useful plasma marker for CRC. However, we only observed higher plasma NGAL levels in patients with CRC than in healthy donors, and higher plasma NGAL levels in patients with advanced-stage CRC and with metastasis. However, the differences were not significant. Although a larger cohort of cases may reveal a stronger trend, the likelihood is small that NGAL could function as a robust plasma marker for CRC.
The prognostic value of NGAL expression in tumor cells is worth further evaluation. NGAL expression has been shown to be an indicator of poor prognosis in primary breast cancer (37) and esophageal squamous cell carcinoma (38). Our multivariate analysis revealed that NGAL expression was an independent predictor for overall survival time of patients with CRC. Moreover, patients with high NGAL expression had a trend toward earlier local or distant recurrence. Therefore, the examination of NGAL expression in tumor cells may be useful for evaluating the prognosis of patients with CRC. At present, adjuvant chemotherapy remains controversial for patients with stage II CRC, in which neither lymph node nor distant metastasis was found (39). In this study, stage II CRC patients with high NGAL expression had more probability to relapse. Furthermore, high NGAL expression was shown to be an adverse prognostic factor not only for worse overall survival but also for worse disease-free survival. Thus, the examination of NGAL expression may be helpful for identifying at-risk patients for individualized therapy.
In summary, our study of NGAL expression in a large set of clinical tissues and animal model experiments provides supporting evidence that NGAL contributes to the tumorigenesis and progression of CRC, which suggests that NGAL might be a potential therapeutic target for CRC. In addition, examining NGAL expression in tumor cells with immunohistochemical staining may prove to be a valuable approach for evaluating the prognosis of and determining suitable therapy for patients with CRC.
Supplementary Material
Translational Relevance.
Neutrophil gelatinase–associated lipocalin (NGAL) is one of the highly interesting and enigmatic proteins involved in the development and progression of tumors. The proposed roles of NGAL in cancers are often contradictory. Using immunohistochemistry on a large set of tissue samples, the current study showed for the first time that NGAL overexpression correlated with colorectal adenoma–carcinoma sequence. Moreover, animal model experiments indicated that NGAL overexpression enhanced tumorigenesis and potentially increased liver metastasis of colorectal cancer (CRC) cells. These findings suggest that NGAL may contribute to initiation and progression of CRC and is a potential therapeutic target for CRC. Furthermore, survival analysis revealed that NGAL expression was an independent predictor for overall survival of patients with CRC and for disease-free survival of patients with stage II CRC, suggesting that NGAL may serve as a novel prognostic factor for CRC and may be helpful for identifying at-risk patients for individualized therapy.
Acknowledgments
The authors thank Isaiah J. Fidler for his support with the animal experiments, Yingmei Wang for her help in ELISA assay, and Lina Zhang for her assistance with preparing the manuscript. They also thank Kristi M. Speights at the Department of Scientific Publications of MD Anderson Cancer Center for her editorial assistance in preparation of the manuscript.
Grant Support
This work was partially supported by a grant from the National Foundation for Cancer Research (W. Zhang and S.R. Hamilton) and in part by the NIH through MD Anderson’s Cancer Center Support Grant CA016672 and by Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) in China and National Key Scientific and Technological Project (2011ZX0 9307-001-04).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Ilyas M, Straub J, Tomlinson IP, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:1986–2002. doi: 10.1016/s0959-8049(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 4.Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011;29:17–24. doi: 10.1200/JCO.2010.30.1077. [DOI] [PubMed] [Google Scholar]
- 5.Sanjoaquin MA, Choodari-Oskooei B, Dolbear C, Putcha V, Sehgal A, Key TJ, et al. Colorectal cancer incidence, mortality and survival in South-east England between 1972 and 2001. Eur J Cancer Prev. 2007;16:10–6. doi: 10.1097/01.cej.0000228398.30235.f5. [DOI] [PubMed] [Google Scholar]
- 6.Triebel S, Blaser J, Reinke H, Tschesche H. A 25 kDa alpha 2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–8. doi: 10.1016/0014-5793(92)81511-j. [DOI] [PubMed] [Google Scholar]
- 7.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 8.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 9.Gwira JA, Wei F, Ishibe S, Ueland JM, Barasch J, Cantley LG. Expression of neutrophil gelatinase-associated lipocalin regulates epithelial morphogenesis in vitro. J Biol Chem. 2005;280:7875–82. doi: 10.1074/jbc.M413192200. [DOI] [PubMed] [Google Scholar]
- 10.Bratt T. Lipocalins and cancer. Biochim Biophys Acta. 2000;1482:318–26. doi: 10.1016/s0167-4838(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 11.Zerega B, Cermelli S, Michelis B, Cancedda R, Cancedda FD. Expression of NRL/NGAL (neu-related lipocalin/neutrophil gelatinase-associated lipocalin) during mammalian embryonic development and in inflammation. Eur J Cell Biol. 2000;79:165–72. doi: 10.1078/s0171-9335(04)70019-9. [DOI] [PubMed] [Google Scholar]
- 12.Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288:10–6. doi: 10.1016/j.canlet.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–8. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11:5390–5. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:3913–8. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009;69:8579–84. doi: 10.1158/0008-5472.CAN-09-1934. [DOI] [PubMed] [Google Scholar]
- 17.Kubben FJ, Sier CF, Hawinkels LJ, Tschesche H, van Duijn W, Zuidwijk K, et al. Clinical evidence for a protective role of lipocalin-2 against MMP-9 autodegradation and the impact for gastric cancer. Eur J Cancer. 2007;43:1869–76. doi: 10.1016/j.ejca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z, et al. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: significant correlation with cell differentiation and tumour invasion. J Clin Pathol. 2007;60:555–61. doi: 10.1136/jcp.2006.039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW, et al. Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer. 2007;120:2426–34. doi: 10.1002/ijc.22352. [DOI] [PubMed] [Google Scholar]
- 20.Tong Z, Kunnumakkara AB, Wang H, Matsuo Y, Diagaradjane P, Harikumar KB, et al. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100–8. doi: 10.1158/0008-5472.CAN-08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang XF, Zhang Y, Zhang XH, Zhou SM, Yang GG, Wang OC, et al. Clinical significance of neutrophil gelatinase-associated lipocalin (NGAL) expression in primary rectal cancer. BMC Cancer. 2009;9:134. doi: 10.1186/1471-2407-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bousserouel S, Kauntz H, y F, Bouhadjar M, Soler L, Marescaux J, et al. Identification of gene expression profiles correlated to tumor progression in a preclinical model of colon carcinogenesis. Int J Oncol. 2010;36:1485–90. doi: 10.3892/ijo_00000635. [DOI] [PubMed] [Google Scholar]
- 23.Hu L, Hittelman W, Lu T, Ji P, Arlinghaus R, Shmulevich I, et al. NGAL decreases E-cadherin-mediated cell-cell adhesion and increases cell motility and invasion through Rac1 in colon carcinoma cells. Lab Invest. 2009;89:531–48. doi: 10.1038/labinvest.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y, Kim JS. Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int J Cancer. 2006;118:2490–7. doi: 10.1002/ijc.21657. [DOI] [PubMed] [Google Scholar]
- 25.Sun B, Sun Y, Wang J, Zhao X, Zhang S, Liu Y, et al. The diagnostic value of SYT-SSX detected by reverse transcriptase-polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) for synovial sarcoma: a review and prospective study of 255 cases. Cancer Sci. 2008;99:1355–61. doi: 10.1111/j.1349-7006.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moniaux N, Chakraborty S, Yalniz M, Gonzalez J, Shostrom VK, Standop J, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98:1540–7. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71. [PubMed] [Google Scholar]
- 28.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–20. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng X, Lin H, Ding T, Wang Y, Wu Y, Klumpp S, et al. Lipocalin 2 is required for BCR-ABL-induced tumorigenesis. Oncogene. 2008;27:6110–9. doi: 10.1038/onc.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng X, Ding T, Arlinghaus R. Requirement of lipocalin 2 for hematopoietic and solid tumor malignancies. Adv Enzyme Regul. 2009;49:142–6. doi: 10.1016/j.advenzreg.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger T, Cheung CC, Elia AJ, Mak TW. Disruption of the Lcn2 gene in mice suppresses primary mammary tumor formation but does not decrease lung metastasis. Proc Natl Acad Sci U S A. 2010;107:2995–3000. doi: 10.1073/pnas.1000101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 33.Nuntagowat C, Leelawat K, Tohtong R. NGAL knockdown by siRNA in human cholangiocarcinoma cells suppressed invasion by reducing NGAL/MMP-9 complex formation. Clin Exp Metastasis. 2010;27:295–305. doi: 10.1007/s10585-010-9327-y. [DOI] [PubMed] [Google Scholar]
- 34.Iannetti A, Pacifico F, Acquaviva R, Lavorgna A, Crescenzi E, Vascotto C, et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-kappaB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci U S A. 2008;105:14058–63. doi: 10.1073/pnas.0710846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li SH, Hawthorne VS, Neal CL, Sanghera S, Xu J, Yang J, et al. Upregulation of neutrophil gelatinase-associated lipocalin by ErbB2 through nuclear factor-kappaB activation. Cancer Res. 2009;69:9163–8. doi: 10.1158/0008-5472.CAN-09-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Provatopoulou X, Gounaris A, Kalogera E, Zagouri F, Flessas I, Goussetis E, et al. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer. 2009;9:390. doi: 10.1186/1471-2407-9-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108:389–97. doi: 10.1007/s10549-007-9619-3. [DOI] [PubMed] [Google Scholar]
- 38.Du ZP, Lv Z, Wu BL, Wu ZY, Shen JH, Wu JY, et al. Neutrophil gelatinase-associated lipocalin and its receptor: independent prognostic factors of oesophageal squamous cell carcinoma. J Clin Pathol. 2011;64:69–74. doi: 10.1136/jcp.2010.083907. [DOI] [PubMed] [Google Scholar]
- 39.Rousseau B, Chibaudel B, Bachet JB, Larsen AK, Tournigand C, Louvet C, et al. Stage II and stage III colon cancer: treatment advances and future directions. Cancer J. 2010;16:202–9. doi: 10.1097/PPO.0b013e3181ddc5bf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.