Abstract
Rationale
Exercise results in beneficial adaptations of the heart that can be directly observed at the ventricular myocyte level. However, the molecular mechanism(s) responsible for these adaptations are not well understood. Interestingly, signaling via neuronal nitric oxide synthase (NOS1) within myocytes results in similar effects as exercise.
Objective
Thus, the objective was to define the role NOS1 plays in the exercise-induced beneficial contractile effects in myocytes.
Methods and Results
After an 8 week aerobic interval training program, exercise-trained (Ex) mice had higher VO2max and cardiac hypertrophy compared to sedentary (Sed) mice. Ventricular myocytes from Ex mice had increased NOS1 expression and nitric oxide production compared to myocytes from Sed mice. Remarkably, acute NOS1 inhibition normalized the enhanced contraction (shortening and Ca2+ transients) in Ex myocytes to Sed levels. The NOS1 effect on contraction was mediated via greater Ca2+cycling that resulted from increased phospholamban phosphorylation. Intriguingly, a similar aerobic interval training program on NOS1 knockout mice failed to produce any beneficial cardiac adaptations (VO2max, hypertrophy, and contraction).
Conclusions
These data demonstrate that the beneficial cardiac adaptations observed after exercise training were mediated via enhanced NOS1 signaling. Therefore, it is likely that beneficial effects of exercise may be mimicked by the interventions that increase NOS1 signaling. This pathway may provide a potential novel therapeutic target in cardiac patients who are unable or unwilling to exercise.
Keywords: myocyte, Ca2+, sarcoplasmic reticulum, high intense treadmill training, nitric oxide
INTRODUCTION
Regular exercise has many beneficial effects [33], including direct adaptations of the heart that result in greater aerobic fitness (VO2max), physiological hypertrophy, enhanced contraction, and accelerated relaxation. These adaptations have clinical implications as exercise has been shown to decrease the risk for the development of cardiomyopathies [37]. In addition, exercise is used as a rehabilitation tool for cardiac patients to improve the quality of life and to decrease mortality [63]. These intrinsic exercise-induced cardiac adaptations can be observed at the myocyte level [25]. For example, ventricular myocytes from exercise-trained mice exhibit increased size, greater contraction due to enhanced sarcoplasmic reticulum (SR) Ca2+ cycling, and faster contraction-relaxation rates. Previous studies have proposed that the contractile adaptations of exercise result from increases in SR Ca2+ATPase (SERCA) expression [23, 24]. However, it has been shown that the exercise-induced cardiac adaptations are still present in SERCA knockout mice [9], suggesting that mechanisms other than SERCA must also contribute to the beneficial cardiac adaptations of exercise.
In skeletal muscle, exercise training has been shown to increase nitric oxide (NO) produced via neuronal NO synthase (NOS1) [56]. Furthermore, exercise increased heart NOS1 expression in a nitric oxide-deficient hypertension model [19]. NOS1 is also constitutively expressed within ventricular myocytes, and its signaling leads to enhanced contraction and accelerated relaxation via increased SR Ca2+cycling [1, 28, 58, 60]. As such, the contractile effects of NOS1 signaling are similar to the contractile adaptations of exercise. However, the contribution of NOS1 signaling to exercise-induced cardiac myocyte adaptations has not been investigated.
Therefore the purpose of the study was to assess whether NOS1 signaling contributes to the exercise-induced adaptations in the myocyte. We hypothesize that exercise training will 1) increase NOS1 expression levels and NO bioavailability, and 2) increase contraction and accelerate relaxation via enhanced SR Ca2+ cycling due to amplified NOS1 signaling in isolated ventricular myocytes.
MATERIALS AND METHODS
To achieve exercise induced adaptations, C57Bl/6 (wildytpe, WT), NOS1 knockout (KO) mice and canines underwent a treadmill training protocol (Table S1 and supplementary material) [18]. The effects of exercise were confirmed by VO2max testing [61]. Post training, ventricular myocytes were isolated and Ca2+ transient and shortening were simultaneously measured as previously described [32, 58]. Inhibition of NOS1 was obtained by incubating isolated myocytes for 60 minutes with a specific NOS1 inhibitor, S-methyl-L-thiocitrulline (SMLT) [10]. Western blot analysis were performed with homogenized ventricular myocytes to measure NOS1 protein expression (Santa Cruz, Santa Cruz, CA) and PLB phosphorylation (Badrilla, Leeds, UK), as previously described [58]. NO bioavailability was measured via DAF-2 AM fluorescence. Data are presented as mean±SEM. Differences between two groups were evaluated for statistical significance (P< 0.05) by unpaired Student's t tests or by one way or two way ANOVA for multiple groups.
RESULTS
Exercise induced adaptations
Following an 8 week high intense, aerobic interval treadmill training (Table S1), exercise-trained (Ex) mice (C57Bl/6) had elevated VO2max levels and physiological hypertrophy (Table 1) as compared to sedentary control (Sed) mice. Therefore, the training protocol had achieved a state of fitness and produced a “physiological” ventricular hypertrophy.
Table 1.
Summary data of maximal oxygen consumption (VO2max) (n=4-6 mice), heart weight to body weight ratio and heart weight to tibia length (n=12 mice) in exercise (Ex) and sedentary (Sed) WT and NOS1KO mice.
| Sed | Ex | Sed-NOS1KO | Ex-NOS1KO | |
|---|---|---|---|---|
| Heart weight (mg) | 121 ± 2 | 129 ± 3 * | 112 ± 3 | 111 ± 4 |
| Body weight (g) | 22.2 ± 0.4 | 20.4 ± 0.2 * | 21.8 ± 0.5 | 20.2 ± 0.5 * |
| Heart to body ratio (mg/g) | 5.5 ± 0.1 | 6.3 ± 0.1 * | 5.2 ± 0.1 | 5.5 ± 0.2 |
| Tibia length (mm) | 22.0 ± 0.1 | 21.9 ± 0.1 | 21.0 ± 0.2 | 21.2 ± 0.4 |
| Heart to tibia ratio (mg/mm) | 5.8 ± 0.1 | 6.2 ± 0.2 * | 5.4 ± 0.1 | 5.2 ± 0.2 |
| VO2max (mL/kg-0.75/min) | 52.7 ± 2.9 | 62.0 ± 1.4 * | 50.4 ± 2.4 | 51.1 ± 1.6 |
P<0.05 vs corresponding control.
Exercise increases myocyte NOS1 protein expression, NO bioavailability, and contraction/relaxation
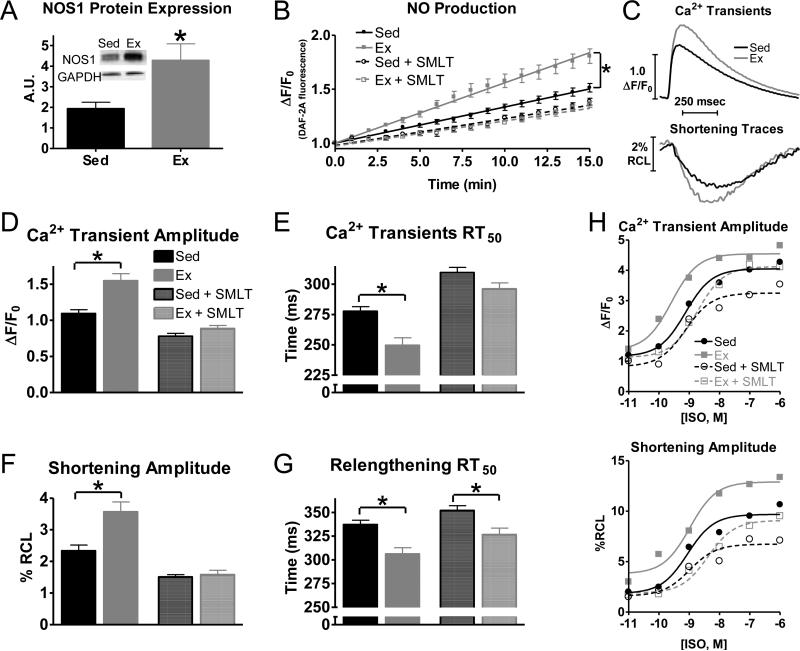
Exercise training elicited a significant increase in NOS1 expression in isolated ventricular myocytes from Ex mice compared to Sed mice (Fig 1A). This was accompanied by an increase in NO production in Ex myocytes (Fig 1B), that could be normalized by acute NOS1 inhibition (SMLT, 10 μM). Our data suggest that in Sed myocytes that NOS1 accounts for ~35% of the cardiomyocyte NO production, while it increases to ~60% in Ex myocytes.
Fig 1.
Exercise increases myocyte NOS1 expression, nitric oxide (NO) production and enhances myocyte contraction, which is normalized by acute NOS1 inhibition. A) Summary data of NOS1 protein expression (A.U.–arbitrary units), n=12 mice/group. B) Summary data of NO production over a 15 minute time period (±acute NOS1 inhibition, SMLT, dashed), n=19-26 cells/3 hearts. C) Representative traces of Ca2+ transients and shortening. Summary data of Ca2+ transient amplitudes (D), Ca2+decline to 50% of its peak (RT50)(E), shortening amplitudes (F), and relengthening RT50 (G) in exercise (Ex-grey) and sedentary (Sed-black)myocytes (±acute NOS1 inhibition, SMLT, striped), n=39-42 cells/7-10 hearts, (H) Summary data of Ca2+ transient (top) and shortening (bottom) amplitudes to various concentrations of ISO, n=12-39 myocytes/4 hearts, *P< 0.05 vs corresponding Sed.
In agreement with previous studies [23, 24, 26], Ex myocytes also exhibited a greater contraction (observed as increased Ca2+transient and shortening amplitudes) and a faster relaxation (measured as time to 50% relaxation, RT50) (faster Ca2+ transient and relengthening RT50, Fig 1C-G). Remarkably, acute NOS1 inhibition (+SMLT) normalized Ex myocyte contraction (Ca2+ transients and cell shortening amplitude) and Ca2+ decline to Sed myocyte levels (Fig 1D-F). In addition, Ex myocytes had a greater functional response (Ca2+ transient and shortening amplitudes) to β-adrenergic receptor (β-AR) stimulation with isoproterenol (ISO) (Fig 1H), consistent with previous studies in animals and humans [34, 41, 53, 55]. As with basal contraction, acute NOS1 inhibition (+SMLT) abolished the increased functional response to low dose ISO in Ex myocytes. At higher doses of ISO with acute NOS1 inhibition (+SMLT), there was still a greater functional response in the Ex compared to Sed myocytes. We believe this is due to the increased expression of Gsα[15].The force-frequency relationship (FFR), another important regulator of contraction [20, 42], was also enhanced via Ex and normalized with acute NOS1 inhibition (Figs S1). These data are consistent with the effects of Ex [26, 62] and NOS1 signaling [28, 58] on FFR.
Effects of Ex and NOS1 signaling on SR Ca2+ cycling
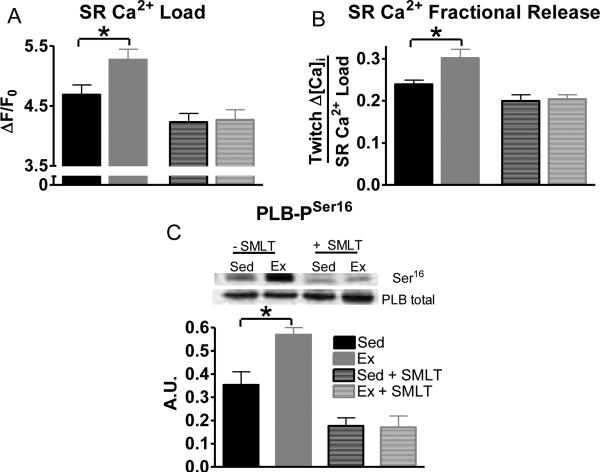
Since exercise enhances SR Ca2+cycling [25], SR Ca2+ load and SR Ca2+ fractional release (twitch Ca2+ amplitude / SR Ca2+ load) were examined. Ex myocytes had both an increased SR Ca2+ load (similar to a previous study [54]), and an increased SR Ca2+ fractional release compared to Sed myocytes. These differences were normalized by acute NOS1 inhibition (Fig 2A and 2B). SR Ca2+ cycling was further examined with post-rest potentiation, which is the enhanced contraction following a rest period (Fig S2). Ex myocytes reached a greater Ca2+ transient and shortening amplitude compared to Sed on the first twitch following short periods of rest, which was also normalized by acute NOS1 inhibition (Fig S2). Thus, the Ex-induced increases in myocyte SR Ca2+ cycling resulted from enhanced NOS1 signaling.
Fig 2.
Exercise enhances SR Ca2+cycling and is normalized by acute NOS1 inhibition. Summary data of SR Ca2+ load (A) and SR Ca2+ fractional release (B), n=26-35 cells/11-12 hearts. C) Summary data of PLB Serine16 phosphorylation (n=6 hearts) in exercise (Ex-grey) and sedentary (Sed-black) myocytes (±acute NOS1 inhibition, SMLT, striped), *P< 0.05 vs corresponding Sed.
Accelerated SR Ca2+ uptake occurs via increasing phosphorylation of phospholamban (PLB) [47, 48]. Previous studies have shown that Ex [36, 39] and NOS1 signaling increase PLB phosphorylation [58, 66]. In the present study, Ex myocytes (compared to Sed myocytes) had increased PLB serine16 phosphorylation, which is the PKA site, but not threonine17 phosphorylation, the CaMKII site (Sed: 0.24±0.03, Ex: 0.27±0.07 A.U.). Once again, acute NOS1 inhibition normalized Ex serine16 phosphorylation to Sed levels (Fig 2C). There was no effect of NOS1 inhibition on threonine17 phosphorylation (Sed+SMLT: 0.28±0.05, Ex+SMLT: 0.28±0.05 A.U.). Thus, these data suggest that the molecular mechanism responsible for the enhanced SR Ca2+ cycling induced by exercise is a NOS1 mediated increase in PLB phosphorylation.
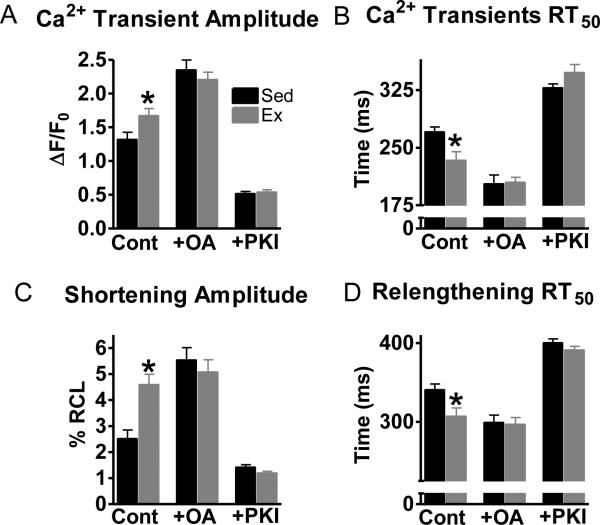
The increase in serine16 phosphorylation with Ex suggest a change in PKA and/or phosphatase activity. Hence, we investigated the roles of PKA and phosphatase 1 and 2a on Ex and Sed myocyte contraction. Interestingly, contraction in Ex myocytes was normalized with either phosphatase inhibition (okadaic acid, OA) or PKA inhibition (PKI) (Fig 3). Thus, these data suggest that exercise shifts the kinase/phosphatase balance in myocytes.
Fig 3.
Protein phosphatase 1 and 2a inhibition (okadaic acid, OA) and PKA inhibition (PKI) abrogated the contractile difference between Sed and Ex myocytes. Summary data of Ca2+ transient amplitudes (A), Ca2+ transient RT50 (B), shortening amplitudes and (C), relengthening RT50 (D) in exercise (Ex-grey) and sedentary (Sed-black) myocytes. n=10-22 cells/4 hearts, *P< 0.05 vs corresponding Sed.
Effects of training NOS1 deficient mice
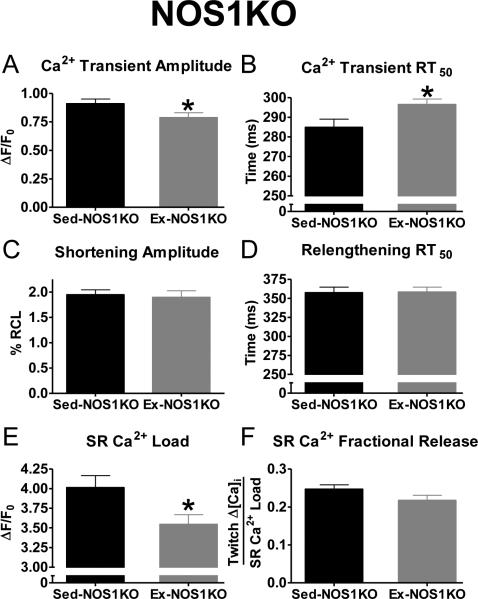
Since acute NOS1 inhibition reversed the adaptive contractile effects of exercise in WT mice, we examined the effects of Ex in a model deficient in NOS1. Thus, the cardiac effects of a similar exercise training protocol were evaluated in NOS1 knockout (KO) mice (Table S1). Notably, Ex-NOS1KO mice did not exhibit any of the cardiac adaptations that were observed in WT mice. That is, Ex-NOS1KO mice did not have increased VO2max or hypertrophy compared to Sed-NOS1KO mice (Table 1). Furthermore, contraction, FFR, and post-rest potentiation were not increased in Ex-NOS1KO myocytes (Fig 4 and S3). Surprisingly, we observed Ca2+ mismanagement in the Ex-NOS1KO myocytes. That is, Ex-NOS1KO myocytes had significantly decreased Ca2+ transient amplitudes, SR Ca2+ loads, and slowed Ca2+transient RT50 (Fig 4). Thus, exercise training did not produce beneficial cardiac adaptations in NOS1KO mice and actually caused deleterious effects on myocyte Ca2+ handling.
Fig 4.
Negative effects of exercising on Ca2+handling in NOS1 deficient myocytes. Summary data of Ca2+ transient amplitudes (A), Ca2+ transient RT50 (B), shortening amplitudes (C), relengthening RT50 (D), SR Ca2+ load (E), and SR Ca2+ fractional release (F) in exercise (Ex-NOS1KO-grey) and sedentary (Sed-NOS1KO-black), n=22-42 cells/5-6 hearts, *P< 0.05 vs corresponding Sed.
Effects of Ex and NOS1 on canines
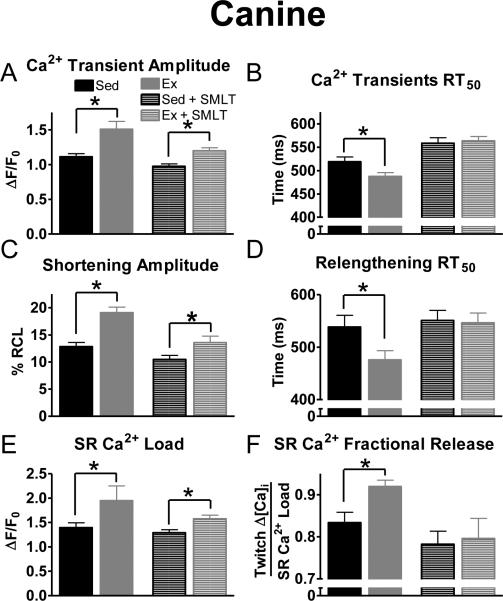
It is well documented that contractility is different between rodents and larger mammals (e.g., dog, humans). Therefore, we evaluated the contribution of the NOS1 signaling pathway to the cardiac adaptive response to exercise training in a canine model (see Supplementary material). Acute NOS1 inhibition (SMLT) had similar effects in isolated canine myocytes as were noted for murine myocytes (Fig 5). These data suggest a more universal role for exercise induced enhancement of NOS1 signaling in both large and small mammalian species.
Fig 5.
Exercise in a trained canine model elicited similar results with NOS1 inhibition on myocyte contraction. Summary data of Ca2+ transient amplitudes (A), Ca2+ transient RT50 (B), shortening amplitudes (C), relengthening RT50 (D), SR Ca2+ load (E), and SR Ca2+ fractional release (F) in exercise (Ex-grey) and sedentary (Sed- black), (±acute NOS1 inhibition, SMLT, striped), n = 10-33 cells/3-4 hearts, *P< 0.05 vs corresponding Sed.
Enhanced role of NOS1 signaling with Ex
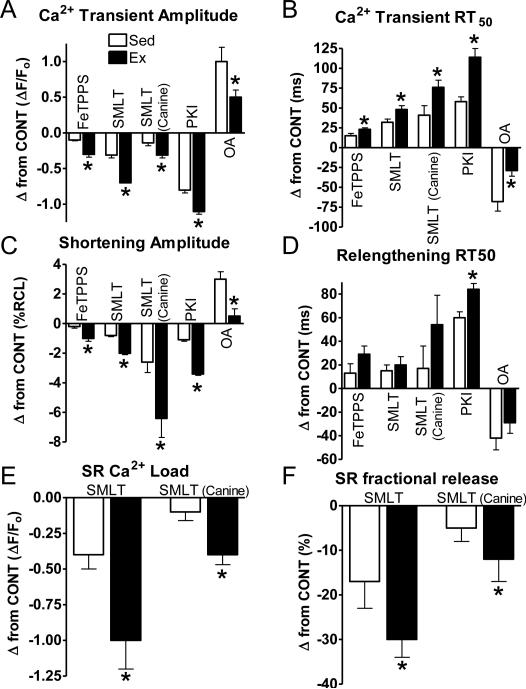
Since NOS1 inhibition normalized contractile function in Ex myocytes to Sed levels, the role of NOS1 with Ex was further investigated. NOS1 signals, in part, via peroxynitrite [31, 58]. FeTPPS, a peroxynitrite decomposition catalyst, had a similar effect on contraction as NOS1 inhibition in both Ex and Sed myocytes (Fig S4). However, the functional effects of FeTPPS were much greater in Ex vs Sed myocytes (Fig 6). This is consistent with the contractile effects of NOS1 inhibition (SMLT in murine and canine myocytes), PKA inhibition with PKI, and phosphatase inhibition with OA (smaller effect consistent with the kinase/phosphatase shift) (Fig 6). NOS1 inhibition also had a greater effect on its downstream target PLB. Specifically, NOS1 inhibition resulted in a greater decrease in serine16 PLB phosphorylation in Ex vs Sed myocytes (-0.40±0.05 vs -0.18±0.03 A.U., P<0.05). Thus, these data suggest that NOS1 and its downstream signaling targets are the major mediators in the enhanced contraction and accelerated Ca2+decline produced by exercise training.
Fig 6.
NOS1 and its downstream signaling targets have a greater effect with exercise. Summary data shown as change from control of Ca2+ transient amplitudes (A),Ca2+decline to 50% of its peak (RT50)(B), shortening amplitudes (C), and relengthening RT50 (D), SR Ca2+ load (E), and SR Ca2+ fractional release (F) in exercise (Ex-grey) and sedentary (Sed-black) myocytes, *P< 0.05 vs corresponding Sed.
DISCUSSION
The molecular mechanism(s) by which the heart adapts to regular exercise is/are not completely known. This study demonstrates that NOS1 signaling plays an essential role in these adaptations via: 1) increased VO2max, hypertrophy, and contraction in WT but not NOS1KO mice; 2) increased ventricular myocyte NOS1 expression and NO production; and 3) normalization and greater contractile effects of inhibiting NOS1 (and its downstream targets) in Ex myocytes.
The role of NOS1 signaling in the exercise induced beneficial cardiac adaptations
The health benefits of exercise and the adverse consequences of inactivity are widely recognized. Regular exercise is associated with a lower risk for both cardiovascular and non-cardiovascular disease and increases the quality of life [33, 37, 63]. It may then be possible to design therapies (either specific exercise protocols or pharmacological targets) that maximize the beneficial aspects of exercise. Thus, it is imperative that the molecular mechanisms responsible for the beneficial cardiac effects of exercise are identified.
Exercise training produces physiological hypertrophy, enhances VO2max and myocyte contraction in both mice [26] and humans [63]. In the present study, in marked contrast to WT mice, aerobic interval training did not elicit these adaptations in NOS1KO mice (Table 1, Fig 1 & 4). While there are many contributing factors to VO2max, it is mainly limited by maximal stroke volume [2]. Endogenous NO is able to modulate stroke volume [45]. Thus, the decreased myocyte contraction caused by NOS1 ablation may have contributed to the lack of improvement in trained NOS1KO. It is widely accepted that physiological hypertrophy is dependent upon the insulin-growth factor/PI3K/AKT pathway [5]. Our current data suggests that NOS1 is also essential for physiological hypertrophy. Interestingly, AKT has been shown to phosphorylate NOS1 in the brain [11], suggesting that NOS1 may be downstream of AKT. Future studies (e.g., IGF1/PI3K/AKT and calcineurin/NFAT pathways) are warranted to investigate the mechanism(s) for the lack of exercise induced hypertrophy in the NOS1KO mice. Overall, our observations strongly suggest that the NOS1 signaling pathway plays an essential role in cardiac adaptation to exercise.
Increased SR Ca2+ cycling and greater fractional shortening are also widely accepted as an adaptive response to exercise [25, 54] (Fig 1-3, 5). Previous studies focused on exercise-induced changes in excitation-contraction coupling protein profiles (i.e. SERCA2a) [24, 36, 39, 52]. The present study expands these findings, demonstrating that the NOS1 signaling pathway is also a key component in the exercise-induced contractile adaptations (Fig 1, 5 and 6). NOS1, which is localized to the SR, modulates SR Ca2+cycling by targeting PLB (Fig 2). Studies have shown that increased PLB phosphorylation is responsible for the exercise induced increase in SR Ca2+ cycling [36, 39]. Our results extend this observation by demonstrating that enhanced NOS1 signaling is responsible for this effect. We believe that the molecular mechanism for the increased phosphorylation is a shift in the kinase/phosphatase balance (Fig 3) These data are consistent with previous reports that demonstrated increased PKA activity with Ex [54], while NOS1 signaling can activate PKA [31, 66] and inhibit phosphatase activity [66]. While Ex has been shown to increase NOS3 expression [14], NO produced via NOS3 does not contribute to the faster relengthening or the other observed contractile effects reported in the present study since NOS3 only modulates contractile function during β-AR stimulation and not basal function [38, 59]. Indeed, basal NO bioavailability was similar between Ex and Sed myocytes after NOS1 inhibition (Fig 1B). Taken together, these data suggest that exercise training increases contraction and accelerates Ca2+ decline at baseline due to augmented NOS1 and its downstream targets (Fig 6).
Training NOS1 deficient mice results in Ca2+mishandling
Not only did exercise training fail to induce adaptive cardiac effects (VO2max and hypertrophy) in the NOS1KO mice, but actually provoked detrimental actions on myocyte Ca2+ handling (Fig 4). As this is a global knockout, it is difficult to delineate the mechanism(s) responsible for this adverse cardiac response to exercise training. For example, the contribution of NO produced via NOS1 in the skeletal muscle cannot be overlooked, as demonstrated in other disease models [7]. In fact, mice deficient in NOS1 have increased skeletal muscle fatigue due to an immediate decrease in vasodilation after brief, mild exercise even though the strength of the muscle was sustained [30]. However, NOS1 signaling may not limit flow during sustained exercise [6]. As such, an increased vulnerability to fatigue could have reduced the exercise capacity (i.e., weekly training speeds) and thereby, potentially limiting the exercise-induced cardiac adaptations. However, this seems unlikely as training intensity was matched to similar levels in both the WT and NOS1KO mice (i.e., mice were trained at ~90% of the speed of the peak oxygen consumption (VO2peak) (Fig S5). Since exercise induced cardiac adaptations are dependent upon training intensity [26], similar intensities between groups one would expect that the exercise training effects should yield similar cardiac adaptive responses in both WT and NOS1KO mice, regardless of any potential skeletal muscle fatigue. However, only the WT mice exhibited exercise-induced enhancement on VO2max.
In a similar manner, NOS1 signaling also increases coronary blood flow [51]. Thus, NOS1KO mice may not have been able to increase coronary blood flow to match the increased myocardial oxygen consumption during exercise, resulting in ischemia and enhanced superoxide production [40]. Hence, implementing the 8 week training protocol in NOS1KO mice may have potentially exacerbated the already high cardiac superoxide levels [27, 29, 65] culminating in deleterious effects on SR Ca2+ cycling. While our data provide evidence that NOS1 is indispensible for the adaptive cardiac effect of exercise, other studies have shown that exercise results in other beneficial adaptations to the heart such as electrical remodeling (e.g., K+ channels, L-type Ca2+ channel) and the mitochondria [22, 64]. While it's known that NO is able to modulate various ion channels and mitochondria function [3, 68], future studies are warranted to determine if electrical remodeling and mitochondrial adaptations with exercise still occur in NOS1KO mice.
Beneficial effects of exercise
By understanding the natural/innate mechanisms by which a normal heart is remodeled into an athlete's heart, one might gain the knowledge necessary to design therapies to heal the diseased heart. Alternates to exercise must be identified to treat those cardiac patients that are mentally unwilling or physically unable to exercise. This might be achieved by pharmacological approaches to target the beneficial signaling pathways that are activated by exercise training in order to mimic “exercise in a pill” [12, 46]. One could term this new-age approach: Exercise-Like Induced Medicine (ELIM). Our results showing that NOS1 is indispensible for the beneficial cardiac effects of exercise and earlier studies demonstrating a cardioprotective role of NO [13, 16, 17, 44, 50] would suggest that manipulation of NOS1 protein expression or activation of components of the NOS1 signaling pathway might represent one such pharmacological approach. In this regards, it is interesting that studies have shown that NOS1 knockout mice do worse (adverse remodeling, contractility, mortality) after myocardial infarction [8, 49] while NOS1 overexpression is protective after ischemia/reperfusion, transverse aortic constriction, and heart failure [4, 35, 43]. It should be noted that the Burkard et al (2010) study [4] showed that NOS1 overexpression resulted in decreased contraction (opposite of our current results). We believe that this is due to the overexpressed NOS1 being localized to the caveolae (instead of the SR). Studies have shown although NO is a diffusible gas, its signaling is highly compartmentalized [67]. Thus, since the overexpressed NOS1 is at the caveolae, its negative effect on contraction is consistent with that of NOS3, which is also localized to caveolae and cardioprotective [21, 57, 59]. These results further suggest that NOS1 overexpression may mimic the beneficial effects of exercise and could potentially be a novel therapeutic (ELIM) treatment.
In conclusion, as far as we are aware, the present study is the first to show that NOS1 signaling contributes to the adaptive cardiac effects of exercise. Specifically, exercise increases ventricular myocyte NOS1 expression and NO bioavailability, which is essential for aerobic fitness, hypertrophy and enhanced contraction/relaxation.
Supplementary Material
ACKNOWLEDGMENTS
None
SOURCES OF FUNDING
This work was supported by the National Institutes of Health (K02HL094692, R01HL079283, M. T. Ziolo).
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. doi: 10.1038/416005a. [DOI] [PubMed] [Google Scholar]
- 2.Bassett DR, Jr., Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3:187–204. doi: 10.1016/j.mito.2003.10.001. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Burkard N, Williams T, Czolbe M, Blomer N, Panther F, Link M, Fraccarollo D, Widder JD, Hu K, Han H, Hofmann U, Frantz S, Nordbeck P, Bulla J, Schuh K, Ritter O. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation. 2010;122:1588–1603. doi: 10.1161/CIRCULATIONAHA.109.933630. doi: 10.1161/CIRCULATIONAHA.109.933630. [DOI] [PubMed] [Google Scholar]
- 5.Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Front Biosci. 2008;13:312–324. doi: 10.2741/2681. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- 6.Copp SW, Hirai DM, Schwagerl PJ, Musch TI, Poole DC. Effects of neuronal nitric oxide synthase inhibition on resting and exercising hindlimb muscle blood flow in the rat. J Physiol. 2010;588:1321–1331. doi: 10.1113/jphysiol.2009.183723. doi: 10.1113/jphysiol.2009.183723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabire H, Barthelemy I, Blanchard-Gutton N, Sambin L, Sampedrano CC, Gouni V, Unterfinger Y, Aguilar P, Thibaud JL, Ghaleh B, Bize A, Pouchelon JL, Blot S, Berdeaux A, Hittinger L, Chetboul V, Su JB. Vascular endothelial dysfunction in Duchenne muscular dystrophy is restored by bradykinin through upregulation of eNOS and nNOS. Basic Res Cardiol. 2012;107:240–246. doi: 10.1007/s00395-011-0240-6. doi: 10.1007/s00395-011-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation. 2005;112:3729–3737. doi: 10.1161/CIRCULATIONAHA.105.539437. doi: 10.1161/CIRCULATIONAHA.105.539437. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson M, Sjaland C, Andersson KB, Sjaastad I, Christensen G, Sejersted OM, Ellingsen O. Exercise training before cardiac-specific Serca2 disruption attenuates the decline in cardiac function in mice. J Appl Physiol. 2010;109:1749–1755. doi: 10.1152/japplphysiol.00282.2010. doi: 10.1152/japplphysiol.00282.2010. [DOI] [PubMed] [Google Scholar]
- 10.Furfine ES, Harmon MF, Paith JE, Knowles RG, Salter M, Kiff RJ, Duffy C, Hazelwood R, Oplinger JA, Garvey EP. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J Biol Chem. 1994;269:26677–26683. doi: 10.1074/jbc.273.15.888. [PubMed] [Google Scholar]
- 11.Gingerich S, Krukoff TL. Activation of ERbeta increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology. 2008;55:878–885. doi: 10.1016/j.neuropharm.2008.06.058. doi: 0.1016/j.neuropharm.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Goodyear LJ. The exercise pill--too good to be true? N Engl J Med. 2008;359:1842–1844. doi: 10.1056/NEJMcibr0806723. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 13.Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–286. doi: 10.1007/s00395-010-0146-8. doi: 10.1007/s00395-010-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 15.Hammond HK, Ransnas LA, Insel PA. Noncoordinate regulation of cardiac Gs protein and beta-adrenergic receptors by a physiological stimulus, chronic dynamic exercise. J Clin Invest. 1988;82:2168–2171. doi: 10.1172/JCI113840. doi: 10.1172/JCI113840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 17.Heusch G, Post H, Michel MC, Kelm M, Schulz R. Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res. 2000;87:146–152. doi: 10.1161/01.res.87.2.146. doi: 10.1161/01.RES.87.2.146. [DOI] [PubMed] [Google Scholar]
- 18.Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:753–760. doi: 10.1097/HJR.0b013e3281eacef1. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 19.Husain K. Physical conditioning modulates rat cardiac vascular endothelial growth factor gene expression in nitric oxide-deficient hypertension. Biochem Biophys Res Commun. 2004;320:1169–1174. doi: 10.1016/j.bbrc.2004.06.058. doi: 10.1016/j.bbrc.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 20.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43:523–531. doi: 10.1016/j.yjmcc.2007.08.012. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, Jans P, Scherrer-Crosbie M, Picard MH, Szelid Z, Gillijns H, Van de Werf F, Collen D, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94:1256–1262. doi: 10.1161/01.RES.0000126497.38281.23. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 22.Kavazis AN, Alvarez S, Talbert E, Lee Y, Powers SK. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am J Physiol Heart Circ Physiol. 2009;297:H144–152. doi: 10.1152/ajpheart.01278.2008. doi: 10.1152/ajpheart.01278.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemi OJ, Ceci M, Condorelli G, Smith GL, Wisloff U. Myocardial sarcoplasmic reticulum Ca2+ ATPase function is increased by aerobic interval training. Eur J Cardiovasc Prev Rehabil. 2008;15:145–148. doi: 10.1097/HJR.0b013e3282efd4e0. doi: 10.1097/HJR.0b013e3282efd4e0. [DOI] [PubMed] [Google Scholar]
- 24.Kemi OJ, Ellingsen O, Ceci M, Grimaldi S, Smith GL, Condorelli G, Wisloff U. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol. 2007;43:354–361. doi: 10.1016/j.yjmcc.2007.06.013. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemi OJ, Ellingsen O, Smith GL, Wisloff U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front Biosci. 2008;13:356–368. doi: 10.2741/2685. doi: 10.2741/2685. [DOI] [PubMed] [Google Scholar]
- 26.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisloff U, Ellingsen O. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67:161–172. doi: 10.1016/j.cardiores.2005.03.010. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fradley M, Shoukas AA, Berkowitz DE, Hare JM. Nitric oxide regulation of myocardial contractility and calcium cycling: independent impact of neuronal and endothelial nitric oxide synthases. Circ Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 29.Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–362. doi: 10.1161/01.RES.0000155331.09458.A7. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohr MJ, Traynham CJ, Roof SR, Davis JP, Ziolo MT. cAMP-independent activation of protein kinase A by the peroxynitrite generator SIN-1 elicits positive inotropic effects in cardiomyocytes. J Mol Cell Cardiol. 2010;48:645–648. doi: 10.1016/j.yjmcc.2010.01.007. doi: 10.1016/j.yjmcc.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases {beta}-adrenergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77:353–361. doi: 10.1093/cvr/cvm018. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation. 2010;122:1637–1648. doi: 10.1161/CIRCULATIONAHA.110.948349. doi: 10.1161/CIRCULATIONAHA.110.948349. [DOI] [PubMed] [Google Scholar]
- 34.Libonati JR, MacDonnell SM. Cardiac beta-adrenergic responsiveness with exercise. Eur J Appl Physiol. 2011;111:2735–2741. doi: 10.1007/s00421-011-1909-0. doi: 10.1007/s00421-011-1909-0. [DOI] [PubMed] [Google Scholar]
- 35.Loyer X, Gomez AM, Milliez P, Fernandez-Velasco M, Vangheluwe P, Vinet L, Charue D, Vaudin E, Zhang W, Sainte-Marie Y, Robidel E, Marty I, Mayer B, Jaisser F, Mercadier JJ, Richard S, Shah AM, Benitah JP, Samuel JL, Heymes C. Cardiomyocyte overexpression of neuronal nitric oxide synthase delays transition toward heart failure in response to pressure overload by preserving calcium cycling. Circulation. 2008;117:3187–3198. doi: 10.1161/CIRCULATIONAHA.107.741702. doi: 10.1161/CIRCULATIONAHA.107.741702. [DOI] [PubMed] [Google Scholar]
- 36.MacDonnell SM, Kubo H, Crabbe DL, Renna BF, Reger PO, Mohara J, Smithwick LA, Koch WJ, Houser SR, Libonati JR. Improved myocardial beta-adrenergic responsiveness and signaling with exercise training in hypertension. Circulation. 2005;111:3420–3428. doi: 10.1161/CIRCULATIONAHA.104.505784. doi: 10.1161/CIRCULATIONAHA.104.505784. [DOI] [PubMed] [Google Scholar]
- 37.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 38.Martin SR, Emanuel K, Sears CE, Zhang YH, Casadei B. Are myocardial eNOS and nNOS involved in the beta-adrenergic and muscarinic regulation of inotropy? A systematic investigation. Cardiovasc Res. 2006;70:97–106. doi: 10.1016/j.cardiores.2006.02.002. doi: 10.1016/j.cardiores.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros A, Rolim NP, Oliveira RS, Rosa KT, Mattos KC, Casarini DE, Irigoyen MC, Krieger EM, Krieger JE, Negrao CE, Brum PC. Exercise training delays cardiac dysfunction and prevents calcium handling abnormalities in sympathetic hyperactivity-induced heart failure mice. J Appl Physiol. 2008;104:103–109. doi: 10.1152/japplphysiol.00493.2007. doi: 10.1152/japplphysiol.00493.2007. [DOI] [PubMed] [Google Scholar]
- 40.Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–219. [PubMed] [Google Scholar]
- 41.Mole PA. Increased contractile potential of papillary muscles from exercise-trained rat hearts. Am J Physiol. 1978;234:H421–425. doi: 10.1152/ajpheart.1978.234.4.H421. [DOI] [PubMed] [Google Scholar]
- 42.Neumann T, Ravens U, Heusch G. Characterization of excitation-contraction coupling in conscious dogs with pacing-induced heart failure. Cardiovasc Res. 1998;37:456–466. doi: 10.1016/s0008-6363(97)00246-0. doi: 10.1016/S0008-6363(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 43.Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, Miller KL, Vandegaer K, Bedja D, Gabrielson KL, Paolocci N, Kass DA, Barouch LA. Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol. 2012;59:1979–1987. doi: 10.1016/j.jacc.2011.12.046. doi: 10.1016/j.jacc.2011.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Post H, Schulz R, Gres P, Heusch G. No involvement of nitric oxide in the limitation of beta-adrenergic inotropic responsiveness during ischemia. Am J Physiol Heart Circ Physiol. 2001;281:H2392–2397. doi: 10.1152/ajpheart.2001.281.6.H2392. [DOI] [PubMed] [Google Scholar]
- 45.Rassaf T, Poll LW, Brouzos P, Lauer T, Totzeck M, Kleinbongard P, Gharini P, Andersen K, Schulz R, Heusch G, Modder U, Kelm M. Positive effects of nitric oxide on left ventricular function in humans. Eur Heart J. 2006;27:1699–1705. doi: 10.1093/eurheartj/ehl096. doi: 10.1093/eurheartj/ehl096. [DOI] [PubMed] [Google Scholar]
- 46.Richter EA, Kiens B, Wojtaszewski JF. Can exercise mimetics substitute for exercise? Cell Metab. 2008;8:96–98. doi: 10.1016/j.cmet.2008.07.004. doi: 10.1016/j.cmet.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Roof SR, Biesiadecki BJ, Davis JP, Janssen PM, Ziolo MT. Effects of increased systolic Ca(2+) and beta-adrenergic stimulation on Ca(2+) transient decline in NOS1 knockout cardiac myocytes. Nitric Oxide. 2012;27:242–247. doi: 10.1016/j.niox.2012.08.077. doi: 10.1016/j.niox.2012.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roof SR, Shannon TR, Janssen PM, Ziolo MT. Effects of increased systolic Ca2+ and phospholamban phosphorylation during beta-adrenergic stimulation on Ca2+ transient kinetics in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1570–1578. doi: 10.1152/ajpheart.00402.2011. doi: 10.1152/ajpheart.00402.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 50.Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:402–413. doi: 10.1016/j.cardiores.2003.09.019. doi: 10.1016/j.cardiores.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 51.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- 52.Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, Bidasee KR. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol. 2009;106:1280–1292. doi: 10.1152/japplphysiol.91280.2008. doi: 10.1152/japplphysiol.91280.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spina RJ, Ogawa T, Coggan AR, Holloszy JO, Ehsani AA. Exercise training improves left ventricular contractile response to beta-adrenergic agonist. J Appl Physiol. 1992;72:307–311. doi: 10.1152/jappl.1992.72.1.307. [DOI] [PubMed] [Google Scholar]
- 54.Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisloff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 55.Takeda N, Dominiak P, Turck D, Rupp H, Jacob R. The influence of endurance training on mechanical catecholamine responsiveness, beta-adrenoceptor density and myosin isoenzyme pattern of rat ventricular myocardium. Basic Res Cardiol. 1985;80:88–99. doi: 10.1007/BF01906747. doi: 10.1007/BF01906747. [DOI] [PubMed] [Google Scholar]
- 56.Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284:L452–457. doi: 10.1152/ajplung.00270.2002. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Bonilla IM, Huang X, He Q, Kohr MJ, Carnes CA, Ziolo MT. Prolonged Action Potential and After Depolarizations Are Not Due to Changes in Potassium Currents in NOS3 Knockout Ventricular Myocytes. J Signal Transduct. 2012:645721. doi: 10.1155/2012/645721. doi: 10.1155/2012/645721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–1575. doi: 10.1152/ajpcell.00367.2007. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Kohr MJ, Wheeler DG, Ziolo MT. Endothelial nitric oxide synthase decreases beta-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am J Physiol Heart Circ Physiol. 2008;294:H1473–1480. doi: 10.1152/ajpheart.01249.2007. doi: 10.1152/ajpheart.01249.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Viatchenko-Karpinski S, Sun J, Gyorke I, Benkusky NA, Kohr MJ, Valdivia HH, Murphy E, Gyorke S, Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588:2905–2917. doi: 10.1113/jphysiol.2010.192617. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wisloff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H1301–1310. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 62.Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54:162–174. doi: 10.1016/s0008-6363(01)00565-x. doi: 10.1016/S0008-6363(01)00565-X. [DOI] [PubMed] [Google Scholar]
- 63.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 64.Yang KC, Foeger NC, Marionneau C, Jay PY, McMullen JR, Nerbonne JM. Homeostatic regulation of electrical excitability in physiological cardiac hypertrophy. J Physiol. 2010;588:5015–5032. doi: 10.1113/jphysiol.2010.197418. doi: 10.1113/jphysiol.2010.197418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang YH, Dingle L, Hall R, Casadei B. The role of nitric oxide and reactive oxygen species in the positive inotropic response to mechanical stretch in the mammalian myocardium. Biochim Biophys Acta. 2009;1787:811–817. doi: 10.1016/j.bbabio.2009.03.020. doi: 10.1016/j.bbabio.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–249. doi: 10.1161/CIRCRESAHA.107.164798. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 67.Ziolo MT, Bers DM. The real estate of NOS signaling: location, location, location. Circ Res. 2003;92:1279–1281. doi: 10.1161/01.RES.0000080783.34092.AF. doi: 10.1161/01.RES.0000080783.34092.AF. [DOI] [PubMed] [Google Scholar]
- 68.Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol. 2008;45:625–632. doi: 10.1016/j.yjmcc.2008.07.015. doi: 10.1016/j.yjmcc.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.