Abstract
Habituation is a basic form of learning that reflects the adaptive reduction in responses to a stimulus that is neither threatening nor rewarding. Extremely shy, or inhibited individuals, are typically slow to acclimate to new people, a behavioral pattern that may reflect slower habituation to novelty. To test this hypothesis, we used functional magnetic resonance imaging to examine habituation to neutral faces in 39 young adults with either an extreme inhibited or extreme uninhibited temperament. Our investigation focused on two key brain regions involved in response to novelty—the amygdala and the hippocampus. Habituation to neutral faces in the amygdala and hippocampus differed significantly by temperament group. Individuals with an uninhibited temperament demonstrated habituation in both the amygdala and hippocampus, as expected. In contrast, in individuals with an inhibited temperament, the amygdala and hippocampus failed to habituate across repeated presentations of faces. The failure of the amygdala and hippocampus to habituate to faces represents a novel neural substrate mediating the behavioral differences seen in individuals with an inhibited temperament. We propose that this failure to habituate reflects a social learning deficit in individuals with an inhibited temperament and provides a possible mechanism for increased risk for social anxiety.
Keywords: habituation, inhibited temperament, amygdala, neuroimaging, hippocampus, social anxiety
INTRODUCTION
Social functioning deficits are common in individuals who are extremely shy, and reduced social functioning may reflect underlying deficits in social learning. Of the many forms of social learning, one of the most basic forms is habituation. Habituation is defined as reduced responses to a stimulus following repeated exposures without meaningful consequences (Rankin et al., 2009). As an individual learns that a stimulus is neither threatening nor rewarding, the stimulus becomes safe and familiar, resulting in habituation at both neural and behavioral levels. Habituation is adaptive because it provides a mechanism for allocating attention resources to novel stimuli, which have unknown valence, over familiar stimuli. Thus, reduced habituation to novel social stimuli may reflect deficits in social learning and contribute to lower social functioning.
Although habituation is a fundamental learning process that begins in infancy (e.g. Bushnell, 1982), individuals differ in their rates of habituation to social stimuli. Individuals who habituate more slowly are likely to find encounters with new people overwhelming and thus avoid novel social experiences, whereas those who habituate more quickly are likely to seek novel social experiences. These two extreme categories of response to novelty have been conceptualized as inhibited and uninhibited temperament, respectively (Kagan, et al., 1987). Individuals with an inhibited temperament are typically shy, cautious, quiet and slow to warm up in new situations. Inhibited individuals are also at increased risk for social anxiety disorder (Schwartz, et al., 1999; Chronis-Tuscano, et al., 2009; Essex, et al., 2010). On the other extreme, individuals with an uninhibited temperament are typically outgoing, talkative and adventurous. Although the two temperament groups display behavioral differences in habituation to social stimuli, differences in habituation of brain responses have yet to be explored.
While neural differences in habituation may occur across the whole brain, the medial temporal lobe is a primary candidate for habituation to novelty. Within the medial temporal lobe, both the amygdala and hippocampus respond to novel stimuli (Fried et al., 1997; Schwartz et al., 2003b; Wright et al., 2003; Rutishauser et al., 2006; Blackford et al., 2010) and habituate with repeated exposure (Breiter et al., 1996; Wright et al., 2001; Fischer et al., 2003; Phan et al., 2003; Yamaguchi et al., 2004; Gonsalves et al., 2005; Blackford et al., 2010). Differences in amygdala and hippocampus function have been demonstrated in individuals with an inhibited temperament relative to those with an uninhibited temperament using functional magnetic resonance imaging (fMRI). For example, individuals with an inhibited temperament show multiple differences in amygdala function compared to those with an uninhibited temperament including: faster amygdala responses to novel faces (Blackford et al., 2011); sustained amygdala responses to recently familiarized faces (Blackford et al., 2010a); and greater amygdala responses to novel faces (Schwartz et al., 2003a; Beaton et al., 2008), expected fear faces (Clauss et al., 2011) and attended fear faces (Perez-Edgar et al., 2007). Although the hippocampus has received less attention in inhibited temperament research, recent findings in non-human primates demonstrate that increased hippocampus activation is a key neural signature of anxious temperament (Oler et al., 2010), a construct similar to inhibited temperament in humans. Based on previous demonstrations of habituation in the amygdala and hippocampus, as well as evidence of temperament differences in the function of these two brain regions, we propose that reduced amygdala and hippocampus habituation mediates the increased avoidance of novelty characteristic of individuals with an inhibited temperament.
In the present study, we used fMRI to examine habituation of the amygdala and hippocampus to repeated presentations of human faces in 39 young adults with either an extreme inhibited or extreme uninhibited temperament. We hypothesized that individuals with an inhibited temperament would have reduced amygdala and hippocampus habituation to faces, compared to individuals with an uninhibited temperament.
METHODS
Participants
Thirty-nine adults, with either an inhibited temperament (n = 19) or an uninhibited temperament (n = 20), participated in the study. Participants were on average 23 years of age (s.d. = 2.88), Caucasian (82%) and right-handed (87%). The two temperament groups were similar on gender, age, ethnicity and handedness (Table 1).
Table 1.
Participant characteristics by temperament group
| Inhibited temperament | Uninhibited temperament | ||
|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | P-value | |
| Temperament | |||
| Retrospective (RSRI) | 3.13 (0.43) | 1.48 (0.18) | <0.0001 |
| Adult (ASRI) | 3.13 (0.37) | 1.66 (0.19) | <0.0001 |
| Demographics | |||
| Age | 23.58 (3.27) | 22.30 (2.36) | ns |
| n (%) | n (%) | P-value | |
| Gender (Females) | 11 (58) | 12 (60) | ns |
| Handedness (Right) | 17 (89) | 17 (85) | ns |
| Ethnicity | |||
| Caucasian | 15 (79) | 16 (84) | ns |
| African-American | 3 (16) | 2 (11) | |
| Asian | 1 (5) | 0 (0) | |
| Other | 0 (0) | 2 (5) |
ns: not significant.
Participants were selected from a larger study of inhibited temperament. For the present study, we selected participants who were between 18 and 30 years of age, had extreme childhood and adult temperament scores, passed an MRI safety screen and were free of: psychoactive medications; substance abuse during the past 6 months; major medical illness and history of brain trauma. Temperament was assessed using two self-report instruments: the Retrospective Self-Report of Inhibition (RSRI; child) and the Adult Self-Report of Inhibition (ASRI; adult). The RSRI consists of 30-items about childhood behaviors on a 1–5 likert scale (1 = uninhibited, 5 = inhibited), such as: ‘Did you enjoy meeting new children your age?’ and ‘Were you scared of the dark?’ The ASRI assesses adult temperament using 31 items (1–5 likert scale) such as: ‘Do you feel comfortable speaking in front of a large group of people?’ and ‘Do open-air high places bother you?’ Both measures have demonstrated reliability and validity in a non-selected sample (Reznick et al., 1992) and excellent reliability in this sample (RSRI α = 0.96; ASRI α = 0.96). We used normative guidelines (Reznick et al., 1992) to define cut-offs for extreme (top and bottom 15%) temperament scores on both of the temperament questionnaires. As expected, the two temperament groups differed significantly on both the retrospective (RSRI) and adult (ASRI) measures of temperament (Table 1).
Participants were not excluded for psychiatric illness because inhibited temperament is strongly associated with increased rates of anxiety. Psychiatric status was assessed by a trained clinical interviewer using the Structured Clinical Interview for DSM-IV (Spitzer et al., 1992). Consistent with other studies (Schwartz et al., 1999; Blackford et al., 2009), inhibited participants had higher rates of current internalizing disorders compared to the uninhibited group. Six inhibited participants met criteria for at least one anxiety or depressive disorder. Across these six participants, the diagnoses included: Social Anxiety Disorder (n = 4); Generalized Anxiety Disorder (n = 3); Specific Phobia (n = 1); Anxiety Not Otherwise Specified (NOS; n = 2); and Dysthymia (n = 2). Of the uninhibited participants, one met criteria for Post Traumatic Stress Disorder and one had comorbid Depression NOS and Anxiety NOS. Data from a portion of these participants in a subsequent task have been previously published (Blackford et al., 2011).
The Vanderbilt University Institutional Review Board approved the study and we obtained written informed consent after providing participants with a complete description of the study.
fMRI task
Task
While in the scanner, participants were told ‘In this study a face will appear in the middle of the screen. Your job is to stay focused on the screen and look at each face. The faces will flash quickly’. Participants passively viewed six neutral faces, presented eight times each across four separate blocks, such that faces were novel in the first presentation and became familiarized with subsequent presentations. We chose a passive viewing task based on evidence that even basic tasks increase cognitive demands and reduce emotional reactions (Taylor et al., 2003; Costafreda et al., 2008; Pessoa 2008; Lieberman et al., 2011).
The fMRI task consisted of a single run, consisting of four 18-s image blocks interleaved with six 10-s fixation blocks (132-s total). Within each image block there were 12 1-s face presentations (each of the six faces presented twice), separated by a 0.5 s blank screen. Face order was randomized both within and between blocks. We used a block design to increase our power to detect activation differences (Birn et al., 2002) . We chose a short session length to capture the initial, rapid amygdala habituation that has been previously demonstrated with repeated exposures to faces (Breiter et al., 1996; Wright et al., 2001; Fischer et al., 2003) and to isolate habituation from longer term processes that may involve other forms of learning or memory.
Stimuli
Stimuli were black and white images of human faces with neutral expressions, selected from two standard sets of emotional expressions (Lundqvist et al., 1998; Gur et al., 2001). All stimuli were edited to ensure uniform face size, eye position and nose position, and all extraneous features (e.g. shirt collars, hair) were removed. Eprime software (Version 1.1, Psychology Software Tools, Pittsburgh, PA, USA) was used to present the stimuli.
MRI data
Anatomical and echo planar imaging (EPI) images were collected on a 3 Tesla Philips magnet (Philips Healthcare, Inc., Best, The Netherlands). High-resolution T1-weighted anatomical images were collected (256 mm FOV, 170 slices, 1 mm slice thickness, 0 mm gap). EPI images were acquired using a sequence optimized for the amygdala: 2 s TR, 22 ms TE; 90° flip angle; 1.8 SENSE, 240 mm FOV; 3 × 3 mm in plane resolution using an 80 × 80 matrix (reconstructed to 128 × 128), and higher order shimming to limit susceptibility artifacts. Each volume contained 36 2.5 mm (0.25 gap) axial oblique slices (tilted 15° anterior higher than posterior relative to the intercommissural plane), which provided complete anterior–posterior coverage and inferior–superior coverage from the bottom of the temporal lobe to the top of the cingulate gyrus.
MRI data were pre-processed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) and Matlab (Version 7.1, The MathWorks, Inc, Natick, MA, USA). Data were slice time corrected, realigned to the first slice, resampled to 3 × 3 × 3 mm voxels, spatially normalized into standard stereotactic space (MNI EPI template), and high pass filtered (128 s). Data were smoothed with a 6 mm FWHM Gaussian kernel to account for individual differences in brain anatomy. EPI images were visually inspected for artifacts, signal dropout and coverage of the amygdala region of interest. All participants had motion within an acceptable range (<3 mm or 3°).
The MRI data were modeled using SPM5. The first level (participant) model was estimated using a general linear model (Friston et al., 1994), with each block as a regressor. For each participant, habituation was defined as the first block > last block. We chose to compare the first (baseline) block with the last block based on evidence that habituation may be non-linear (Britton et al., 2008). Also, comparison of the first and last blocks maximized power to detect habituation differences across the short session length. To test for temperament differences in habituation, a second-level (group) t-test was performed between the two temperament groups (inhibited/uninhibited) on the habituation contrast.
Data analysis
First, to validate our task, we tested for a significant effect of habituation across all participants using SPM. Next, to test for temperament group differences in habituation we performed between-groups t-tests within each of our regions of interest. The bilateral amygdala and hippocampus regions of interest (ROI) were defined using the AAL templates (Tzourio-Mazoyer et al., 2002) in WFU Pick Atlas (Version 2.4; Maldjian et al., 2003). Type I error was controlled using cluster-based thresholding, a method that enhances sensitivity while controlling for error by setting spatial extent or cluster size requirements (Friston et al., 1993; Poline and Mazoyer, 1993). AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) was used to perform simulations based on region dimensions and smoothing to estimate cluster-based thresholds for a specific uncorrected voxel-level P-value. Using a voxel P-value of 0.05, the following cluster sizes provide for a corrected family wise error rate of α = 0.05 in each region: 11 voxels (297 mm3, left amygdala); 11 voxels (297 mm3, right amygdala); 21 (567 mm3, left hippocampus); and 19 (513 mm3, right hippocampus).
Post hoc analyses were performed for all significant SPM analyses in order to interpret the interactions. Specifically, percent signal change in BOLD signal (vs baseline) were extracted from each significant cluster using MarsBar (Brett et al., 2002). Paired t-tests were performed within each temperament group to test for habituation. In addition, independent t-tests were performed to test for temperament group differences at the first (baseline) block and at the last (habituation) block. Analyses were performed using SAS statistical software (Version 9.1, SAS Institute Inc., Cary, NC, USA).
To directly test for laterality effects (c.f. Davidson and Irwin, 1999), we used the MarsBar transform function (flip L/R) to create a mirror image of each significant cluster in the opposite hemisphere. Percent signal change was extracted from both the left and right hemisphere clusters. Repeated measures analysis of variance were used to explicitly test for a Temperament Group × Hemisphere interaction on habituation using SAS (α = 0.05).
To identify temperament group effects on habituation in other brain regions, we performed exploratory analyses across the brain. As with the ROI analyses, voxel-wise t-tests were performed using SPM. For the whole brain, a voxel P-value of 0.005 and a cluster size of 25 provided a corrected family-wise error rate of α = 0.05.
RESULTS
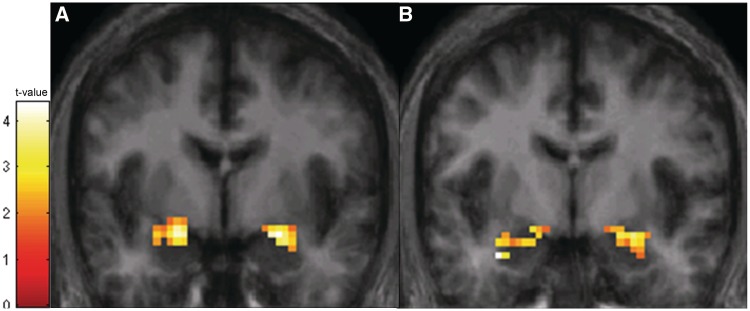
To demonstrate that our task measured habituation in both the amygdala and hippocampus, we first tested for a change in BOLD signal between the first and last block across all participants. The BOLD signal decreased significantly across the two blocks in the bilateral amygdala and bilateral hippocampus (Figure 1), demonstrating habituation in both brain regions.
Fig. 1.
BOLD response habituates over time in both the amygdala and hippocampus. Across all participants, BOLD response decreased over time in the bilateral amygdala and bilateral hippocampus. (A) In the amygdala, significant habituation was observed in clusters in both the left amygdala (cluster size = 42 voxels; peak voxel: x = −21, y = −6, z = −18; z-score = 4.01, P < 0.001) and right amygdala (cluster size = 56 voxels; peak voxel: x = 24, y = −3, z = −18; z-score = 3.89, P < 0.001). The activation map is superimposed on a coronal slice (y = 0) of an averaged brain thresholded at P < 0.05 and 11 voxels. (B) In the hippocampus, significant habituation was observed in a cluster in the left hippocampus (cluster size = 128 voxels; peak voxel: x = −33, y = −6, z = −27; z-score = 3.93, P < 0.001) and the right hippocampus (cluster size = 55 voxels; peak voxel: x = 27, y = −6, z = −18; z-score = 3.27, P = 0.001). The activation map is superimposed on a coronal slice (y = −3) of an average participant brain (averaged across study participants) thresholded at P < 0.05 and 19 voxels.
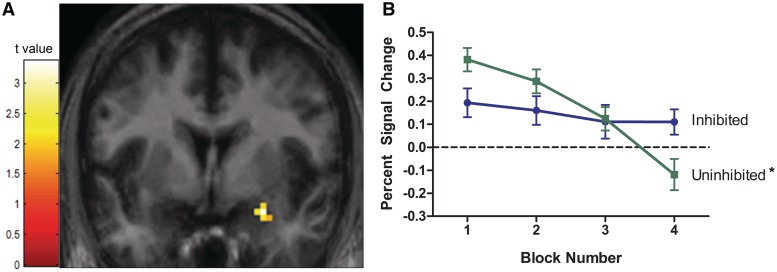
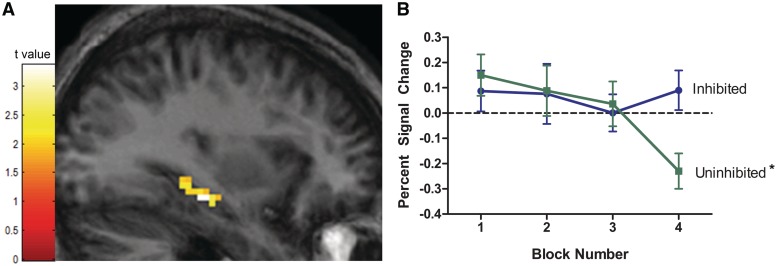
To test for temperament differences in habituation, we compared BOLD signal reduction between the inhibited and uninhibited temperament groups in each of the regions of interest. Habituation differed by temperament group in both the right amygdala (Figure 2) and the left hippocampus (Figure 3).
Fig. 2.
Amygdala BOLD response fails to habituate in individuals with an inhibited temperament. (A) The two temperament groups had different rates of habituation in a cluster of 15 voxels in the right amygdala (peak voxel: x = 27, y = −3, z = −21; z-score = 3.12, P = 0.001). (B) In the uninhibited group, amygdala BOLD response habituated as expected (*P < 0.05). However, in the inhibited group, amygdala BOLD response failed to habituate. The activation maps are superimposed on coronal slices of an average participant brain thresholded at P < 0.05 and 11 voxels. Line graphs show average percent signal change values (relative to baseline) for each block of faces by temperament group. Vertical lines represent standard error or the mean.
Fig. 3.
Hippocampus BOLD response fails to habituate in individuals with an inhibited temperament. (A) Degree of habituation differed by temperament group in the left hippocampus (cluster size = 57 voxels; peak voxel: x = −33, y = −24, z = −15; z-score = 3.19, P = 0.001). (B) In the uninhibited group, hippocampus BOLD response habituated (*P < 0.05). In contrast, in the inhibited group, hippocampus BOLD response failed to habituate. The activation maps are superimposed on coronal slices of an average participant brain (averaged across study participants), thresholded at P < 0.05 and 19 voxels. Line graphs show average percent signal change values (relative to baseline) for each block of faces by temperament group. Vertical lines represent standard error of the mean.
To understand the nature of the interactions, several post hoc tests were performed on the percent signal change values extracted from each significant cluster. Consistent with the SPM analyses, habituation differed by temperament group, F(1, 37) = 10.98, P = 0.002. Specifically, in the uninhibited individuals, the right amygdala habituated over time, as expected, F(1, 19) = 32.48, P < 0.0001. However, in individuals with an inhibited temperament, the right amygdala did not habituate, F(1,18) = 0.85, P = 0.37. When comparing the two temperament groups at each time point, amygdala BOLD signal was significantly higher in the inhibited group relative to the uninhibited group in the last block, F(1, 37) = 5.55, P = 0.03, but was not significantly different in the first block (P = 0.08). There were no significant temperament group differences in habituation in the left amygdala.
A similar pattern of temperament differences in habituation was seen in the left hippocampus (Figure 3). Again, post hoc analyses were performed to aid in interpreting the interaction. In the left hippocampus cluster, habituation differed by temperament group, F(1, 37) = 23.04, P < 0.0001. Hippocampus BOLD response habituated in individuals with an uninhibited temperament, F(1, 19) = 14.70, P = 0.002, but did not habituate in individuals with an inhibited temperament, F(1, 18) = 0.00, P = 0.96. When comparing the two temperament groups at each time point, the inhibited group had significantly higher hippocampus BOLD signal relative to the uninhibited group in the last block, F(1, 37) = 10.92, P = 0.003, but was not significantly different in the first block (P = 0.53). There were no significant temperament group differences in habituation in right hippocampus.
Temperament differences in habituation were restricted to the right amygdala and left hippocampus; however, lack of significant results in one hemisphere does not provide evidence for lateralization (Davidson and Irwin, 1999). Therefore, we explicitly tested for lateralization by comparing percent signal change between the two hemispheres. In the amygdala, habituation did not differ by hemisphere or the interaction of hemisphere × temperament group, suggesting no lateralization effect. Confirming the SPM analysis, habituation was greater in the uninhibited group across both amygdalae [main effect of group, F(1, 37) = 5.55, P = 0.03]. In the hippocampus, there was a significant interaction of hemisphere and temperament group. In the uninhibited group, percent signal change was significantly greater in the left hippocampus compared to the right hippocampus [F(1, 19) = 5.74, P = 0.01]; however, there was no evidence of habituation in either hemisphere for the inhibited group. As with the amygdala analysis, habituation across both hemispheres was greater in the uninhibited group, F(1, 37) = 4.32, P = 0.05.
Given that several of the study participants met criteria for a psychiatric diagnosis, it is important to determine the degree to which the current findings reflect the temperament trait vs the presence of a psychiatric disorder. Therefore, we tested for habituation differences in the subsample of participants without a psychiatric diagnosis. Both the amygdala and hippocampus habituation differences remained significant in the healthy subsample (although cluster sizes were slightly smaller), suggesting that the findings reflect differences in the temperament trait.
To identify other brain regions where habituation differed by temperament group, we compared habituation between the inhibited and uninhibited groups across the whole brain (P < 0.005, corrected). Group differences were not observed in any additional brain regions.
DISCUSSION
The present study demonstrates temperament group differences in amygdala and hippocampus habituation to faces. In individuals with an uninhibited temperament, both the amygdala and hippocampus habituated to repeatedly presented faces, consistent with other studies in healthy controls (Breiter et al., 1996; Wright et al., 2001; Fischer et al., 2003; Phan et al., 2003; Yamaguchi et al., 2004). In contrast, those with an inhibited temperament demonstrated a failure to habituate normally to the faces, evidenced by stable amygdala and hippocampus responses, even following repeated presentations. This failure to habituate provides a novel neural mechanism for understanding the shy and cautious behavior characteristic of inhibited individuals. We propose that the failure to habituate normally reflects a social learning deficit that underlies both the characteristic behavioral avoidance of novelty and the increased risk for social anxiety disorder in those with an inhibited temperament.
Previous neuroimaging studies have reported either increased (Schwartz et al., 2003a) or sustained (Blackford et al., 2011) amygdala BOLD responses to faces in individuals with an inhibited temperament. Based on the findings from this study, we propose that a habituation failure underlies both the increased and sustained amygdala responses previously reported. In studies that average BOLD signal over time, a larger BOLD signal does not indicate that the BOLD response is larger at any given time point. We propose that individuals with an inhibited temperament do not have an increased initial amygdala response to novel stimuli, but instead have a sustained amygdala response caused by reduced habituation. Future studies should use single event approaches with multiple stimuli to test for temperament-based differences in the initial amygdala response to a novel stimuli as well as the time course of habituation.
Individuals with an inhibited temperament also showed a failure to habituate in the hippocampus, a brain structure involved with multiple memory processes including both the detection and subsequent recollection of novel events (e.g. Ranganath and Rainer, 2003). In the uninhibited group, the left hippocampus demonstrated short-term habituation, similar to previous studies in healthy controls (Strange et al., 1999; Brozinsky et al., 2005; Blackford et al., 2010). Habituation of the hippocampus response in the uninhibited group likely reflects the decreased salience of the novel faces as they become familiar. The failure to habituate in the inhibited group suggests that the faces remained salient, even after multiple exposures. Taken together, the habituation failures in the amygdala and hippocampus suggest slower information processing or learning processes such that the previously seen faces are still considered to be relatively novel and salient, resulting in continued BOLD response in the amygdala and hippocampus.
Habituation failure in the amygdala and/or hippocampus may also be associated more broadly with social dysfunction. Reduced amygdala and/or hippocampus habituation has been reported in other groups with characteristics of social dysfunction including autism spectrum disorders (Kleinhans et al., 2009) and schizophrenia (Holt et al., 2005). Findings of reduced habituation in other disorders might suggest that a habituation failure in the amygdala or hippocampus underlies a dimension of social dysfunction and may not be specific to inhibited temperament.
This study’s focus on short-term habituation isolated habituation from longer term processes that may involve other forms of learning or memory; however, this method also had limitations. Since we examined short-term habituation, we were not able to estimate the rate of habituation in the inhibited group; therefore, it remains unknown whether habituation in inhibited individuals is slowed, delayed or completely fails. Future studies should measure habituation over a longer period of time to assess rate of habituation, and should also explore other aspects of habituation, such as dishabituation (recovery of the habituated response) and long-term habituation (persistence of habituation over hours, days or weeks, see Rankin et al., 2009). Also, changes in BOLD signal were estimated from change in two single blocks, which may be less reliable or statistically powerful than traditional fMRI designs which average BOLD signal over many trials. However, while averaging over trials enhances reliability, averaging can also obscure important temporal data, like habituation or sensitization. Reliable estimates of BOLD signal can be estimated from single trials using high field scanners (Ugurbil et al., 1999), and rapid habituation of the hippocampus has been demonstrated using even a single trial approach (Yamaguchi et al., 2004). Another limitation of this study is that attention to the stimuli cannot be directly confirmed because our task did not require participants to perform a task. However, attentional concerns were minimized by the use of a block design and short session length; also activation in visual areas was confirmed for each participant, providing indirect evidence for visual attention during the task. Finally, in this study we only examined habituation to faces, therefore it remains unknown whether the failure to habituate is specific to social stimuli or reflects a general dysfunction in the amygdala and hippocampus that would also be evident with non-social stimuli.
In summary, the results from this study suggest a novel mechanism for inhibited temperament. We propose that a habituation failure in two medial temporal lobe structures, the amygdala and the hippocampus, reflects a social learning deficit that contributes to the behavioral avoidance of novelty characteristic of individuals with an inhibited temperament. Furthermore, this social learning deficit may be a path through which inhibited temperament increases risk for social anxiety disorder.
Conflict of Interest
None declared.
Acknowledgments
We thank Jerome Kagan and Margaret Benningfield for helpful comments on the article and Kristi Simmons for research assistance. Portions of this work were presented at the Cognitive Neuroscience Society meeting, San Francisco, April 2011 and the Society for Biological Psychiatry, San Francisco, May 2011. This work was supported in part by funding from the National Institutes of Health [MH083052 to J.U.B., DA015137 to R.L.C., DA020149 to R.L.C., MH073800 to R.L.C., RR024975 to the Vanderbilt Institute for Clinical and Translational Research]; the American Academy of Child and Adolescent Psychiatry [Summer Medical Student Fellowship to A.H.A.]; and the Vanderbilt University Institute of Imaging Science.
REFERENCES
- Beaton EA, Schmidt LA, Schulkin J, Antony MM, Swinson RP, Hall GB. Different neural responses to stranger and personally familiar faces in shy and bold adults. Behavioral Neuroscience. 2008;122:704–9. doi: 10.1037/0735-7044.122.3.704. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: Choosing the optimal stimulus timing. Neuroimage. 2002;15:252–64. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience. 2011;6(5):621–29. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the amygdala in novelty detection. Neuroimage. 2010;50:1188–93. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] Neuroimage. 2002;16:s497. [Google Scholar]
- Britton J, Shin L, Barrett L, Rauch S, Wright C. Amygdala and fusiform gyrus temporal dynamics: Responses to negative facial expressions. BMC Neuroscience. 2008;9:44. [Google Scholar]
- Brozinsky CJ, Yonelinas AP, Kroll NEA, Ranganath C. Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus. 2005;15:557–61. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- Bushnell IWR. Discrimination of faces by young infants. Journal of Experimental Child Psychology. 1982;33:298–308. doi: 10.1016/0022-0965(82)90022-4. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–35. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Cowan RL, Blackford JU. Expectancy and temperament modulate amygdala and dorsal anterior cingulate responses to fear faces. Cognitive Affective & Behavioral Neuroscience. 2011;11:13–21. doi: 10.3758/s13415-010-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Science. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. American Journal of Psychiatry. 2010;167:40–6. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Research Bulletin. 2003;59:387–92. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1994;2:189–210. [Google Scholar]
- Friston K, Worsley K, Frackowiak R, Mazziotta J, Evans A. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1993;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–61. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, et al. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–76. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biological Psychiatry. 2005;57:1011–19. doi: 10.1016/j.biopsych.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–73. [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of Psychiatry. 2009;166:467–75. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Lieberman M, Inagaki T, Tabibnia G, Crockett M. Subjective responses to emotional stimuli during labeling, reappraisal and distraction. Emotion. 2011;11:468–80. doi: 10.1037/a0023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. Department of Clinical Neuroscience, Psychology section, Karolinska Institute; 1998. The Karolinska Directed Emotional Faces - KDEF. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–8. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28:1344–50. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- Poline JB, Mazoyer BM. Analysis of individual positron emission tomography activation maps by detection of high signal-to-noise-ratio pixel clusters. Journal of Cerebral Blood Flow and Metabolism. 1993;13:425–37. doi: 10.1038/jcbfm.1993.57. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nature Reviews Neuroscience. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92:135–8. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Development and Psychopathology. 1992;4:301–21. [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–13. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–15. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003a;300:1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, et al. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003b;53:854–62. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RNA, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4034–9. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Hu XP, Chen W, Zhu XH, Kim SG, Georgopoulos A. Functional mapping in the human brain using high magnetic fields. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1999;354:1195–213. doi: 10.1098/rstb.1999.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney S, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18:660–9. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. Journal of Neuroscience. 2004;24:5356–63. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]