Abstract
Studies on culture-related differences in cognition have shown that Westerners attend more to object-related information, whereas East Asians attend more to contextual information. Neural correlates of these different culture-related visual processing styles have been reported in the ventral-visual and fronto-parietal regions. We conducted an fMRI study of East Asians and Westerners on a visuospatial judgment task that involved relative, contextual judgments, which are typically more challenging for Westerners. Participants judged the relative distances between a dot and a line in visual stimuli during task blocks and alternated finger presses during control blocks. Behaviorally, East Asians responded faster than Westerners, reflecting greater ease of the task for East Asians. In response to the greater task difficulty, Westerners showed greater neural engagement compared to East Asians in frontal, parietal, and occipital areas. Moreover, Westerners also showed greater suppression of the default network—a brain network that is suppressed under condition of high cognitive challenge. This study demonstrates for the first time that cultural differences in visual attention during a cognitive task are manifested both by differences in activation in fronto-parietal regions as well as suppression in default regions.
Keywords: culture, default network, fMRI, visuo-spatial processing
INTRODUCTION
Studies on cultural differences in cognition have shown that whereas Westerners display an analytic visual processing style and attend more to features of an object in a picture, East Asians use a more holistic approach, preferentially attending to contextual information (Masuda and Nisbett, 2001, 2006; Nisbett et al., 2001; Nisbett, 2003; Nisbett and Masuda, 2003; Chua et al., 2005; Nisbett and Miyamoto, 2005; Miyamoto et al., 2006; Masuda et al., 2008; Boduroglu et al., 2009; Goh et al., 2009). These studies have suggested that culture-related differences in analytic and holistic visual processing styles operate as biases in visual attention and perceptual processes that act on incoming visual stimuli. Indeed, neural correlates of these culture-related differences in visual processing have been observed in the fronto-parietal attentional system (Hedden et al., 2008) and ventral-visual perceptual system (Gutchess et al., 2006; Goh et al., 2007; Goh and Park, 2009; Jenkins et al., 2010), that are consistent with an analytic visual processing style in Westerners and a holistic visual processing style in East Asians.
In this present study, we postulated that culture-related differences in visual processing should also be associated with another important brain system that is sensitive to differential attention to external stimuli—the default-network (Greicius et al., 2003; Buckner et al., 2008; Miller et al., 2008; Anticevic et al., 2010; Hayden et al., 2010; Spreng et al., 2010). Specifically, we hypothesized that, due to a more analytic processing style, Westerners would have more difficulty and require greater attentional effort compared to East Asians in certain kinds of visuospatial tasks, in particular tasks that involve relative judgments. In contrast, East Asians would perform relative judgments with greater ease than Westerners due to their preference for holistic processing. We expected that attentional differences between Westerners and East Asians during such cognitive tasks would not only be associated with differences in fronto-parietal attention systems, as found in Hedden et al. (2008), but also extends further even to the default network.
The default network is a set of brain regions that are suppressed or deactivated during task conditions that require attention to external stimuli compared to rest conditions, across many different types of experimental tasks (Raichle et al., 2001; Greicius et al., 2003). The brain regions that have been implicated as part of the default network include medial frontal, posterior cingulate, bilateral angular and at times the medial temporal regions. Findings from default-network studies suggest that it is involved in processing more introspective aspects of cognition such as autobiographical memory and other self-referential information (Gusnard et al., 2001; Mason et al., 2007; Andrews-Hanna et al., 2010). These introspective processes are suppressed when a task directs attention toward external stimuli. Thus, because greater deactivation of the default network during an experimental task is associated with the attentional resources directed to process external stimuli (Anticevic et al., 2010; Benjamin et al., 2010; Hayden et al., 2010; Mayer et al., 2010), it should be a sensitive measure of cultural differences in allocation of visual attention to task demands.
There is evidence that neural activity differs as a function of analytic and holistic visual processing styles in Westerners and East Asians, respectively. Using a visuospatial line judgment task, Hedden et al. (2008) showed that Westerners engage greater fronto-parietal processing than East Asians when making relative judgments, whereas East Asians engaged these regions more when making absolute judgments. These findings suggest that participants recruit greater attentional resources when processing visual stimuli in a manner that conflicts with their culturally preferred visual processing style. Interestingly, Jenkins et al. (2010) also showed that East Asians engaged greater activity in lateral occipital regions than Westerners when object-background contextual relationships were incongruent [see also Goto et al. (2010) for a similar finding using ERP]. This finding is consistent with greater attentional involvement in East Asians when their default holistic processing style encounters difficulty in processing contextual information in the visual stimuli.
Taking the above findings together, in this present study, we hypothesized that culture-related differences in visual processing style would engage different levels of attentional effort in Westerners compared to East Asians during a relative visual judgment task, and that this would be reflected in the degree of suppression of the default network system. We used a visual judgment task utilized by Park et al. (2010) that yielded strong evidence for modulated default network activity. Participants made judgments about the distance between a dot and a bar relative to the length of a reference line. We hypothesized that this relative comparison of two visual properties would be more effortful for Westerners than East Asians due to their analytic bias to attend to each object independently, but would be less difficult for East Asians due to the holistic bias to encode relationships between objects. We expected that the increased difficulty for Westerners would be reflected in a greater neural response in frontal-parietal regions, similar to results obtained by Hedden et al. (2008) on a related task. Critically, we expected that Westerners would also show greater suppression in default network regions compared to East Asians, reflecting the greater engagement of attentional effort and greater suppression of introspective processes. We also considered the relationship of neural activity to differences in cultural values, specifically, to individualism in Western culture and collectivism in East Asian cultures (Nisbett et al., 2001; Nisbett, 2003; Goh and Park, 2009). These individualistic and collectivistic culture value systems are associated with distinctive social interactions and physical environments that differentially shape the cognitive processing of individuals within these cultural environments (Nisbett & Miyamoto, 2005; Miyamoto et al., 2006). Moreover, cultural studies of social cognition and self-concept have shown that default-network activity reflects individual differences in subscribing to individualistic and collectivistic values (Han & Northoff, 2008; Chiao et al., 2009, 2010). Thus, we obtained measures of individualism and collectivism (Schwartz, 1992) in each participant and examined the relationship of these measures to neural activity in specific brain regions.
MATERIALS AND METHODS
Participants
Ninety-seven participants were scanned in this block-design functional magnetic resonance imaging (fMRI) experiment. The participants consisted of 50 Westerners (US: 25 males and 25 females; mean age: 22.1 years; range: 20–29 years), and 47 East Asians (SG: 25 males and 22 females; mean age: 24.2 years; range: 20–30 years). Westerners were American students from the University of Illinois at Urbana-Champaign, USA. East Asians were Chinese Singaporeans recruited from local universities in Singapore. Singapore is a multiracial nation, consisting of Chinese, Malay and Indian cultural groups that predominantly subscribe to East Asian values (Hofstede, 2001). To keep our sample comparable to other studies, all East Asians were ethnic Chinese born in Singapore. Visual acuity in the scanner was corrected to at least 20/40 on the Snellen scale. Participants gave informed consent approved by local institutional review boards at the two sites and were remunerated after the study. Those who had counter-indications for scanning were excluded.
Visual reproduction test and Schwartz value survey
As an additional independent measure of visual processing style, participants completed the Visual Reproduction Test (Wechsler, 1997) outside the scanner that assessed their ability to recall details about abstract line drawings as a proxy for where attentional resources have been directed. Participants were given 10 s to view each of four drawings, after which the drawing was removed and the participant had to reproduce it on a blank sheet of paper. Participants’ drawings were scored such that higher scores indicated more detailed reproduction of the drawings, reflecting a processing style that attends more to visual features.
Participants also completed the Schwartz Value Survey (SVS; Schwartz, 1992) that measured the degree to which they subscribed to individualistic and collectivistic values. For each participant, we obtained ratings of the subjective importance of sub-scale values of the SVS, which consisted of the individualistic values power, achievement, hedonism, stimulation and self-direction and the collectivistic values universalism, benevolence, tradition, conformity and security. We also computed summarized scores of individualism and collectivism using the averages across the respective associated SVS sub-scales.
fMRI scan stimuli and procedure
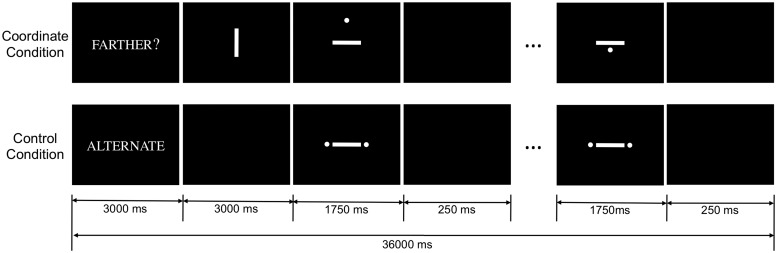
In the blocked-design fMRI experiment, participants performed a variant of the visuospatial judgment task used by Baciu et al. (1999) and Park et al. (2010). Participants completed a single run (7 min 12 s) that consisted of two types of task blocks: the coordinate task and the control task (Figure 1). At the beginning of each task block, participants were shown a cue for 3 s that informed them about which task they were to perform. In the coordinate task, participants were first shown a reference vertical line for 3 s at the beginning of each block of trials and were to remember the length of the line. When the block began, participants viewed stimuli consisting of a dot that was either above or below a horizontal bar, and their task was to decide and respond using button presses whether the dot was farther away from the bar than the length of the reference line for each trial. Each bar and dot trial remained on screen for 1750 ms followed by a 250 ms blank screen. There were six coordinate task blocks consisting of 15 trials each with half of the trials consisting of dots that were farther. Trial types were randomly distributed within each block, and each block (including cue, reference, and task trials) lasted 36 s. In the control task, participants saw stimuli consisting of a horizontal bar between two dots and were to respond by alternately pressing two buttons upon the appearance of each stimulus. This control task was designed to engage minimal cognitive processing and act as a baseline by controlling for motor responses. The number of blocks, trials, and duration for control task blocks were identical to the coordinate task. All stimuli remained on screen until the participant responded or until the end of the stimulus duration. Coordinate and control blocks alternated across the single-run of this experiment. Stimuli were presented using E-Prime software (http://www.pstnet.com/eprime.cfm) with back-projection through the scanner bore and a mirror mounted on the head coil.
Fig. 1.
A sample of one block of stimuli presentation timings during the fMRI experiment.
Imaging protocol
Functional images of the brain were acquired using two identical 3 T Siemens Allegra scanner (Siemens, Erlangen, Germany) systems located at the Cognitive Neuroscience Lab, Singapore, and the University of Illinois at Urbana-Champaign, USA. At both sites, 32 axial slices were acquired for each participant along the anterior and posterior commissural axis, with slice thickness of 4 mm (0.4 mm gap), and 3.437 × 3.437 mm in-plane voxel sizes, 64 × 64 matrix, giving an in-plane FOV of 220 × 220 mm. 216 functional scans were obtained, using TR 2 s and TE 32 ms. Co-planar structural T2 images (TR 7000 ms, TE 98 ms, flip angle 150°, 0.859 × 0.859 mm in-plane voxel size, slice thickness 4 mm with 0.4 mm gap, 256 × 256 matrix and FOV 220 × 220 mm) were acquired to register and overlay the functional images to a 3D-MPRAGE T1 structural image (TR 2000 ms, TE 2.22 ms, flip angle 8°, 1.0 × 1.0 × 1.0 mm voxel size, 192 × 192 × 192 matrix and FOV 240 × 240 × 240 mm). Extensive tests were conducted at the two sites prior to this study that demonstrated clearly that the functional signals obtained for this study were comparable between the two magnets (Sutton et al., 2008). Briefly, the same four participants underwent several sessions of functional scanning involving visual and motor tasks at both the Singapore and USA sites, using the same scanning sequences and hardware. Data analysis revealed that the individual differences and task-related differences in neural responses greatly exceeded the small amount of variance contributed by scanners. Moreover, phantoms were scanned at both sites on a daily basis once this study began to ensure that the calibration between magnets was maintained.
Image analysis
Functional images in this present study were analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). The images were preprocessed with slice-timing and motion correction, and normalized to MNI space. Images were then smoothed with a FWHM 8 mm Gaussian kernel. In the first-level voxel-by-voxel statistical analysis, general linear models (GLM) were computed for each participant that consisted of the onsets of each of the coordinate and control blocks convolved with the hemodynamic response function (HRF), along with motion correction parameters. Contrasts were then performed on these first-level individual GLMs to obtain response estimates for the difference between responses to coordinate and control tasks in each voxel (coordinate > control contrast) for each participant. These individual response estimates were then used as the dependent variable in a second group-level analysis consisting of a factorial model with culture groups (US and SG) as the between-subject variable.
Group-level whole brain contrasts were then performed on the factorial model to identify voxels that showed significant cultural differences in the brain responses to the coordinate vs control conditions within task-active and default-network regions, as follows. To examine culture-related differences in task-active regions, we created a mask based on voxels that showed significantly greater responses to coordinate relative to control conditions (coordinate > control contrast) at P < 0.001, with a cluster size threshold of 20 voxels. Within this mask of task-active regions with reduced number of voxels for consideration, we then identified voxels that showed significant cultural differences (US > SG or SG > US) at P < 0.005, cluster size threshold 20 voxels, in the coordinate relative to control responses. Similarly, we examined culture-related differences in default-network regions by creating a mask based on the control > coordinate contrast (P < 0.001, cluster size threshold of 20 voxels), and then identifying voxels showing significant cultural differences within this mask at P < 0.005, cluster size threshold 20 voxels. The cluster size threshold of 20 voxels was determined using Monte Carlo simulations (as implemented in Slotnick et al., 2003) based on our imaging parameters and corrected P value < 0.05.
We additionally assessed whether trial-by-trial responses times (RT), an index of time-on-task and cognitive effort (Yarkoni et al., 2009), would contribute to culture-related differences in brain responses to coordinate vs ccontrol conditions. We performed another group-level analysis that was identical to the one above, except that individual brain response estimates were obtained using first-level GLMs that included trial-by-trial RTs, convolved with the HRF, as a covariate for each participant. To the extent that cultural differences in coordinate vs control processing are related to cognitive effort, RT as a covariate should account for and reduce group differences in brain response estimates to these conditions.
We also performed regions-of-interest (ROI) analysis as a follow-up of the whole-brain analysis to relate brain responses to the SVS measures as well as behavioral responses in the coordinate task and performance in the Visual Reproduction Test. ROIs were based on regions that showed significant culture group differences in the whole-brain analysis. Specifically, ROIs were specified as contiguous significant voxels within a 10 mm radius around the peaks identified in the whole-brain contrasts for culture-related differences in task-active and default-network areas. Estimates of the responses to coordinate relative to control conditions (coordinate > control contrast) were then extracted from these ROIs for each participant and submitted to two-tailed independent t-tests with homogeneity of variances across culture-groups assumed, and Pearson's correlations with two-tailed tests of significance.
RESULTS
Behavioral performance and values
Table 1 shows the accuracy levels and response times during the coordinate task for the US and SG groups separately. Whereas both groups performed equally well in the coordinate task [t(95) = 0.43, n.s.], as expected, the SG group had a significantly faster mean response time than the US group [t(95) = 2.68, P < 0.01]. This behavioral data suggest that the US group may have encountered greater difficulty when making relative visuospatial judgments compared to the SG group. In addition, the US group had significantly higher scores than the SG group in the Visual Reproduction Test [mean (s.e.): US = 96.3 (1.1), SG = 89.2 (0.8); t(95) = 5.31, P < 0.01], suggesting that the US group had a visual processing style that involved more detailed processing of visual features relative to the SG group.
Table 1.
Accuracy (s.e.) and response times during the coordinate task for US and SG groups
| Behavioral Measures | US | SG |
|---|---|---|
| Mean (s.e.) | Mean (s.e.) | |
| Accuracy (%) | 83.7 (1.4) | 82.9 (1.0) |
| Response Time (ms) | 799.7 (12.1) | 756.3 (10.6) |
With respect to cultural differences in values, group differences in SVS sub-scale ratings have been reported for this sample in a previous study (Goh et al, 2010) and only significant results are listed here. Briefly, the US group gave higher ratings than the SG group for Hedonism (an individualistic value) [US = 9.0 (0.3), SG = 8.1 (0.4); t(95) = 1.89, P < 0.05], but the SG group rated benevolence [US = 23.6 (0.5), SG = 25.4 (0.5); t(95) = 2.66, P < 0.01], Tradition [US = 12.8 (0.7), SG = 18.3 (0.5); t(95) = 6.32, P < 0.01], conformity [US = 15.1 (0.5), SG = 17.9 (0.5); t(95) = 3.72, P < 0.01] and security [US = 18.3 (0.5), SG = 22.1 (0.6); t(95) = 5.46, P < 0.01] (collectivistic values) higher than did the US group. Whereas there was no significant difference in value ratings of individualism between the US and SG groups [mean (s.e.): US = 14.5 (0.31), SG = 14.9 (0.32); t(95) = 1.01, n.s.], the SG group gave significantly higher ratings for collectivism than the US group [mean (s.e.): US = 20.9 (0.40), SG = 23.8 (0.41); t(95) = 5.03, P < 0.01].
Whole-brain analysis
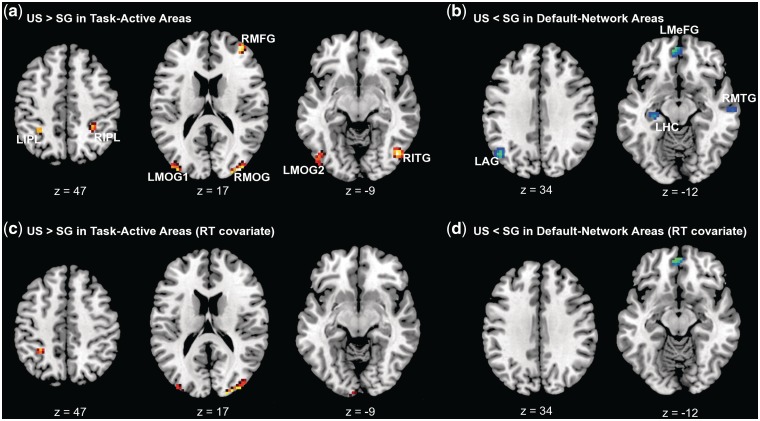
Figure 2 shows the results of the whole-brain analysis of cultural differences in the response to coordinate relative to control conditions in task-active and default-network regions and Table 2 lists the peak voxels of this analysis (see Supplementary Table S1 for locations of peak voxels for the task-active and default-network regions). The locations of task-active and default-network regions found in this study were consistent with those reported in Park et al.(2010) and prior literature. In all task-active areas listed in Table 2 and seen in Figure 2A, the US group showed greater responses to the coordinate condition than the SG group in attention-related regions that included bilateral inferior parietal (LIPL and RIPL) and middle occipital regions (LMOG1, LMOG2 and RMOG), right middle frontal (RMFG) and inferior temporal regions (RITG). There were no regions in the task-active areas that showed greater coordinate responses in the SG compared to the US group.
Fig. 2.
Axial slices showing voxels with significant culture-related differences in responses to the coordinate relative to the control tasks in (A) task-active areas and (B) default-network areas. (C and D) Showing the same contrasts when response time (RT) is included as a covariate.
Table 2.
Peak voxels that showed culture-related differences in responses to the coordinate relative to control tasks in task-active and default-network areas
| Contrast | Region | BA | x | y | z | t |
|---|---|---|---|---|---|---|
| Task-active areas, US > SG | L. inf. Parietal lobule | 40 | −33 | −48 | 51 | 3.88 |
| R. inf. parietal lobule | 40 | 30 | −42 | 45 | 3.47 | |
| L. mid. occipital gyrus I | 18 | −36 | −93 | 9 | 4.15 | |
| R. mid. occipital gyrus | 19 | 36 | −90 | 18 | 4.14 | |
| R. mid. frontal gyrus | 46 | 36 | 51 | 18 | 3.72 | |
| L. mid. occipital gyrus II | 19 | −36 | −72 | 3 | 3.34 | |
| R. inf. temporal gyrus | 19 | 45 | −72 | −9 | 3.86 | |
| Default-network areas, US < SG | L. angular gyrus | 39 | −45 | −66 | 36 | 3.68 |
| L. med. frontal gyrus | 11 | −6 | 51 | −12 | 3.61 | |
| R. mid. temporal gyrus | 21 | 63 | −15 | −15 | 3.35 | |
| L. hippocampus | 20 | −27 | −24 | −12 | 3.19 | |
| L. parahippocampal gyrus | 30 | −24 | −36 | −18 | 3.06 |
In contrast to the task-active areas, in default-network areas, the US group showed greater deactivation than the SG group during the coordinate task in the left angular (LAG), medial frontal (LMeFG), parahippocampal (LPHC), hippocampal (LHC) and right middle temporal regions (RMTG) (Figure 2B and Table 2). There were no regions in the default network that showed less deactivation in the US compared to the SG group.
These group differences in task-active and default-network areas were somewhat reduced when RT was used as a covariate in the whole-brain analyses (see ‘Materials and Methods’ section; Figure 2C and D). Specifically, group differences in LIPL, LMOG1, and RMOG in task-active areas, and in LMeFG in default-network areas remained. Thus, although some of the culture effect observed in the task-active and default-network was due to longer time-on-task in US subjects, culture differences in neural activity generally remained consistent. Overall, the findings are consistent with the notion that, compared to East Asians, Westerners engaged greater processing in attention networks and suppressed default network processing more, even after time-on-task was controlled.
ROI analysis
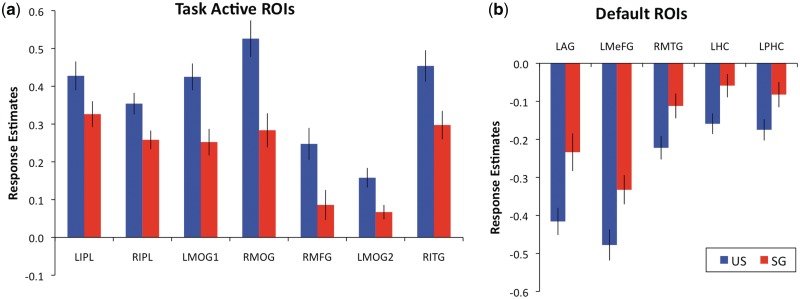
To follow-up the findings from the whole-brain analysis, we examined the responses during the coordinate relative to control conditions in the task-active and default-network ROIs that were associated with culture-related differences reported above. Figure 3 shows the extracted responses from each ROI that reflect the whole-brain analysis. Consistent with the whole-brain analyses, during the coordinate task, the US group showed greater responses in task-active ROIs (Figure 3A) and greater suppression in default-network ROIs (Figure 3B), compared to the SG group.
Fig. 3.
Responses during the coordinate relative to control tasks in (A) task-active and (B) default-network ROIs that showed significant culture-related differences. All group differences are significant at P < 0.05 and error bars show standard errors.
While correlations between behavior and brain responses in these ROIs were relatively weak, some significant relationships bear mention. We found that higher visual reproduction scores were associated with greater functional responses in task-active regions [RIPL: r(95) = 0.20, P < 0.05; LMOG1: r(95) = 0.27, P < 0.05; RMOG: r(95) = 0.26, P < 0.05; RITG: r(95) = 0.21, P < 0.05] as well as greater suppression in default-network regions [LAG: r(95) = −0.24, P < 0.05; LMeFG: r(95) = −0.25, P < 0.05; RMTG: r(95) = −0.23, P < 0.05]. These correlations suggest that participants who attend more to visual features as a preferential processing style had greater difficulty in performing relative visual judgments and thus required greater neural effort to do so. In addition, slower response times in the relative visual judgment task in the scanner was marginally correlated with greater functional responses in the RMOG [r(95) = 0.17, P < 0.10], a task-active region, which also supports the notion that greater responses in visual attention regions reflects greater task difficulty.
With respect to SVS values, higher ratings of Hedonism, an individualistic value, was associated with greater suppression in the default network [LMeFG: r(95) = −0.25, P < 0.05; LHC: r(95) = −0.24, P < .05]. Also, higher ratings of Tradition, a collectivistic value, was associated with less suppression in the LMeFG [r(95) = 0.21, P < 0.05] in the default-network, and reduced responses in task-active areas [LMOG1: r(95) = −0.22, P < 0.05; LMOG2: r(95) = −0.23, P < 0.05; RITG: r(95) = −0.27, P < 0.05]. Greater collectivism was marginally correlated with less suppression of the left angular gyrus (LAG: r = 0.17, P < 0.10). These findings are consistent with the association between culture-related biases and neural function such that Westerners who are more individualistic tend to engage greater responses in task-active regions and suppress default-network activity during this relative visuospatial task more than East Asians who are more collectivistic.
DISCUSSION
The results in this study are consistent with greater attentional processing in Westerners than East Asian when making relative visuospatial judgments that is associated with greater recruitment of task-active regions and, importantly, greater suppression of default-network regions. Compared to East Asians, Westerners engaged greater neural processing during the coordinate relative to control task in bilateral inferior parietal, occipital and right middle frontal and inferior temporal regions, but greater suppression in left angular, medial frontal, medial temporal and right middle temporal regions. Behaviorally, Westerners took longer to respond than East Asians with comparable accuracy. Group differences in brain responses remained, even after accounting for time-on-task, consistent with culture-related differences in task difficulty and attentional effort when making relative visuospatial judgments. Compared to East Asians, Westerners showed higher visual reproduction performance indicating a processing style that involves greater attention to visual features. Moreover, higher visual reproduction performance was associated with greater responses in task-active regions along with greater suppression in default-network regions in the relative visuospatial judgment task. Interestingly, individual differences in cultural values were associated with responses in task-active regions and suppression in default-network regions as well, although these correlations were small in size. Compared to Westerners, East Asians rated individualistic values lower and collectivistic values higher. And, whereas higher ratings of individualistic values were associated with greater default-network suppression and task-active responses, higher ratings of collectivistic values were associated with less suppression of the default-network regions and lower functional responses in task-active regions.
Our findings show that culture-related differences in visual processing extends to the default-network, adding to studies that have found functional differences between Westerners and East Asians in other brain regions. Culture-related differences in perceptual processing of visual stimuli have been reported in the ventral-visual cortex (Gutchess et al., 2006; Goh et al., 2007, 2010; Goh & Park, 2009; Jenkins et al., 2010). Specifically, consistent with a more analytic visual processing style in Westerners, Westerners tend to show greater object-processing responses in the lateral occipital complex and temporal regions compared to East Asians when processing naturalistic pictures consisting of objects and scenes (Gutchess et al., 2006; Goh et al., 2007). In addition, Goh et al. (2010) found that Westerners also show more analytic processing of face stimuli associated with bilateral fusiform involvement in comparison with more holistic face processing in East Asians associated with more right-lateralized fusiform responses (Sergent, 1982; Rhodes, 1985; Rossion et al., 2000; Rotshtein et al., 2007). As previously mentioned, culture-related differences in visual processing styles have also been associated with differences in attentional control mechanisms in fronto-parietal (Hedden et al., 2008) and occipital regions (Jenkins et al., 2010). Taken together with our findings of culture-related differences in default-network function, the extent to which cultural differences are present in brain function may now be seen to be more widespread and ingrained in neurocognitive processes than previously thought.
Culture-related differences in default-network function can be attributed to differences in general attentional demand associated with the level of difficulty Westerners and East Asians had during the relative visuospatial judgment task. Thus, the default-network responses observed may index downstream effects of cultural differences in the level of engagement of visual processing elsewhere in the brain. It is also plausible that the default-network differences observed reflects culture-related differences in top-down processes that bias the way visual stimuli are perceived and attended to, independent of processing difficulty. That is, different ability to suppress default-network processing in Westerners and East Asians may determine how they subsequently attend to different elements in a visual scene. Greater default-network suppression may facilitate analytic processing in Westerners through a greater but narrower focus of attention on specific visual object features, whereas less default-network suppression may bias East Asians to adopt a wider attentional focus to integrate information between multiple visual elements.
Initial clues to these subtle but important causal distinctions in the mechanism of cultural differences in default-network processing may be seen in studies that have related default-network processing to individual differences (Chiao et al., 2009, 2010) and cultural differences (Zhu et al., 2007) in self-processing. In Chiao et al. (2009, 2010), greater responses in the medial frontal regions during general and contextual judgments predicted whether individuals subscribed to individualistic or collectivistic styles of self-construal, respectively. Importantly, Chiao et al. (2010) showed that manipulating individualistic or collectivistic biases in participants through priming modulated medial frontal responses and subsequently their self-construal values, suggesting that differences in medial frontal processing affected the biases that participants expressed in their judgments. In our study involving visual cognition and attention, we find some evidence suggesting that greater collectivism in East Asians was associated with less suppression of the default-network regions, compared to Westerners. Thus, we cautiously speculate that perhaps some aspect of individual differences in cultural values may be related to differences in visual cognitive processing in Westerners and East Asians and that the default-network regions are important for the connection between these psychological domains. To effectively characterize the association between cultural values, cognition and default-network processing, however, would require much more definitive evaluations of the acquisition of cultural values over the lifespan and more temporally sensitive measures of neural function. At the very least, our findings show that culture-related differences in default-network processing are present during a cognitive task that does not involve self or social processing, suggesting that a more general mechanism is involved in default-network activity that manifests across the domains of cognitive, self and social processing.
In sum, the results of this study are consistent with the notion that culture-related differences in analytic and holistic visual processing styles are tied to differences in attentional processes. These cultural differences in visual processing involve a bias to attend to different aspects of the visual stimulus, thus processing them in a culturally non-preferred way requires more neural effort to compensate for or overcome the attentional bias. Critically, the neural signature of these culture-related differences in attentional bias can be seen in regions involved in perceptual processing, attentional control, and now, even in default-network processing. The widespread nature of these cultural differences in brain function reflects the plasticity of the brain and provides impetus to reconsider the heterogeneity of human cognition in future studies and everyday situations.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
Marion Reeds, Peggy Charney, Eric Leshikar, Blair Flicker, Lucas Jenkins, Karren Chen, Karen Chan, and Sunny Kort assisted in data collection.
National Institutes of Health (R-01-AG015047-09 to D.C.P, R-37-AG006265-26 to D.C.P.).
REFERENCES
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology. 2010;104(1):322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage. 2010;49(3):2638–48. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M, Koenig O, Vernier MP, Bedoin N, Rubin C, Segebarth C. Categorical and coordinate spatial relations: fMRI evidence for hemispheric specialization. Neuroreport. 1999;10(6):1373–8. doi: 10.1097/00001756-199904260-00040. [DOI] [PubMed] [Google Scholar]
- Benjamin C, Lieberman DA, Chang M, Ofen N, Whitfield-Gabrieli S, Gabrieli JDE, Gaab N. The influence of rest period instructions on the default mode network. Frontiers in Human Neuroscience. 2010;4:218. doi: 10.3389/fnhum.2010.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boduroglu A, Shah P, Nisbett RE. Cultural differences in allocation of attention in visual information processing. Journal of Cross-Cultural Psychology. 2009;40(3):349–60. doi: 10.1177/0022022108331005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, et al. Neural basis of individualistic and collectivistic views of self. Human Brain Mapping. 2009;30(9):2813–20. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, et al. Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience. 2010;22(1):1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chua HF, Boland JE, Nisbett RE. Cultural variation in eye movements during scene perception. Proceedings of the National Academy of Sciences USA. 2005;102(35):12629–33. doi: 10.1073/pnas.0506162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, et al. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cognitive, Affective & Behavioral Neuroscience. 2007;7(1):44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Goh JOS, Leshikar ED, Sutton BP, et al. Culture differences in neural processing of faces and houses in the ventral visual cortex. Social Cognitive and Affective Neuroscience. 2010;5(2–3):227–35. doi: 10.1093/scan/nsq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Park DC. Culture sculpts the perceptual brain. Progress in Brain Research. 2009;178:95–111. doi: 10.1016/S0079-6123(09)17807-X. [DOI] [PubMed] [Google Scholar]
- Goh JO, Tan JC, Park DC. Culture modulates eye-movements to visual novelty. PloS One. 2009;4(12):e8238. doi: 10.1371/journal.pone.0008238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto SG, Ando Y, Huang C, Yee A, Lewis RS. Cultural differences in the visual processing of meaning: detecting incongruities between background and foreground objects using the N400. Social Cognitive and Affective Neuroscience. 2010;5(2–3):242–53. doi: 10.1093/scan/nsp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences USA. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Boduroglu A, Park DC. Cultural differences in neural function associated with object processing. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(2):102–9. doi: 10.3758/cabn.6.2.102. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: a transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9(8):646–54. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Cognitive control signals in posterior cingulate cortex. Frontiers in Human Neuroscience. 2010;4:223. doi: 10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Ketay S, Aron A, Markus HR, Gabrieli JDE. Cultural influences on neural substrates of attentional control. Psychological Science. 2008;19(1):12–7. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- Hofstede G. Culture's Consequences: Comparing Values, Behaviors, Institutions, and Organizations Across Nations. Thousand Oaks, CA, USA: Sage Publications; 2001. [Google Scholar]
- Jenkins LJ, Yang Y, Goh J, Hong Y, Park DC. Cultural differences in the lateral occipital complex while viewing incongruent scenes. Social Cognitive and Affective Neuroscience. 2010;5(2–3):236–41. doi: 10.1093/scan/nsp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Gonzalez R, Kwan L, Nisbett RE. Culture and aesthetic preference: comparing the attention to context of East Asians and Americans. Personality and Social Psychology Bulletin. 2008;34(9):1260. doi: 10.1177/0146167208320555. [DOI] [PubMed] [Google Scholar]
- Masuda T, Nisbett RE. Attending holistically versus analytically: comparing the context sensitivity of Japanese and Americans. Journal of Personality and Social Psychology. 2001;81(5):922–34. doi: 10.1037//0022-3514.81.5.922. [DOI] [PubMed] [Google Scholar]
- Masuda T, Nisbett RE. Culture and change blindness. Cognitive Science. 2006;30:1–19. doi: 10.1207/s15516709cog0000_63. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Roebroeck A, Maurer K, Linden DEJ. Specialization in the default mode: task-induced brain deactivations dissociate between visual working memory and attention. Human Brain Mapping. 2010;31(1):126–39. doi: 10.1002/hbm.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the USA. 2008;105(6):2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Nisbett RE, Masuda T. Culture and the physical environment: holistic versus analytic perceptual affordances. Psychological Science. 2006;17(2):113–9. doi: 10.1111/j.1467-9280.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- Nisbett RE. The Geography of Thought: How Asians and Westerners think Differently—and Why. New York: Free Press; 2003. [Google Scholar]
- Nisbett RE, Masuda T. Culture and point of view. Proceedings of the National Academy of Sciences USA. 2003;100(19):11163–70. doi: 10.1073/pnas.1934527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, Miyamoto Y. The influence of culture: holistic versus analytic perception. Trends in Cognitive Sciences. 2005;9(10):467–73. doi: 10.1016/j.tics.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Peng K, Choi I, Norenzayan A. Culture and systems of thought: holistic versus analytic cognition. Psychological Review. 2001;108(2):291–310. doi: 10.1037/0033-295x.108.2.291. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Frontiers in Human Neuroscience. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. Lateralized processes in face recognition. British journal of Psychology. 1985;76(2):249–71. doi: 10.1111/j.2044-8295.1985.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Rossion B, Dricot L, Devolder A, et al. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 2000;12(5):793–802. doi: 10.1162/089892900562606. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Geng JJ, Driver J, Dolan RJ. Role of features and second-order spatial relations in face discrimination, face recognition, and individual face skills: behavioral and functional magnetic resonance imaging data. Journal of Cognitive Neuroscience. 2007;19(9):1435–52. doi: 10.1162/jocn.2007.19.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH. Universals in the content and structure of values: Theoretical advances and empirical tests in 20 countries. Advances in Experimental Social Psychology. 1992;25:1–62. [Google Scholar]
- Sergent J. About face: left-hemisphere involvement in processing physiognomies. Journal of Experimental Psychology: Human Perception and Performance. 1982;8(1):1–14. doi: 10.1037//0096-1523.8.1.1. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53(1):303–17. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton BP, Goh J, Hebrank A, Welsh RC, Chee MWL, Park DC. Investigation and validation of intersite fMRI studies using the same imaging hardware. Journal of Magnetic Resonance Imaging. 2008;28(1):21–8. doi: 10.1002/jmri.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale– Third Edition (WMS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: a multi-study fMRI analysis. PloS One. 2009;4(1):e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. NeuroImage. 2007;34(3):1310–16. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.