Abstract
Ruthenium-catalyzed transfer hydrogenation of diverse π-unsaturated reactants in the presence of aldehydes provides products of carbonyl addition. Dehydrogenation of primary alcohols in the presence of the same π-unsaturated reactants provides identical products of carbonyl addition. In this way, carbonyl addition is achieved from the alcohol or aldehyde oxidation level in the absence of stoichiometric organometallic reagents or metallic reductants. In this account, the discovery of ruthenium-catalyzed C–C bond-forming transfer hydrogenations and the recent development of diastereo- and enantioselective variants are discussed.
Keywords: C–C bond formation, catalysis, ruthenium catalysis, transfer hydrogenation
INTRODUCTION
Whereas hydrogenation is principally employed as a method for C–H bond formation, alkene hydroformylation, the largest volume application of homogeneous catalysis, reveals that hydrogenation also may serve as a method for reductive C–C coupling [1,2]. Inspired by the atom economy and selectivity of hydrogenation and hydroformylation, our laboratory launched a systematic effort to develop C–C bond-forming hydrogenations beyond hydroformylation [3,4]. Toward this end, cationic rhodium and iridium catalysts were found to promote reductive C–C coupling of π-unsaturated reactants to carbonyl compounds and imines under hydrogenation conditions. More recently, under the conditions of ruthenium- and iridium-catalyzed transfer hydrogenation employing isopropanol or formic acid as terminal reductant, π-unsaturated reactants and aldehydes were found to engage in reductive C–C coupling. A significant outgrowth of the ruthenium- and iridium-catalyzed C–C bond-forming transfer hydrogenations relates to the ability to engage primary alcohols as both hydrogen donors and aldehyde precursors, enabling carbonyl addition directly from the alcohol oxidation level. In this account, the formation of C–C bonds under the conditions of ruthenium-catalyzed transfer hydrogenation is reviewed, including the recent development of diastereo- and enantioselective variants [5–7].
RESULTS AND DISCUSSION
Carbonyl allylation
Carbonyl allylation is one of the foremost methods used for the construction of polyketide natural products. Accordingly, many chiral allyl metal reagents for enantioselective carbonyl allylation have been developed [8].To avoid the use of stoichiometric chiral modifiers, chiral Lewis acids and chiral Lewis bases have been employed as catalysts for enantioselective carbonyl addition employing allylmetal reagents [8]. Other catalytic methods for carbonyl allylation include the reduction of metallo-π-allyls derived from allylic alcohols and allylic carboxylates [9] and asymmetric variants of Fürstner's modification of the Nozaki–Hiyama reaction [10,11]. However, the large-scale implementation of these reductive methods is impeded by their use of terminal reductants that are metallic, pyrophoric, or highly mass intensive.
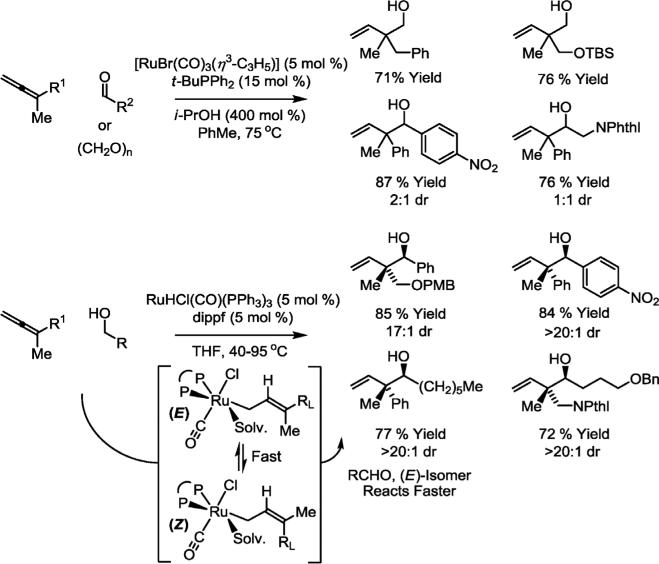
The development of hydrogen-mediated carbonyl allylations began with the observation that iridium-catalyzed hydrogenation of 1,1-dimethylallene in the presence of aldehydes promotes reductive coupling to form products of tert-prenylation [12a]. Subsequently, iridium-catalyzed carbonyl allylations employing allenes or dienes as allyl donors were achieved under transfer hydrogenative conditions employing isopropyl alcohol as the terminal reductant, or through direct redox neutral C–C couplings of primary alcohols, wherein the alcohol serves as both hydrogen source and aldehyde precursor [12b,13]. In parallel with the exploration of iridium-catalyzed C–C bond-forming transfer hydrogenations, analogous ruthenium-catalyzed processes were developed. For example, under the conditions of ruthenium-catalyzed transfer hydrogenation, isopropanol-mediated reductive coupling of 1,1-disubstituted allenes to formaldehyde or higher aldehydes occurs efficiently to provide homoallylic alcohols [14a]. For higher aldehydes, the diastereoselectivity is sensitive to the oxidation level of the coupling partner. Whereas reactions conducted from the aldehyde oxidation level exhibit poor diastereoselectivity, reactions performed from the alcohol oxidation level often deliver a single diastereomer. The collective data suggest that for reactions performed from the alcohol oxidation level, high diastereoselectivities arise in response to Curtin–Hammett effects associated with turn-over limiting carbonyl addition of the allylruthenium intermediate to the transient aldehyde (Scheme 1) [14b].
Scheme 1.
Ruthenium-catalyzed hydrohydroxyalkylation of 1,1-disubstituted allenes from the alcohol or aldehyde oxidation level. dippf = bis(diisopropylphosphino)ferrocene; NPthl = N-phthalimido.
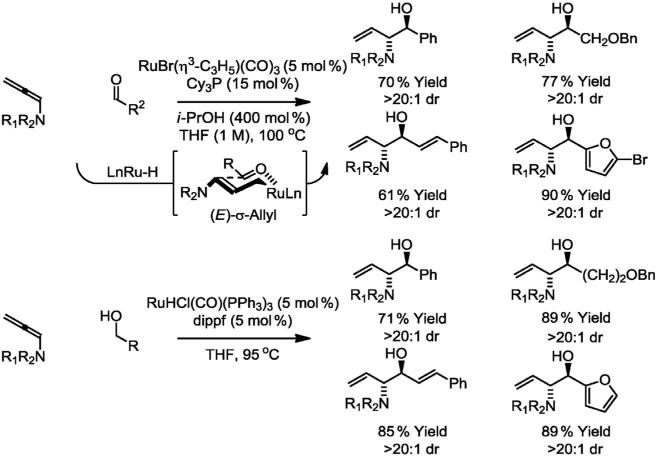
Rather than relying on Curtin–Hammett effects, a more general method for controlling diastereoselectivity is based on the hypothesis that carbonyl addition occurs stereospecifically through closed chair-like transition structures and, hence, can be influenced through complete partitioning of (E)- and (Z)-σ-allylruthenium intermediates on the basis of steric effects. For example, N,N-disubstituted allenamides, which are easily prepared through base-catalyzed isomerization of propargyl amines, participate in reductive coupling with aldehydes under the conditions of ruthenium-catalyzed transfer hydrogenation to furnish the products of carbonyl anti-aminoallylation as single diastereomers [15a]. Mechanistic studies suggest the observed anti-diastereoselectivity is a consequence of stereospecific carbonyl addition from the transient (E)-σ-allylruthenium intermediate through a chair-like transition state. Identical products of anti-aminoallylation are obtained upon ruthenium-catalyzed hydrogen exchange between N,N-disubstituted allenamides and primary alcohol with equivalent levels of diastereocontrol (Scheme 2) [15b].
Scheme 2.
Anti-diastereoselective ruthenium catalyzed hydrohydroxyalkylation of allenamides from the alcohol or aldehyde oxidation level. R1 = 4-nitrobenzenesulfonyl, R2 = 2,4-(MeO)2Bn.
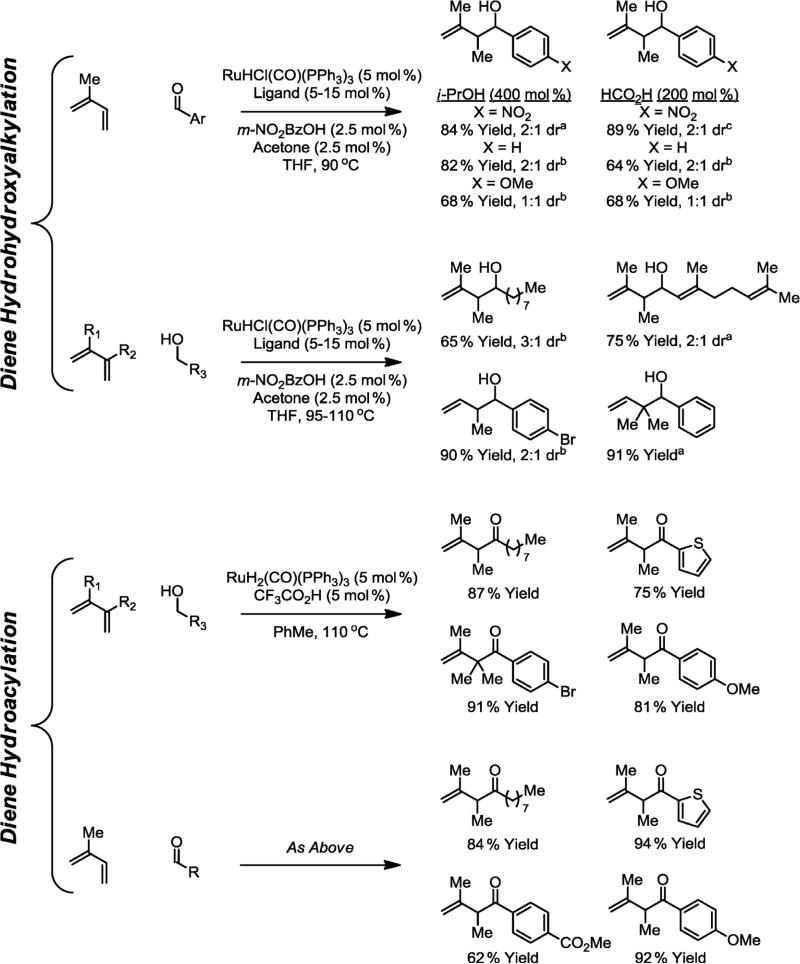
Simple acyclic 1,3-dienes also participate in ruthenium-catalyzed C–C bond-forming transfer hydrogenation to give products of diene hydrohydroxyalkylation. For example, butadiene, isoprene, and 2,3-dimethylbutadiene reductively couple to aldehydes when RuHCl(CO)(PPh3)3 is employed as a pre-catalyst and isopropyl alcohol or formic acid are present as the hydrogen source [16a]. Identical products of carbonyl allylation are obtained from the alcohol oxidation level in the absence of exogenous terminal reductant. As observed in the preceding examples, the alcohol reactants readily undergo dehydrogenation under the reaction conditions, whereas the alcohol-containing products do not. Product dehydrogenation is suppressed by coordination of the homoallylic olefin to ruthenium to generate a coordinatively saturated homoallylic ruthenium alkoxide complex that is incapable of β-hydride elimination. However, through the use of the coordinatively unsaturated precatalyst Ru(O2CCF3)(H)(CO)(PPh3)2, which is generated in situ through the acid–base reaction of Ru(H)2(CO)(PPh3)3 and HO2CCF3, further oxidation of the initially formed alcohol products to furnish β,γ-unsaturated ketones occurs efficiently to provide the β,γ-enone products [16b,c]. Thus, through careful selection of the reaction conditions, products of diene hydrohydroxyalkylation or hydroacylation are accessible from either the alcohol or aldehyde oxidation levels (Scheme 3) [17].
Scheme 3.
Hydrohydroxyalkylation and hydroacylation of acyclic dienes from the alcohol or aldehyde oxidation level. aLigand = (p-MeOPh)3P, bLigand = rac-BINAP, cNo ligand added.
While reductive coupling of 2-substituted 1,3-dienes mediated by neutral ruthenium catalysts furnishes branched disubstituted homoallylic alcohols, catalysts possessing a degree of cationic character furnish regioisomeric products of carbonyl addition incorporating all carbon quaternary centers. Cationic ruthenium complexes allow for rapid interconversion between isomeric π-allyls, leading to carbonyl addition from a terminally disubstituted (E)-σ-allylruthenium intermediate. Hydro -hydroxymethylation of 2-substituted dienes with paraformaldehyde in the presence of a cationic ruthenium complex generated in situ through the acid–base reaction of RuH2(CO)(PPh3)3 and HO2CC7F15 results in the formation of primary neopentyl alcohols [18a]. Similarly, exposure of ethanol to 2-substituted dienes results in diastereoselective hydrohydroxymethylation to form secondary neopentyl alcohols with modest levels of regioselectivity (Scheme 4) [18b].
Scheme 4.
Hydrohydroxyalkylation of 2-substituted dienes to form all-carbon quaternary centers.
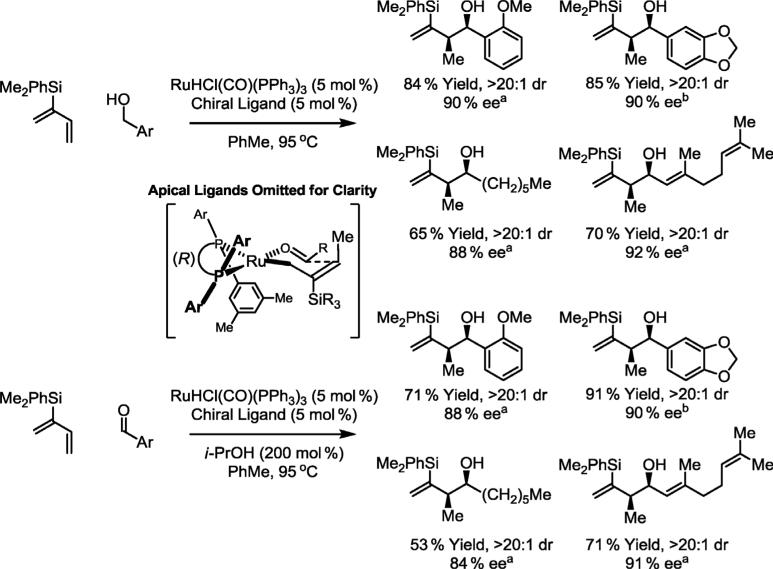
Although the diastereo- and enantioselective hydrohydroxyalkylation of butadiene would represent a completely atom economic approach to carbonyl crotylation, difficulties in generating geometrically defined σ-allylruthenium intermediates from butadiene have, thus far, foiled development of highly diastereoselective processes. However, 2-trialkylsilyl-substituted crotylmetal species are known to adopt a “pseudo-(Z)-σ-crotylmetal” geometry by virtue of allylic strain [19]. Exposure of alcohols or aldehydes to the indicated 2-silyl-substituted diene, prepared from chloroprene, in the presence of ruthenium catalysts modified by (R)-SEGPHOS or (R)-DM-SEGPHOS leads to products of syn-diastereo- and enantioselective carbonyl crotylation (Scheme 5). Elaboration of these products through diastereoselective hydroboration-Suzuki cross-coupling followed by Tamao–Fleming oxidation of the C–Si bond furnishes anti,syn-stereotriads found in scores of polyketide natural products [16d].
Scheme 5.
syn-Diastereo- and enantioselective carbonyl crotylation via hydrohydroxyalkylation of 2-silylbutadienes from the alcohol or aldehyde oxidation level. aChiral ligand = (R)-DM-SEGPHOS. bChiral ligand = (R)-SEGPHOS.
Carbonyl vinylation
A highly convergent route for the enantioselective preparation of allylic alcohols is the catalytic asymmetric addition of vinylmetal reagents to aldehydes [20]. Most often, the vinylmetal reagents required for such additions are prepared through stoichiometric alkyne hydrometallation. Direct catalytic reductive couplings of alkynes and aldehydes bypass discrete generation of vinylmetal reagents [21–25], however, such transformations typically employ pyrophoric or mass-intensive terminal reductants (e.g., ZnR2, BEt3, HSiR3). In contrast, completely atom-economical carbonyl vinylation is achieved through hydrogen-mediated reductive couplings of alkynes and aldehydes [26–28].
Recently, it was found that redox neutral carbonyl vinylation may be achieved under the conditions of ruthenium-catalyzed transfer hydrogenation through the use of primary alcohols reactants as hydrogen donors and, thus, aldehyde precursors. Using Ru(O2CCF3)2(CO)(PPh3)2 as a precatalyst, hydrogen is transferred from a primary alcohol to 2-butyne to generate aldehyde-vinylruthenium pairs, which react to give allylic alcohols [29]. At higher reaction temperatures, the initially formed allylic alcohols undergo dehydrogenation to form α,β-unsaturated ketones. Thus, intermolecular alkyne hydroacylation is achieved from the alcohol oxidation level (Scheme 6) [29b].
Scheme 6.
Coupling of alcohols with 2-butyne to provide products of alkyne hydrohydroxyalkylation or hydroacylation.
Nonsymmetric alkynes pose additional challenges in terms of regioselectivity. It was found that the specific combination of iodide and carboxylate counterions at ruthenium enabled highly regioselective reductive to occur under transfer hydrogenation conditions employing formic acid as terminal reductant [29a]. Related regioselective carbonyl vinylations from the alcohol oxidation level are currently under investigation (Scheme 7).
Scheme 7.
Hydrohydroxyalkylation of nonsymmetric internal alkynes from the aldehyde oxidation level.
Ruthenium-catalyzed transfer hydrogenative couplings of nonsymmetric alkynes exhibit regioselectivities that complement those displayed by nickel catalysts [29d]. For example, whereas formic acid-mediated reductive coupling of arylpropynes to paraformaldehyde under the conditions of ruthenium catalysis results in regioselective C–C coupling proximal to the aryl moiety, the analogous nickel-catalyzed reactions lead to exclusive formation of the alternate regioisomers. In the latter case, the coupling products form as the formate esters, which are cleaved to the allylic alcohol upon basic work-up. An exogenous reductant is not required in this case as paraformaldehyde serves both as electrophile and reductant (Scheme 8).
Scheme 8.
Regiodivergence in ruthenium- and nickel-catalyzed reductive couplings of nonsymmetric disubstituted alkynes to paraformaldehyde.
Carbonyl propargylation
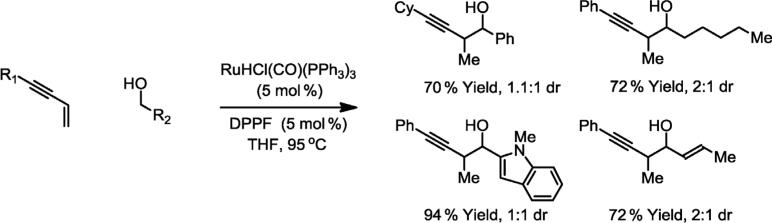
The synthesis of homopropargylic alcohols is typically accomplished through the addition of allenylmetal reagents to carbonyl compounds [30]. In contrast, completely atom-economical carbonyl propargylation is achieved upon ruthenium-catalyzed transfer hydrogenation of 1,3-enynes in the presence of benzylic, allylic, and aliphatic alcohols. Here, hydrogen transfer from the primary alcohol to the 1,3-enyne generates aldehyde-allenylruthenium pairs, which combine to form products of carbonyl propargylation directly from the alcohol oxidation level [31] (Scheme 9). Identical propargylation products are achieved through enyne-aldehyde coupling in the presence of isopropyl alcohol as the terminal reductant. This protocol bypasses the use of discrete allenylmetal reagents. Diastereo- and enantioselective variants of this process are under development in our laboratory.
Scheme 9.
Ruthenium-catalyzed hydrohydroxyalkylation of 1,3-enynes from the alcohol oxidation level. DPPF = 1,1'-bis(diphenylphosphino)ferrocene.
CONCLUSION AND OUTLOOK
The C–C coupling of π-unsaturates with carbonyl electrophiles under the conditions of ruthenium-catalyzed transfer hydrogenation enables carbonyl allylation, vinylation, and propargylation from the aldehyde or alcohol oxidation levels in the absence of stoichiometric organometallic reagents. For transfer hydrogenative couplings from the aldehyde oxidation level, isopropyl alcohol or formic acid serve as terminal reductants, while from the alcohol oxidation level, exogenous reductant is not required. The ongoing development of diastereo- and enantioselective variants of these processes should assist a departure from the use of premetallated reagents in the chemistry of carbonyl addition.
Several key challenges remain. The development of conditions applicable to α-chiral aldehydes would broaden the utility of these methods in natural product synthesis. Extending the electrophile scope to encompass imine addition from the amine oxidation level would provide direct, atom-economical routes to chiral amines. Finally, the isolation of well-defined chiral ruthenium precatalysts may facilitate development of stereoselective processes. Given these challenges, the emerging field of transfer hydrogenative C–C coupling promises to remain exciting for years to come, and the central role of ruthenium in the continued evolution from classical carbanion chemistry to such transfer hydrogenative technologies is clear.
ACKNOWLEDGMENTS
Acknowledgment is made to the Robert A. Welch Foundation (F-0038), Johnson & Johnson, Eli Lilly, Merck, Firmenich the NIH-NIGMS (RO1-GM69445), and the ACS-GCI, for partial support of the research described in this account. Drs. Yasunori Ino, Wataru Kuriyama, and Taichiro Touge of Takasago are thanked for the generous donation of SEGPHOS ligands. J. M. thanks the National Sciences and Engineering Research Council of Canada (NSERC) for a postdoctoral fellowship.
Footnotes
Pure Appl. Chem. 84, xxx–xxx (2012). A collection of invited papers based on presentations at the 16th International Symposium on Organometallic Chemistry Directed Towards Organic Synthesis (OMCOS-16), Shanghai, China, 24–28 July 2011.
REFERENCES
- 1.a Frohning CD, Kohlpaintner CW. In: Applied Homogeneous Catalysis with Organometallic Compounds. Cornils B, Herrmann WA, editors. Vol. 1. Wiley-VCH; Weinheim: 1996. pp. 29–104. [Google Scholar]; b van Leeuwen PWNM. Homogeneous Catalysis: Understanding the Art. Kluwer; Dordrecht: 2004. [Google Scholar]
- 2.For a review of ruthenium-catalyzed alkene hydroformylation, see: Kalck P, Peres Y, Jenck J. Adv. Organomet. Chem. 1991;32:121.
- 3.For recent reviews on C–C bond-forming hydrogenation and transfer hydrogenation, see: Patman RL, Bower JF, Kim IS, Krische MJ. Aldrichim. Acta. 2008;41:95.Bower JF, Kim IS, Patman RL, Krische MJ. Angew. Chem., Int. Ed. 2009;48:34. doi: 10.1002/anie.200802938.Han SB, Kim IS, Krische MJ. Chem. Commun. 2009:7278. doi: 10.1039/b917243m.Bower JF, Krische MJ. Top. Organomet. Chem. 2011;43:107. doi: 10.1007/978-3-642-15334-1_5.
- 4.In related “hydrogen auto-transfer” reactions, alcohol dehydrogenation and nucleophile generation occur independently. Hence, pre-activated nucleophiles are required. Such processes promote formal alcohol substitution rather than carbonyl addition. For selected reviews, see: Nixon TD, Whittlesey MK, Williams JMJ. Dalton Trans. 2009:753. doi: 10.1039/b813383b.Dobereiner GE, Crabtree RH. Chem. Rev. 2010;110:681. doi: 10.1021/cr900202j.Guillena G, Ramón DJ, Yus M. Chem. Rev. 2010;110:1611. doi: 10.1021/cr9002159.
- 5.For a previous review on ruthenium-catalyzed transfer hydrogenative C–C bond formation, see: Shibahara F, Krische MJ. Chem. Lett. 2008;37:1102. doi: 10.1246/cl.2008.1102.
- 6.For selected reviews of ruthenium-catalyzed C–C coupling, see: Trost BM, Toste FD, Pinkerton AB. Chem. Rev. 2001;101:2067. doi: 10.1021/cr000666b.Kondo T, Mitsudo T.-a. Curr. Org. Chem. 2002;6:1163.Derien S, Monnier F, Dixneuf PH. Top. Organomet. Chem. 2004;11:1.
- 7.For related C–C couplings that occur by way of nucleophilic ruthenium π-allyls, see: Tsuji Y, Mukai T, Kondo T, Watanabe Y, Organomet J. Chem. C. 1989;369:51.Kondo T, Ono H, Satake N, Mitsudo T.-a., Watanabe Y. Organometallics. 1995;14:1945.Yu C-M, Lee S, Hong Y-T, Yoon S-K. Tetrahedron Lett. 2004;45:6557.Omura S, Fukuyama T, Horiguchi J, Murakami Y, Ryu I. J. Am. Chem. Soc. 2008;130:14094. doi: 10.1021/ja806929y.Denmark SE, Nguyen ST. Org. Lett. 2009;11:781. doi: 10.1021/ol8028725.
- 8.For reviews on enantioselective carbonyl allylation, see: Ramachandran PV. Aldrichim. Acta. 2002;35:23.Denmark SE, Fu J. Chem. Rev. 2003;103:2763. doi: 10.1021/cr020050h.Yu C-M, Youn J, Jung H-K. Bull. Kor. Chem. Soc. 2006;27:463.Marek I, Sklute G. Chem. Commun. 2007:1683. doi: 10.1039/b615042j.Hall DG. Synlett. 2007:1644.Lachance H, Hall DG. Org. React. 2008;73:1.
- 9.For selected reviews of carbonyl allylation based on the reductive coupling of metallo-π-allyls derived from allylic alcohols, ethers, or carboxylates, see: Masuyama Y. Palladium-catalyzed carbonyl allylation via π-allylpalladium complexes. In: Liebeskind LS, editor. Advances in Metal-Organic Chemistry. Vol. 3. JAI Press; Greenwich: 1994. pp. 255–303.Tamaru Y. Palladium-catalyzed reactions of allyl and related derivatives with organo -electrophiles. In: Negishi E.-i., de Meijere A., editors. Handbook of Organopalladium Chemistry for Organic Synthesis. Vol. 2. John Wiley; New York: 2002. pp. 1917–1943.Tamaru Y. J. Organomet. Chem. 1999;576:215.Kondo T, Mitsudo T.-a. Curr. Org. Chem. 2002;6:1163.Tamaru Y. Eur. J. Org. Chem. 2005;13:2647.Zanoni G, Pontiroli A, Marchetti A, Vidari G. Eur. J. Org. Chem. 2007;22:3599.
- 10.Fürstner A, Shi N. J. Am. Chem. Soc. 1996;118:2533. [Google Scholar]
- 11.For selected reviews on catalytic Nozaki–Hiyama coupling, see: Avalos M, Babiano R, Cintas P, Jiménez JL, Palacios JC. Chem. Soc. Rev. 1999;28:169.Bandini M, Cozzi PG, Umani-Ronchi A. Chem. Commun. 2002:919. doi: 10.1039/b109945k.Hargaden GC, Guiry PJ. Adv. Syn. Catal. 2007;349:2407.Inoue M, Suzuki T, Kinoshita A, Nakada M. Chem. Rec. 2008;8:169. doi: 10.1002/tcr.20148.
- 12.a Skucas E, Bower JF, Krische MJ. J. Am. Chem. Soc. 2007;129:12678. doi: 10.1021/ja075971u. [DOI] [PubMed] [Google Scholar]; b Bower JF, Skucas E, Patman RL, Krische MJ. J. Am. Chem. Soc. 2007;129:15134. doi: 10.1021/ja077389b. [DOI] [PubMed] [Google Scholar]
- 13.a Bower JF, Patman RL, Krische MJ. Org. Lett. 2008;10:1033. doi: 10.1021/ol800159w. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Han SB, Kim IS, Han H, Krische MJ. J. Am. Chem. Soc. 2009;131:6916. doi: 10.1021/ja902437k. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zbieg JR, Fukuzumi T, Krische MJ. Adv. Synth. Catal. 2010;352:2416. doi: 10.1002/adsc.201000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a Ngai M-Y, Skucas E, Krische MJ. Org. Lett. 2008;10:2705. doi: 10.1021/ol800836v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zbieg JR, McInturff EL, Leung JC, Krische MJ. J. Am. Chem. Soc. 2011;133:1141. doi: 10.1021/ja1104156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a Skucas E, Zbieg JR, Krische MJ. J. Am. Chem. Soc. 2009;131:5054. doi: 10.1021/ja900827p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zbieg JR, McInturff EL, Krische MJ. Org. Lett. 2010;12:2514. doi: 10.1021/ol1007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.a Shibahara F, Bower JF, Krische MJ. J. Am. Chem. Soc. 2008;130:6338. doi: 10.1021/ja801213x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shibahara F, Bower JF, Krische MJ. J. Am. Chem. Soc. 2008;130:14120. doi: 10.1021/ja805356j. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Omura S, Fukuyama T, Horiguchi J, Murakami Y, Ryu I. J. Am. Chem. Soc. 2008;130:14094. doi: 10.1021/ja806929y. [DOI] [PubMed] [Google Scholar]; d Zbieg JR, Moran J, Krische MJ. J. Am. Chem. Soc. 2011;133:10582. doi: 10.1021/ja2046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For intermolecular ruthenium-catalyzed hydroacylation, see: Isnard P, Denise B, Sneeden RPA, Cognion JM, Durual P. J. Organomet. Chem. 1982;240:285.Isnard P, Denise B, Sneeden RPA, Cognion JM, Durual P. J. Organomet. Chem. 1983;256:135.Kondo T, Tsuji Y, Watanabe Y. Tetrahedron Lett. 1987;28:6229.Kondo T, Akazome M, Tsuji Y, Watanabe Y. J. Org. Chem. 1990;55:1286.Kondo T, Hiraishi N, Morisaki Y, Wada K, Watanabe Y, Mitsudo T-A. Organometallics. 1998;17:2131.
- 18.a Smejkal T, Han H, Breit B, Krische MJ. J. Am. Chem. Soc. 2009;131:10366. doi: 10.1021/ja904124b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Han H, Krische MJ. Org. Lett. 2009;12:2844. doi: 10.1021/ol101077v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotylmagnesium and crotylzinc reagents with Me3Si-substituents at the 2-position react with aldehydes to give racemic syn-adducts: Sato F, Kusakabe M, Kobayashi Y. J. Chem. Soc., Chem. Commun. 1984:1131.Helm MD, Mayer P, Knochel P. Chem. Commun. 2008:1916. doi: 10.1039/b802157k.
- 20.For a recent review of carbonyl vinylation, see: Kauffman MC, Walsh PJ. In: Science of Synthesis, Stereoselective Synthesis. Molander GA, editor. Vol. 2. Thieme; Stuttgart: 2011. pp. 449–495.
- 21.Ojima I, Tzamarioudaki M, Tsai C-Y. J. Am. Chem. Soc. 1994;116:3643. [Google Scholar]
- 22.Crowe WE, Rachita MJ. J. Am. Chem. Soc. 1995;117:6787. for an aligned study, see: Kablaoui NM, Buchwald SL. J. Am. Chem. Soc. 1995;117:6785.
- 23.a Oblinger E, Montgomery J. J. Am. Chem. Soc. 1997;119:9065. [Google Scholar]; b Tang X-Q, Montgomery J. J. Am. Chem. Soc. 1999;121:6098. [Google Scholar]; c Tang X-Q, Montgomery J. J. Am. Chem. Soc. 2000;122:6950. [Google Scholar]; d Mahandru GM, Liu G, Montgomery J. J. Am. Chem. Soc. 2004;126:3698. doi: 10.1021/ja049644n. [DOI] [PubMed] [Google Scholar]; e Knapp-Reed B, Mahandru GM, Montgomery J. J. Am. Chem. Soc. 2005;127:13156. doi: 10.1021/ja054590i. [DOI] [PubMed] [Google Scholar]; f Chaulagain MR, Sormunen GJ, Montgomery J. J. Am. Chem. Soc. 2007;129:9568. doi: 10.1021/ja072992f. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Malik HA, Sormunen GJ, Montgomery J. J. Am. Chem. Soc. 2010;132:6304. doi: 10.1021/ja102262v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.a Huang W-S, Chan J, Jamison TF. Org. Lett. 2000;2:4221. doi: 10.1021/ol006781q. [DOI] [PubMed] [Google Scholar]; b Miller KM, Huang W-S, Jamison TF. J. Am. Chem. Soc. 2003;125:3442. doi: 10.1021/ja034366y. [DOI] [PubMed] [Google Scholar]; c Miller KM, Jamison TF. Org. Lett. 2005;7:3077. doi: 10.1021/ol051075g. [DOI] [PubMed] [Google Scholar]
- 25.Takai K, Sakamoto S, Isshiki T. Org. Lett. 2003;5:653. doi: 10.1021/ol0272996. [DOI] [PubMed] [Google Scholar]
- 26.a Kong J-R, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2006;128:718. doi: 10.1021/ja056474l. [DOI] [PubMed] [Google Scholar]; b Cho C-W, Krische MJ. Org. Lett. 2006;8:3873. doi: 10.1021/ol061485k. [DOI] [PubMed] [Google Scholar]; c Hong Y-T, Cho C-W, Skucas E, Krische MJ. Org. Lett. 2007;9:3745. doi: 10.1021/ol7015548. [DOI] [PubMed] [Google Scholar]; d Komanduri V, Krische MJ. J. Am. Chem. Soc. 2006;128:16448. doi: 10.1021/ja0673027. [DOI] [PubMed] [Google Scholar]
- 27.a Liu P, Krische MJ, Houk KN. Chem.-Eur. J. 2011;17:4021. doi: 10.1002/chem.201002741. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Musashi Y, Sakaki S. J. Am. Chem. Soc. 2002;124:7588. doi: 10.1021/ja020063c. [DOI] [PubMed] [Google Scholar]; c Williams VM, Kong J-R, Ko B-J, Mantri Y, Brodbelt JS, Baik M-H, Krische MJ. J. Am. Chem. Soc. 2009;131:16054. doi: 10.1021/ja906225n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong J-R, Cho C-W, Krische MJ. J. Am. Chem. Soc. 2005;127:11269. doi: 10.1021/ja051104i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.a Patman RL, Chaulagain MR, Williams VM, Krische MJ. J. Am. Chem. Soc. 2009;131:2066. doi: 10.1021/ja809456u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Williams VM, Leung JC, Patman RL, Krische MJ. Tetrahedron. 2009;65:5024. doi: 10.1016/j.tet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bausch CC, Patman RL, Breit B, Krische MJ. Angew. Chem., Int. Ed. 2011;50:5686. doi: 10.1002/anie.201101496. [DOI] [PubMed] [Google Scholar]; d Leung JC, Patman RL, Sam B, Krische MJ. Chem.-Eur. J. 2011;17:12437. doi: 10.1002/chem.201101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.For reviews encompassing carbonyl propargylation employing allenyl metal reagents, see: Moreau J-L. In: The Chemistry of Ketenes, Allenes and Related Compounds. Patai S, editor. John Wiley; New York: 1980. pp. 363–413.Marshall JA. Chem. Rev. 1996;96:31. doi: 10.1021/cr950037f.Gung BW. Org. React. 2004;64:1.Marshall JA, Gung BW, Grachan ML. In: Modern Allene Chemistry. Krause N, Hashmi ASK, editors. Wiley-VCH; Weinheim: 2004. pp. 493–592.Marshall JA. J. Org. Chem. 2007;72:8153. doi: 10.1021/jo070787c.
- 31.Patman RL, Williams VM, Bower JF, Krische MJ. Angew. Chem., Int. Ed. 2008;47:5220. doi: 10.1002/anie.200801359. [DOI] [PMC free article] [PubMed] [Google Scholar]