Abstract
Post-traumatic stress disorder (PTSD) is associated with impaired memory performance coupled with functional changes in brain areas involved in declarative memory and emotion regulation. It is not yet clear how symptom severity and comorbidity affect neurocognitive functioning in PTSD. We performed a functional magnetic resonance imaging (fMRI) study with an emotional declarative memory task in 28 Complex PTSD patients with comorbid depressive and personality disorders, and 21 healthy non-trauma-exposed controls. In Complex PTSD patients—compared to controls—encoding of later remembered negative words vs baseline was associated with increased blood oxygenation level dependent (BOLD) response in the left ventral anterior cingulate cortex (ACC) and dorsal ACC extending to the dorsomedial prefrontal cortex (dmPFC) together with a trend for increased left hippocampus activation. Patients tended to commit more False Alarms to negative words compared to controls, which was associated with enhanced left ventrolateral prefrontal and orbitofrontal cortex (vlPFC/OFC) responses. Severity of child abuse was positively correlated with left ventral ACC activity and severity of depression with (para) hippocampal and ventral ACC activity. Presented results demonstrate functional abnormalities in Complex PTSD in the frontolimbic brain circuit also implicated in fear conditioning models, but generally in the opposite direction, which may be explained by severity of the trauma and severity of comorbid depression in Complex PTSD.
Keywords: anterior cingulate cortex, hippocampus, memory, complex post-traumatic stress disorder, childhood abuse
INTRODUCTION
Intrusive memories (flashbacks, nightmares) and inability to recall aspects of the traumatic events (psychogenic amnesia) are among the key features of post-traumatic stress disorder (PTSD), impairing personal and social functioning (APA, 2000). In neuropsychological studies, performance on declarative memory tests with neutral stimuli has been found to be consistently impaired in PTSD (Brewin et al., 2007; Johnsen and Asbjornsen, 2008). In addition, there is evidence for attention bias toward trauma-relevant information in PTSD (Moradi et al., 2000; McNally, 2006; Moore, 2009), which may be related to intrusions. Declarative memory performance and emotion processing in PTSD are associated with functional changes in brain areas (Elzinga and Bremner, 2002), that are all part of a network with extensive reciprocal connections (Liberzon and Sripada, 2008). According to fear conditioning models, PTSD is characterized by an exaggerated response of the amygdala to threat-related stimuli—associated with increased appraisal of danger and fear responses, whereas activity is decreased in the medial prefrontal (PFC), orbitofrontal (OFC) and anterior cingulate cortex (ACC)—associated with fear extinction and diverting attention from trauma-related stimuli, as well as decreased activity in the hippocampus, which is associated with declarative memory and contextualization of fear responses (Rauch et al., 2006).
PTSD is a heterogeneous disorder with varying trauma types, symptom severity and comorbid disorders. Childhood physical abuse and rape in women are far more likely to result in PTSD than other types of trauma (Kessler et al., 1995) and are associated with psychiatric symptoms that extend beyond DSM-IV classic PTSD, such as problems in affect regulation (e.g. alteration between rage and affective emptiness, and impulsivity), dissociative symptoms (inability to remember certain periods of life, losing track of time, ‘spacing out’, feeling unreal), problems in self-perception (feelings of guilt and shame) and relationships (inability to trust), and somatization, a syndrome therefore labeled as Complex PTSD (Herman, 1992; Zlotnick et al., 1996; Pelcovitz et al., 1997; Ford, 1999). Complex PTSD is associated with high co-morbidity on DSM-IV axes I and II, especially depressive disorders on axis I and borderline personality disorder (BPD) on axis II (Ford and Kidd, 1998), resulting in population heterogeneity. It is not clear to which extent trauma and symptom severity, and comorbidity, may affect neurocognitive functioning.
In symptom provocation studies in PTSD, symptom severity was correlated positively with amygdala activity and/or negatively with hippocampus and/or medial PFC activity (Shin et al., 2004a; Armony et al., 2005; Shin et al., 2005). During encoding of forgotten faces a negative correlation has been found between symptom severity and ventromedial PFC activity (Dickie et al., 2008), but not during retrieval (Bremner et al., 2003a; Shin et al., 2004b). Two symptom provocation studies among PTSD patients found that depression comorbidity may account for more bilateral ACC activity (BA 24) and relatively less left insula (Lanius et al., 2007), amygdala and medial prefrontal activity compared to PTSD-only patients (Kemp et al., 2007). Comorbid dissociative symptoms were associated with increased activity during script-driven imagery in left ACC (BA 32) and bilateral insula and decreased activity in right parahippocampal gyrus in patients with BPD and comorbid PTSD (Ludascher et al., 2010).
Summarizing, impaired memory and associated functional brain changes have been found in PTSD with various types of trauma, but imaging studies investigating memory function in PTSD have been comparatively sparse. Also, it is unclear how symptom severity of PTSD and of comorbid disorders affect neurocognitive functioning. The aim of the present study was to investigate (emotional) memory function and regional blood oxygenation level-dependent (BOLD) signal changes in a population of patients with Complex PTSD with a history of child sexual and/or physical abuse and with high rates of high comorbid depressive and personality disorders and dissociative symptoms, extending a previous pilot study (Thomaes et al., 2009). We performed whole-brain event-related functional magnetic resonance imaging (fMRI) during a verbal declarative memory task with neutral and negative words. We compared performance (reaction times and error rates) and BOLD signal changes between subjects with Complex PTSD and normal controls. In addition, we explored whether any abnormalities were correlated with PTSD, trauma severity and comorbid psychopathology, such as depressive, dissociative and borderline personality symptoms. Based on the fear conditioning model, we formulated the following hypotheses: (i) Complex PTSD patients perform worse than controls during a verbal declarative memory task, in particular for neutral relative to negative words and (ii) This will be reflected by an increased BOLD response—especially during encoding—to negative words contrasted with baseline in the amygdala, and a decreased BOLD response in the medial PFC, OFC and ACC, and in the hippocampus compared to controls.
METHODS
Participants
Thirty-three female outpatients of Dutch mental health institutes from four sites (Amsterdam, Alkmaar, Castricum and Utrecht) with both ‘classic’ PTSD (i.e. according to DSM-IV-criteria) and Complex PTSD after childhood sexual and/or physical abuse and 30 healthy non-trauma-exposed controls, matched for age, gender and handedness, participated in the study. One patient panicked during scanning, and 2 patients and 5 controls had too many omissions, resulting in behavioral data from 30 patients and 25 controls. In addition, the scans of 2 patients and 4 controls had to be discarded due to technical problems, leaving 28 patients and 21 controls for MRI data analysis. Task performance of this subgroup was similar to those of the larger group.
Patients were interviewed by trained mental health workers with the Structured Trauma Interview (STI) (Draijer, 1989). Child sexual abuse was defined as repeated, forced sexual contact with a perpetrator in an intimate relationship before the age of 16 years; subjects who reported moderate (touching and groping) to highly severe (penetration) abuse were eligible for the study. Child physical abuse was defined as repeated maltreatment, which could have harmed the child, from moderate (i.e. sometimes injured) to severe (frequently injured, confinement, battering). Severity of child abuse was further classified as: (i) physical only, (ii) sexual only and (iii) both physical and sexual abuse. Presence and severity of ‘classic’ PTSD was assessed with the Clinician Administered PTSD Scale (CAPS) (Hovens et al., 1994; Blake et al., 1995) and ‘Complex’ PTSD with the Structured Clinical Interview for Disorders of Extreme Stress NOS (SIDES) (Van der Kolk et al., 1992). Comorbid disorders were assessed with the Structured Clinical Interview for DSM-IV axis I disorders (SCID-IV-I) (First et al., 1995) and axis II disorders (SIDP-IV) (Pfohl and Zimmerman, 1997). Symptom severity was measured with the Beck Depression Inventory (BDI) (Beck et al., 1998), Dissociative Experiences Scale (DES) (Bernstein and Putnam, 1986) and Borderline Personality Disorder Severity Index (BPDSI) (Arntz et al., 2003). Controls were recruited via advertisements in local newspapers, interviewed with the STI, CAPS, SIDES, SCID-I-IV and completed the BDI and DES. The Medical Ethical Committee of the VU University Medical Center, Amsterdam, approved the study. Written informed consent was obtained from each participant.
Because Complex PTSD patients participated in a randomized controlled trial patients with antisocial personality disorder, recurrent psychoses or dissociative identity disorder (DID; based on the Structured Clinical Interview for Dissociative Disorders, SCID-D) (Steinberg et al., 1990) were excluded from the study. Other exclusion criteria were current alcohol or drug dependence or abuse (meeting DSM-IV criteria, i.e. during the last month); the use of psychotropic medication other than selective serotonin reuptake inhibitors (SSRI) in stable dosage for at least a month or low dosage benzodiazepines (max. 20 mg oxazepam-equivalent); major neurological and/or internal disorders; retained metal (e.g. pacemaker or surgical clips), and pregnancy. Because a high proportion of patients used SSRIs (64%) or low-dose benzodiazepines (48%), correlations of medication use with memory performance were investigated.
Stimuli and activation paradigm
We adapted an existing verbal declarative memory task with neutral and negative words (de Ruiter et al., 2007). Series of 100 neutral and 100 negative words were selected to be comparable in length, frequency in Dutch language, proportion of verbs, nouns and adjectives and semantic cohesion. During encoding, stimuli were presented in an event-related design, consisting of a randomized selection of 50 neutral (e.g. river, table), 50 negative words (e.g. panic, rape) and 50 baseline stimuli (‘push left’ or ‘push right button’), in semi-random order (i.e. randomization per block). Negative words were related to negative emotions (50%), aggression and sex (35%) and illness and death (15%). A semantic classification instruction was used addressing the personal meaning of the word. Subjects were asked to respond as fast as possible by pressing a button. Presentation time depended on the reaction time of the patient with a maximum of 5 s. In the recognition phase, all 200 words (50 OLD neutral and 50 OLD negative words out of the encoding series; 50 new neutral and 50 new negative words) and 50 baseline stimuli (‘push left’, ‘– middle’ or ‘–right button’) were presented in semi-random order. During recognition, subjects were asked to rate each word as (i) certainly seen during encoding, (ii) probably seen or (iii) novel. Only ‘certainly seen’ trials were analyzed, because these are more likely to activate the medial temporal lobe and more likely to reflect episodic retrieval, similar to remember/know judgments (Wixted, 2007). Moreover, there were too few ‘probably seen’ responses to analyze (hits neutral: 6.8, s.d. 5.7; hits fear: 6.4, s.d. 6.0; False Alarms neutral: 6.3, s.d. 5.9; and False Alarms fear: 7.3, s.d. 4.6). Net performance was computed as proportion hits minus proportion False Alarms (FAs). Low response bias, computed as [FAs/(1 − net performance)], indicated a cautious tendency. In contrast to studies in healthy adults with a subsequent memory paradigm (contrasting later remembered with missed items) and old–new recognition (contrasting hits with forgotten items) (Spaniol et al., 2009), we investigated successful and unsuccessful recognition by contrasting these separately to a non-mnemonic baseline condition, because it has recently been argued that subtracting unsuccessful trials from successful trials entails a risk of false-negative results, for example, because forgotten items may reflect unsuccessful retrieval activity but also successful encoding (Daselaar et al., 2003).
MRI acquisition and data pre-processing
MR imaging was performed on a 1.5-T Sonata MR system (Siemens, Erlangen, Germany) with a standard circularly polarized head coil at the VU University Medical Center. To reduce motion artifacts, the subject's head was immobilized using foam pads. Whole-brain T2*-weighted echo-planar images sensitive to BOLD-contrast (35 coronal slices, between-plane resolution 5 mm, in-plane resolution 3 × 3 mm; TR = 2920 ms, TE = 45 ms, slice order ascending) were acquired while subjects performed the encoding and the recognition task (i.e. two functional MR sessions). In between, a coronal 3D gradient-echo T1-weighted MR image (flip angle = 8°; TR = 2700 ms; TE = 4 ms; TI = 950 ms; BW = 190 Hz/pixel, matrix = 256 × 256, voxel size = 1 × 1 × 1.5 mm, 160 slices) was performed.
Stimuli were projected on a screen at the end of the scanner table, which was seen through a mirror mounted above the subject's head. One magnet-compatible four-key response button box recorded subject's accuracy and response times (RT). Each MRI-session lasted ∼45 min, which included the functional encoding (max. 4 min) and recognition run (max. 10 min) with in between a structural MRI (7.5 min), an emotional Stroop task (∼10 min) and a Go/No Go task with emotional faces (∼10 min), results of which will be reported elsewhere. At four time points in between tasks—inside the scanner—participants were asked to indicate their level of subjective fear on a scale of 0–100. Before and after the scan session—outside the scanner—the Clinician-Administered Dissociative States Scale (CADDS) (Bremner et al., 1998) was administered.
Image pre-processing was performed using SPM5 software (Wellcome Department of Imaging Neuroscience, www.fil.ion.ucl.ac.uk/spm, running in MATLAB 7.0). DICOM images were converted to Analyze format, followed by manual reorienting to the anterior commissure. Functional images were realigned, and the mean image was co-registered to the structural MR image. Spatial normalization into MNI (Montreal Neurological Institute) space was performed using the co-registered MR image. For spatial smoothing a 6 mm FWHM filter was used.
Statistical analysis
Demographic data were analyzed with Mann–Whitney tests in case of non-normal distribution and a χ2 test on ordinal data. Accuracy and reaction times were assessed by repeated measures analyses of variance (ANOVA) with factors Group and Valence, using a one-sided threshold of P < 0.05. In case of significant interactions post hoc tests were performed. Data from any respondent were discarded in case of >10% omissions.
Subjects' responses during performance of the recognition task allowed post hoc classification of events as correctly remembered words (hits) and FAs and correlated with functional MR data. Functional MR data were analyzed in the context of the general linear model, using delta functions convolved with a canonical hemodynamic response to model responses during each condition, with movement parameters modeled as regressors of no interest. The resulting contrast images containing parameter estimates for main effects entered into a second-level (random effects) analysis, using one-way ANOVAs for each contrast (encoding: later remembered negative words vs baseline, later remembered neutral words vs baseline; recognition: negative hits vs baseline, neutral hits vs baseline, negative FAs vs baseline, neutral FAs vs baseline).
For group differences in our a priori regions of interest, we adopted a threshold of P < 0.05 false discovery rate (FDR), corrected for multiple comparisons employing the small volume correction (SVC) option implemented in SPM5 with 3.5 ml for the hippocampus (each side), 10 ml for dorsal ACC (bilateral), 5 ml for the ventral/subcallosal ACC (bilateral) and 7.5 ml for the lateral OFC (each side), similar to previous research (Shad et al., 2006; Kasai et al., 2008). For other regional group differences, we set a threshold of whole-brain FDR corrected P < 0.05. To explore—within the patient group—whole-brain voxel-wise correlations of BOLD responses in our brain regions of interest with symptom severity (severity child abuse, CAPS, BDI, DES and BPDSI), we adopted a threshold of P SVC corrected <0.05.
RESULTS
Participants
The majority of patients (N = 33) had experienced both physical and sexual abuse (19 i.e. 58%), a third sexual abuse (11 i.e. 33%) and a minority physical abuse only (3 i.e. 9%). Patients had multiple comorbid disorders: 23 (70%) other anxiety disorders; 21 (64%) major depressive disorder; 3 (9%) eating disorders; 3 (9%) dysthymia and/or bipolar II disorder. The majority of patients had one or more DSM-IV axis II personality disorders: 11 (33%) BPD; 10 (33%) cluster C PD; 3 (9%) both BPD and cluster C PD. Patients’ symptom ratings were indicative for severe PTSD (CAPS: mean 88.5, s.d. 15.0; range 0–136; >40 indicative for PTSD), severe depressive (BDI: 29.3, s.d. 10.0; range 0–63; >30 indicative for severe depression), and moderate to severe dissociative symptoms [DES: 23.9, s.d. 14.5; range 0–100; 9 (27%) DES > 35, i.e. ‘highly dissociative’, indicative for dissociative disorder NOS], and borderline symptoms (BPDSI: 22.9, s.d. 7.9; range 0–90; >15 indicative for BPD). Controls (N = 30; 11.4, s.d. 2.0) had more years of education compared to patients (10.8, s.d. 2.5), but this difference, although statistically significant (M–W: z = −2.1, P = 0.03), was small. Controls had no current axis-I diagnoses and very low, non-significant symptom ratings.
Task performance
During encoding, patients rated negative words more often as negative compared to controls [patients 91%, controls 79%; Group × Valence F(1, 53) = 12.4, P = 0.001]. Patients reacted slower to neutral words than controls, but relatively fast to negative words [Valence × Group F(1, 53) = 8.0, P = 0.007; post hoc independent t-test on reaction time (RT) neutral words: controls 1029 ms vs patients 1197 ms, t = −2.1, df = 47.9, P = 0.04; RT negative words: controls 1100 ms vs patients 1128 ms, P > 0.05]. Post hoc, we found that medication using patients (N = 21) responded slower during encoding (neutral words: t-test, t = −3.4, df = 27.5, P = 0.002; negative: t-test, t = −3.1, df = 28, P = 0.005) compared to non-users (N = 9).
In Table 1, proportions of Hits, False Alarms, Net Performance and Response Bias of ‘Certainly seen’ responses are shown. Patients generated as many Hits as controls, both to neutral and negative words. There was a trend for patients to commit more FAs to negative words than controls, resulting in a worse Net Performance. Proportions Hits and FAs did not differ significantly between medicated and non-medicated patients.
Table 1.
Mean proportions (s.d.) of Hits, FAs, net performance and response bias for patients with Complex PTSD (N = 30) and non-trauma-exposed healthy controls (N = 25) for different word valences
| Group | Patients | Controls | ||
|---|---|---|---|---|
| Word valence | Neutral | Negative | Neutral | Negative |
| Hits | 0.59 (0.17) | 0.71 (0.19) | 0.58 (0.18) | 0.72 (0.16) |
| False alarms | 0.11 (0.09) | 0.32 (0.22)a | 0.09 (0.08) | 0.23 (0.16)a |
| Net performance | 0.48 (0.16) | 0.40 (0.16)b | 0.49 (0.14) | 0.49 (0.13)b |
| Response bias | 0.22 (0.17) | 0.51 (0.29) | 0.20 (0.18) | 0.46 (0.27) |
Hits = correctly recognized words; FAs = False Alarms, i.e. falsely ‘recognized’ new words; Net Performance = hits minus FAs;
Response Bias = FAs/(1 – net performance) = low bias indicates a cautious tendency i.e. the tendency to give ‘not seen’ responses.
aGroup F(1,53) = 2.1, P = 0.08 and Valence × Group F(1,53) = 2.1, P = 0.08; post hoc t-test, t = −1.6, df = 53, P = 0.07 (trend).
bGroup F(1,53) = 2.0, P = 0.08 and Valence × Group F(1,53) = 4.2, P = 0.02; post hoc t-test, t = 2.3, df = 53, P = 0.01.
State dissociation and subjective fear during imaging
State dissociation (CADDS) was higher in patients than controls, and was also higher after scanning—i.e. outside the scanner—than before the scan session, especially in patients [Group: F(1,59) = 20.1, P < 0.001; Time F(1,59) = 11.6, P = 0.001; Group × Time F(1,59) = 11.6, P = 0.001; post hoc paired t-test t = 3.3, df = 31, P = 0.002]. Absent-mindedness during the scan session—inside the scanner—decreased significantly over time in patients [Group: F(1,60) = 23.3, P < 0.001, Time: F(1,5) = 2.1, P = 0.07; Time × Group: F(1,5) = 3.2, P = 0.007]. Patients reported significantly more subjective fear during MR imaging than controls [Group: F(1,60) = 17.6, P < 0.001], which also decreased over time in both patients and controls [Time: F(1,5) = 9.8, P < 0.001; Time × Group P > 0.05]. Subjective fear was not linearly associated with FAs to negative words (Pearson r = −0.33, P = 0.07), however, plotting of these variables suggested a non-linear (quadratic) correlation, which was confirmed in a post hoc analysis [R2 = 0.23, F(2, 27) = 4.1, P = 0.03], indicating that both low and high scores of subjective fear were associated with a high False Alarm rate. State dissociation correlated positively with subjective fear (r = 0.37, P = 0.04) but not significantly with False Alarm rate (r = −0.12, P = 0.55).
BOLD response during encoding and recognition in Complex PTSD patients and controls
Across groups, we observed robust effects of encoding and recognition of negative and neutral words compared to baseline in the primary and secondary visual cortex, superior temporal cortex, ventral and lateral PFC and dorsal ACC. These effects were bilateral, but with a left-hemispheric preponderance (data not shown).
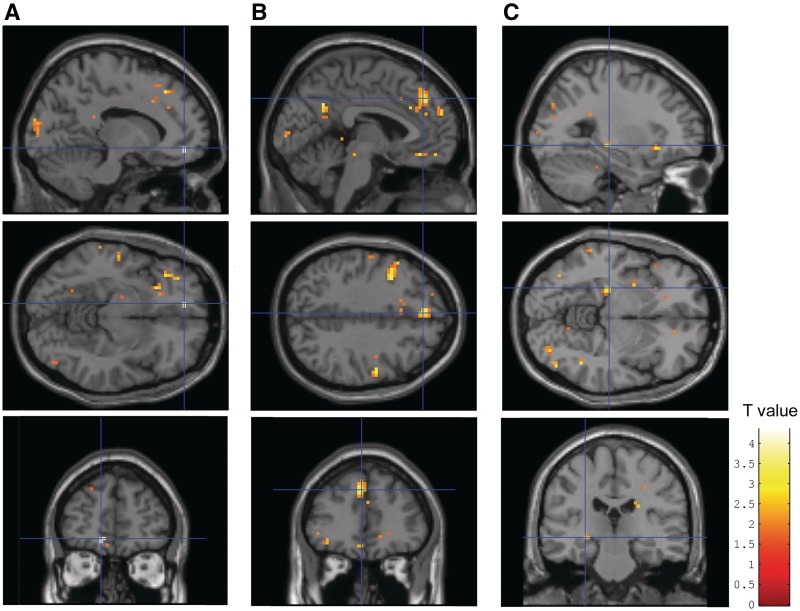
Between groups, patients showed an increased response in the left ventral/subcallosal ACC, the left dorsal ACC extending to the dorsomedial PFC (dACC/dmPFC) and the left posterior hippocampus (trend) during encoding of later remembered negative words vs baseline compared to controls (Table 2 and Figure 1). Post hoc analyses revealed no significant differences between medicated (N = 20) and non-medicated patients (N = 8) in left ventral/subcallosal ACC and hippocampal BOLD responses. The BOLD response in the left dACC/dmPFC tended to be higher in non-medicated compared to medicated patients (MNI: 0, 36, 33, z = 3.04, P uncorrected = 0.001). However, medicated patients still showed a significantly increased BOLD response in this region compared to controls (z = 4.20, P SVC corrected = 0.005), so that medication use apparently did not affect our main findings. During encoding of later remembered neutral words vs baseline, or negative vs neutral words, patients showed no differential activation compared to controls.
Table 2.
Brain regions (Brodmann area and MNI-coordinates for peak intensity) with stronger BOLD responses in Complex PTSD patients (N = 28) compared to healthy non-trauma-exposed controls (N = 21)
| Brain regions | Brodmann area | x, y, z (mm) | Z-value | P uncorrected | P SVC voxel FDR corrected* |
|---|---|---|---|---|---|
| Encoding phase: later remembered negative words vs baseline | |||||
| Left ventral/subcallosal ACC | 32/12 | −12, 48, −9 | 3.99 | 0.00003 | 0.001* |
| Left dorsal ACC/dmPFC | 32/8 | −3, 39, 39 | 3.40 | 0.00033 | 0.044* |
| Left hippocampus posterior | −27, −24, −6 | 3.21 | 0.00067 | 0.082† | |
| Recognition phase: False Alarms to negative words vs baseline | |||||
| Left vlPFC/OFC | 47/11 | −42, 30, −15 | 3.82 | 0.00007 | 0.008* |
For group differences threshold set at an FDR corrected P < 0.05, masked with total group.
*For a priori regions of interest a SVC voxel FDR corrected P < 0.05 or †P < 0.10 (trend).
Fig. 1.
Increased BOLD response in (A) ventral/subcallosal ACC, (B) dorsal ACC extending to the dorsomedial PFC (dmPFC) and (C) left posterior hippocampus during encoding of later remembered negative words vs baseline in Complex PTSD patients (N = 28) compared to healthy non-trauma-exposed controls (N = 21). Activations are shown at P < 0.05, k = 0 for illustrative purposes. See Table 2 for coordinates, k-, Z- and P-values.
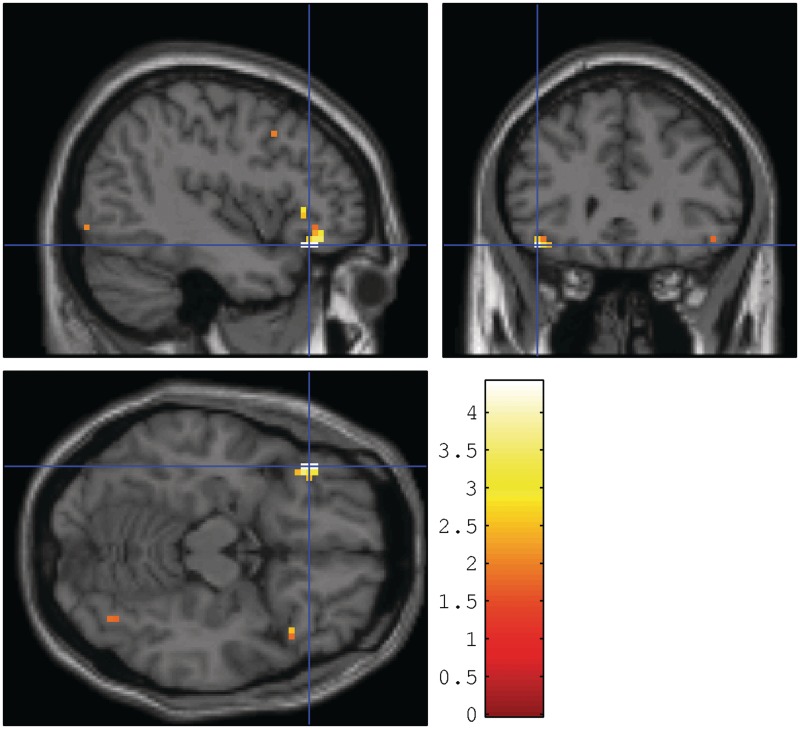
During the retrieval phase, hits to neutral or negative words vs baseline revealed no significant group effects at our a priori threshold. During FAs to negative words vs baseline patients showed a stronger response in the left ventrolateral PFC (vlPFC) extending to the lateral OFC compared to controls (Table 2 and Figure 2). Neutral FAs vs baseline did not give rise to differential BOLD responses between groups.
Fig. 2.
Increased BOLD response in the left ventrolateral PFC extending to the lateral OFC (MNI: −42, 30, −15, z = 3.82, p SVC corrected voxel FDR = 0.0008) during False Alarms to negative words vs baseline in Complex PTSD patients (N = 28) compared to healthy non-trauma-exposed controls (N = 21). Activations are shown at P < 0.05, k = 0 for illustrative purposes.
We performed post hoc analyses with the WFU_BPM toolbox (Casanova et al., 2007) to correct voxel-wise for regional GM density differences, which however revealed similar results.
Correlations of symptom severity with BOLD response during encoding of negative words in Complex PTSD patients
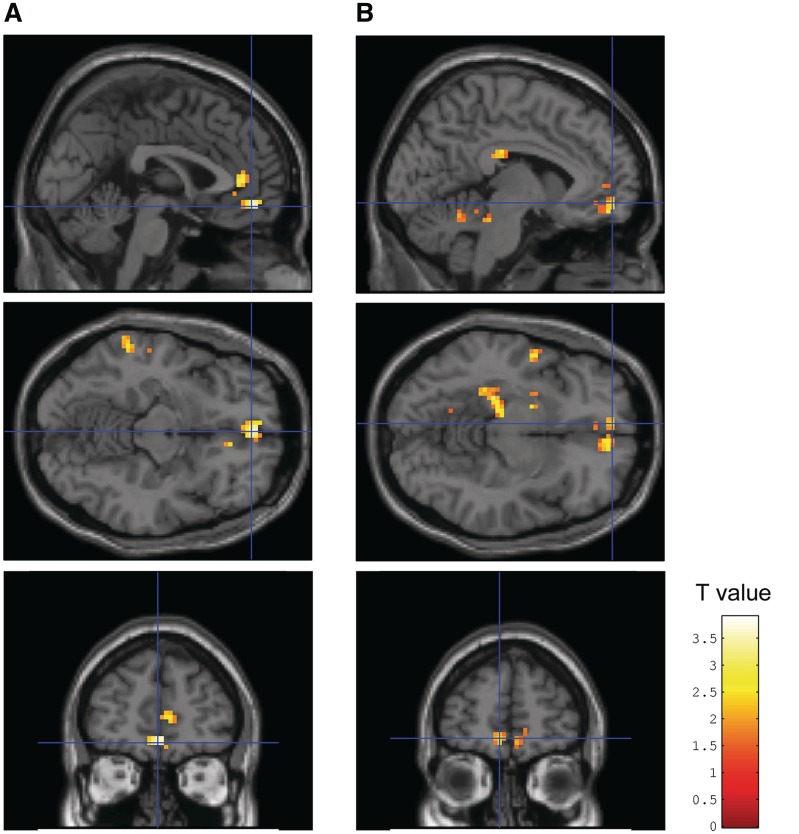
Figure 3 illustrates that severity of child abuse correlated positively with the BOLD response during encoding of negative words in the left ventral/subcallosal ACC. We also observed a significant correlation of severity of depressive symptoms (BDI) with BOLD response during encoding of negative words in the left parahippocampal gyrus extending to the hippocampus and a near-significant positive correlation with the response in the left ventral/subcallosal ACC.
Fig. 3.
In Complex PTSD patients (N = 28) during encoding of negative words: (A) positive correlation of severity of child abuse with BOLD response in the left ventral/subcallosal ACC (vACC) (MNI: 0, 48, −12, z = 3.42, p SVC corrected voxel FDR = 0.020; Spearman correlation 0.53); (B) Positive correlation of severity of depressive symptoms (measured with the BDI) with BOLD response in the left parahippocampal gyrus extending to the hippocampus (MNI: −30, −39, −12, z = 3.49, p SVC voxel FDR corrected = 0.025; Spearman correlation 0.63) and a trend for a positive correlation with the BOLD response in the left vACC (MNI: −6, 54, −9, z = 3.10, p SVC voxel FDR corrected = 0.058; Spearman correlation 0.53). Activations are shown at P < 0.05 and k > 50, respectively, k > 100 for illustrative purposes.
Severity of PTSD symptoms (CAPS), dissociative (DES) or borderline personality symptoms (BPDSI) was not significantly correlated with activity in any of the brain regions of interest (P SVC corrected >0.05).
DISCUSSION
We aimed to investigate neurophysiological correlates of emotional verbal declarative memory in female patients with complex PTSD and to explore whether any abnormalities were correlated with severity of comorbid psychopathology.
During encoding, patients reacted relatively fast on negative compared to neutral words, which could not be explained by medication use. The faster reaction to negative words was associated with subjective fear ratings during scanning and with a higher False Alarm rate to negative words during recognition, as described further in section 4.2. We found increased hippocampus activity during encoding of later remembered negative words, which was a replication of findings of our previous smaller pilot study focusing specifically on the medial temporal lobe (Thomaes et al., 2009). In healthy controls, increased hippocampal activity is likely to reflect successful encoding (Spaniol et al., 2009). In the present study, however, patients’ correct recognition was similar to controls, and net performance was lower due to a high False Alarm rate. Considering the fear conditioning model, impaired memory would be expected to be associated with decreased hippocampus activity. Previous functional imaging studies in various trauma populations (child abuse and veterans) have mostly shown decreased hippocampal activations in PTSD during deep compared to shallow encoding of a neutral text (Bremner et al., 2003a) and during recognition (trauma recall and retrieval of negative and neutral word pairs, respectively) (Bremner et al., 1999; 2003b; Geuze et al., 2008). However, hyperactivation has been found during encoding of neutral stimuli in various trauma populations (Shin et al., 2004b; Sakamoto et al., 2005; Geuze et al., 2008; Werner et al., 2009a), during recall of neutral stimuli (Shin et al., 2004b) and negative pictures (Brohawn et al., 2010). It is not clear if these discrepancies are the result of type of trauma, comorbidity or memory paradigms (e.g. neutral or trauma stimuli, encoding or retrieval phase) employed in these studies. Possibly, hippocampal function is less efficient in Complex PTSD, requiring increased effort to allow similar performance as in controls. Alternatively, due to its role in contextualization of fear responses (Rauch et al., 2006) increased hippocampal activity could reflect impaired extinction of fear responses across contexts, and may result in over-generalized responses to negative words, predisposing to intrusive errors or FAs as observed in the present study and also in the emotional Stroop fMRI study of Shin and coworkers (Shin et al., 2001).
The increased, rather than decreased, activity in the ACC could result from difficulty to divert attention from trauma-related words. Considering its role in detection of response conflicts (Carter and van Veen, 2007), increased ACC activity may also represent a conflict between responding to certainly seen words only vs responding to all trauma-related words. Alternatively, it may be argued that increased hippocampal activations are associated with high dissociation levels of as has been found in highly dissociative students (de Ruiter et al., 2007). Moreover, several independent studies revealed a dissociative subtype of PTSD patients showing increased activation in the ACC/medial PFC along with decreased activations in limbic regions during exposure to a traumatic script, and an intrusive subtype with decreased ACC and increased limbic activations (Lanius et al., 2002, 2010c; Hopper et al., 2007; Kemp et al., 2009; Ludascher et al., 2010). Hyperactivity was also found in the dorsal part or cognitive division of the ACC during dissociative states and during performance of an emotional Stroop paradigm (Shin et al., 2001, 2007; Bremner et al., 2004). In the present highly dissociative population, we would have expected an association between dissociation scores and brain activations, but we did not.
BOLD response in the ventral ACC during encoding of negative words was correlated with severity of child abuse, but not with PTSD severity, probably due to uniformly high scores within our patient group (i.e. ceiling effects). Severity of depression (BDI) was correlated with BOLD response during encoding of negative words in the left parahippocampal gyrus extending to the hippocampus, and approached significance for the response in the left ventral ACC. This is in line with results in PTSD patients with comorbid depression (Lanius et al., 2007) and consistent with previous reports of cognitive abnormalities in PTSD being linked to comorbid depression (Moore, 2009). In MDD, increased activations of the ventral ACC (Drevets et al., 2008) and hippocampus (Videbech et al., 2002) have also been found, although not consistently (Werner et al., 2009b). Glucocorticoid receptors in the ventral ACC are likely to have a key role in the negative feedback mechanism of cortisol secretion and impaired ACC function may contribute to cortisol hypersecretion in during stress (Drevets et al., 2008). In BPD patients, cortisol and ACTH responses were associated with experiences of child abuse but not with comorbid PTSD or MDD (Rinne et al., 2002). It may be argued that depression comorbidity in our sample is inherent to the Complex PTSD diagnosis and probably reflects severity of Complex PTSD. No significant BOLD correlations were found with severity of borderline personality symptoms, although in other studies impulsivity and anger in BPD patients was found to be negatively correlated with hippocampus and ACC activity (Leyton et al., 2001; Oquendo et al., 2005; Silbersweig et al., 2007). This may result from the heterogeneity of our population with borderline but also cluster C personality disorders.
Complex PTSD patients correctly recognized as many neutral and negative words as controls, but had an increased tendency to erroneously recognize newly presented negative words as old (FAs), resulting in decreased net performance for negative words. Medication use did not affect recognition performance. An increased False Alarm rate was not reported in systematic reviews on memory impairment in PTSD, probably because memory performance is usually defined as correct recognition (hits) or net performance (i.e. hits minus FAs), without documenting FAs separately both in neuropsychological (Brewin et al., 2007; Johnsen and Asbjornsen 2008; Moore, 2009) and in imaging studies (Spaniol et al., 2009). However, high False Alarm rates of trauma words, as opposed to neutral words, has been shown in several PTSD studies (Brennen et al., 2007; Hayes et al., 2011) and of neutral words in diverse trauma PTSD populations, which has been interpreted as resulting from disinhibition and hyperarousal associated with dysfunction of the (pre)frontal lobe (Vasterling et al., 1998; Bremner et al., 2000; Zoellner et al., 2000; Lindauer et al., 2006; Johnsen and Asbjornsen, 2009). Our data provide some evidence for this hypothesis because patients showed increased levels of subjective fear during imaging, faster reaction times and FAs to negative words. This was associated with increased activity in the lateral OFC, a brain area supposedly involved in affective decision making and response inhibition (Rolls and Grabenhorst, 2008), which may reflect attempts of the patients to inhibit false-positive errors. Alternatively, FAs could be interpreted as intrusive errors, with trauma-related stimuli activating autobiographical memories confounding task performance (Whalley et al., 2009). The OFC activation was extending into the left ventrolateral PFC (including Broca's area), a region found to be activated in most memory studies, more in successful encoding than in successful recognition (Spaniol et al., 2009). The left ventrolateral PFC has been implicated in semantic rather than declarative memory, stressing the importance of processing the meaning of the stimuli and the interdependence of encoding and retrieval processes (Rugg et al., 2002).
Limitations of the study
Because we did not include a trauma-exposed comparison group we cannot determine whether reported findings are related to trauma-exposure or (Complex) PTSD or both. Also, because we studied only PTSD patients with comorbid disorders, we cannot directly compare PTSD with and without comorbid psychopathology. It should be noted that our patients were found to have decreased gray matter volume relative to control subjects in hippocampus, dorsal ACC and medial OFC, as reported previously (Thomaes et al., 2010). Post hoc analyses correction of our main results to voxel wise for regional GM density differences revealed similar results. Therefore, differences in regional brain volume did not affect our functional results.
Furthermore, in contrast to the fear conditioning model, we found no increased amygdala responses in this study, presumably because we employed a verbal and no visual or imagery instruction paradigm (Costafreda et al., 2008), or because of the use of SSRIs in over half of our population, which may dampen amygdala activation (Lanius et al., 2010b). Also, it may be argued that increased baseline amygdala activity, as has been found in a group of PTSD patients with mainly incidental trauma (Chung et al., 2006), might result in ceiling effects, confounding group by task interaction analyses. However, increased baseline amygdala activity has not been observed in patients with chronic PTSD who experienced trauma >20 years ago, comparable to findings in BPD (Molina et al., 2010) and chronic (probably Complex) female PTSD patients with early trauma showed decreased resting state amygdala connectivity (Bluhm et al., 2009; Lanius et al., 2010a).
CONCLUSION
In conclusion, Complex PTSD patients reacted relatively fast on negative compared to neutral words, associated with enhanced activity in the left ventral and dorsal ACC extending to the dorsomedial PFC, which may indicate difficulty with divide attention from negative words, and in the left posterior hippocampus, reflecting increased effort and/or contextual coupling. Furthermore, Complex PTSD patients showed enhanced left ventrolateral PFC and OFC activity, associated with impaired response inhibition, resulting in a tendency for more FAs to negative words, presumably due to memory intrusions. Severity of child abuse and of depressive symptoms were positively correlated to activity in the ventral ACC and left posterior hippocampus, respectively. The sample size in the present study (28 Complex PTSD subjects and 21 controls) is fairly high for fMRI studies, and the child abuse-related Complex PTSD population with high comorbidity of axes I and II diagnoses, which was extensively assessed in the present study, is understudied. As such, our results indicate that the fear conditioning model of PTSD falls short in explaining the neurophysiological correlates of memory function as found in the present Complex PTSD population. Future research should aim to further investigate the role of trauma, PTSD and (comorbid) depression, dissociation and borderline personality severity by directly comparing diagnostic groups. Also, we stress the importance of studying treatment options for this specific Complex PTSD group and its effects on brain activity. There is evidence—still sparse though intriguing—which pharmacotherapy and psychotherapy may influence hippocampal volume (Vermetten et al., 2003; Lindauer et al., 2005), and prefrontal activity in PTSD (Levin et al., 1999; Fernandez et al., 2001). Results of the present study demonstrate functional abnormalities in Complex PTSD in the frontolimbic brain circuit also implicated in fear conditioning models, but generally in the opposite direction, which may be explained by severity of the trauma and severity of comorbid disorders in Complex PTSD.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank further the trained psychologists N. Ran, R.J. de Vries, M. Burger-Hupkes, and the trained psychiatry residents S.A.N. Renkema and F.E. de Vries for performing extensive assessments, and especially all patients and controls for participating in this study. All authors contributed substantially to conception and design, or analysis and interpretation of data and revised the article critically for important intellectual content and gave final approval. This study was funded by ZonMw, the Netherlands organization for health research and innovation (100-002-020); GGZ InGeest, Institute for Clinical and Experimental Neurosciences (ICEN) and the VU University Medical Center, Amsterdam, The Netherlands.
REFERENCES
- APA. Diagnostic and Statistical Manual of Mental Disorders IV - Text Revision (DSM-IV-TR) Washington DC: American Psychiatric Press Incorporated; 2000. [Google Scholar]
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. American Journal of Psychiatry. 2005;162:1961–3. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Arntz A, van den HM, Cornelis J, Verheul R, van den Bosch WM, de Bie AJ. Reliability and validity of the borderline personality disorder severity index. Journal of Personality Disorders. 2003;17:45–59. doi: 10.1521/pedi.17.1.45.24053. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory. Clinical Psychology Review. 1998;42:841–65. [Google Scholar]
- Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. Journal of Nervous & Mental Disease. 1986;174:727–35. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry & Neuroscience. 2009;34:187–94. [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) Journal of Traumatic Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Shobe KK, Kihlstrom JF. False memories in women with self-reported childhood sexual abuse: an empirical study. Psychological Science. 2000;11:333–7. doi: 10.1111/1467-9280.00266. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological Psychiatry. 2004;55:612–20. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 2003a;160:924–32. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry. 2003b;53:879–89. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Brennen T, Dybdahl R, Kapidzic A. Trauma-related and neutral false memories in war-induced Posttraumatic Stress Disorder. Consciousness and Cognition. 2007;16:877–85. doi: 10.1016/j.concog.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for emotionally neutral information in posttraumatic stress disorder: a meta-analytic investigation. Journal of Abnormal Psychology. 2007;116:448–63. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biological Psychiatry. 2010;68:1023–30. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:367–79. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, et al. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–43. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YA, Kim SH, Chung SK, et al. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clinical Neurophysiology. 2006;117:637–42. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Veltman DJ, Phaf RH, van Dyck R. Negative words enhance recognition in nonclinical high dissociators: an fMRI study. Neuroimage. 2007;37:323–34. doi: 10.1016/j.neuroimage.2007.04.064. [DOI] [PubMed] [Google Scholar]
- Draijer N. Structured Trauma Interview. Amsterdam: Free University, Department of Psychiatry; 1989. [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Pissiota A, Frans O, von Knorring L, Fischer H, Fredrikson M. Brain function in a patient with torture related post-traumatic stress disorder before and after fluoxetine treatment: a positron emission tomography provocation study. Neuroscience Letters. 2001;297:101–4. doi: 10.1016/s0304-3940(00)01674-8. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV diagnoses (SCID-I) Washington DC: American Psychiatric Press Incorporated; 1995. [Google Scholar]
- Ford JD. Disorders of extreme stress following war-zone military trauma: associated features of posttraumatic stress disorder or comorbid but distinct syndromes? Journal of Consulting & Clinical Psychology. 1999;67:3–12. doi: 10.1037//0022-006x.67.1.3. [DOI] [PubMed] [Google Scholar]
- Ford JD, Kidd P. Early childhood trauma and disorders of extreme stress as predictors of treatment outcome with chronic posttraumatic stress disorder. Journal of Traumatic Stress. 1998;11:743–61. doi: 10.1023/A:1024497400891. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HG. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42:659–69. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Labar KS, McCarthy G, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatry Research. 2011;45:660–9. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. Journal of Traumatic Stress. 1992;5:377–91. [Google Scholar]
- Hopper JW, Frewen PA, Van Der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of Traumatic Stress. 2007;20:713–25. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Hovens JE, van der Ploeg HM, Klaarenbeek MT, Bramsen I, Schreuder JN, Rivero VV. The assessment of posttraumatic stress disorder: with the Clinician Administered PTSD Scale: Dutch results. Journal of Clinical Psychology. 1994;50:325–40. doi: 10.1002/1097-4679(199405)50:3<325::aid-jclp2270500304>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Johnsen GE, Asbjornsen AE. Consistent impaired verbal memory in PTSD: a meta-analysis. Journal of Affective Disorders. 2008;111:74–82. doi: 10.1016/j.jad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Johnsen GE, Asbjornsen AE. Verbal learning and memory impairments in posttraumatic stress disorder: the role of encoding strategies. Psychiatry Research. 2009;165:68–77. doi: 10.1016/j.psychres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry. 2008;63:550–6. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Felmingham K, Das P, et al. Influence of comorbid depression on fear in posttraumatic stress disorder: an fMRI study. Psychiatry Research. 2007;155:265–9. doi: 10.1016/j.pscychresns.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Felmingham KL, Liddell BJ, Bryant RA, Williams LM. Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: an fMRI study. Psychiatry Research. 2009;174:158–61. doi: 10.1016/j.pscychresns.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica. 2010a;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Brewin CR, Bremner JD, et al. Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication-free patients? Journal of Psychiatry & Neuroscience. 2010b;35:80–9. doi: 10.1503/jpn.090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Research. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. American Journal of Psychiatry. 2010c;167:640–7. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52:305–11. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Levin P, Lazrove S, van der KB. What psychological testing and neuroimaging tell us about the treatment of Posttraumatic Stress Disorder by Eye Movement Desensitization and Reprocessing. Journal of Anxiety Disorders. 1999;13:159–72. doi: 10.1016/s0887-6185(98)00045-0. [DOI] [PubMed] [Google Scholar]
- Leyton M, Okazawa H, Diksic M, et al. Brain Regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. American Journal of Psychiatry. 2001;158:775–82. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Progress in Brain Research. 2008;167:151–69. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biological Psychiatry. 2006;59:171–7. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, et al. Effects of psychotherapy on hippocampal volume in out-patients with post-traumatic stress disorder: a MRI investigation. Psychological Medicine. 2005;35:1421–31. doi: 10.1017/S0033291705005246. [DOI] [PubMed] [Google Scholar]
- Ludascher P, Valerius G, Stiglmayr C, et al. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder: a pilot study. Journal of Psychiatry & Neuroscience. 2010;35:177–84. doi: 10.1503/jpn.090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends in Cognitive Sciences. 2006;10:271–7. doi: 10.1016/j.tics.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Molina ME, Isoardi R, Prado MN, Bentolila S. Basal cerebral glucose distribution in long-term post-traumatic stress disorder. World Journal of Biological Psychiatry. 2010;11:493–501. doi: 10.3109/15622970701472094. [DOI] [PubMed] [Google Scholar]
- Moore SA. Cognitive abnormalities in posttraumatic stress disorder. Current Opinion in Psychiatry. 2009;22:19–24. doi: 10.1097/YCO.0b013e328314e3bb. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Taghavi R, Neshat-Doost HT, Yule W, Dalgleish T. Memory bias for emotional information in children and adolescents with posttraumatic stress disorder: a preliminary study. Journal of Anxiety Disorders. 2000;14:521–34. doi: 10.1016/s0887-6185(00)00037-2. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Krunic A, Parsey RV, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge in major depressive disorder with and without borderline personality disorder. Neuropsychopharmacology. 2005;30:1163–72. doi: 10.1038/sj.npp.1300689. [DOI] [PubMed] [Google Scholar]
- Pelcovitz D, van der KB, Roth S, Mandel F, Kaplan S, Resick P. Development of a criteria set and a structured interview for disorders of extreme stress (SIDES) Journal of Traumatic Stress. 1997;10:3–16. doi: 10.1023/a:1024800212070. [DOI] [PubMed] [Google Scholar]
- Pfohl N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV) Washington DC: American Psychiatric Press Incorporated; 1997. [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den BW. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biological Psychiatry. 2002;52:1102–12. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in Neurobiology. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philosophical Transactions of the Royal Society B: Biological Sciences. 2002;357:1097–110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26:813–21. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Keshavan MS. Prefrontal subregions and dimensions of insight in first-episode schizophrenia–a pilot study. Psychiatry Research. 2006;146:35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shin LM, Bush G, Whalen PJ, et al. Dorsal anterior cingulate function in posttraumatic stress disorder. Journal of Traumatic Stress. 2007;20:701–12. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004a;61:168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004b;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, et al. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. American Journal of Psychiatry. 2007;164:1832–41. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Steinberg M, Rounsaville B, Cicchetti DV. The Structured Clinical Interview for DSM-III-R Dissociative Disorders: preliminary report on a new diagnostic instrument. American Journal of Psychiatry. 1990;147:76–82. doi: 10.1176/ajp.147.1.76. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NP, et al. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: A pilot study. Psychiatry Research. 2009;171:44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, et al. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. Journal of Clinical Psychiatry. 2010;71:1636–44. doi: 10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- Van der Kolk BA, Pelcovitz D, Herman JL, Roth S, Kaplan S, Spitzer RL. The Disorders of Extreme Stress Inventory. 1992 Unpublished measure. [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–33. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biological Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen TH, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatrica Scandinavica. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Engel RR, et al. Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2009a;43:309–18. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Materne J, et al. Functional MRI study of memory-related brain regions in patients with depressive disorder. Journal of Affective Disorders. 2009b;119:124–31. doi: 10.1016/j.jad.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Whalley MG, Rugg MD, Smith AP, Dolan RJ, Brewin CR. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain and Cognition. 2009;69:98–107. doi: 10.1016/j.bandc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychology Review. 2007;114:152–76. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Zakriski AL, Shea MT, et al. The long-term sequelae of sexual abuse: support for a complex posttraumatic stress disorder. Journal of Traumatic Stress. 1996;9:195–205. doi: 10.1007/BF02110655. [DOI] [PubMed] [Google Scholar]
- Zoellner LA, Foa EB, Brigidi BD, Przeworski A. Are trauma victims susceptible to “false memories”? Journal of Abnormal Psychology. 2000;109:517–24. doi: 10.1037/0021-843X.109.3.517. [DOI] [PubMed] [Google Scholar]