Abstract
The recognition of threatening faces is important for making social judgments. For example, threatening facial features of defendants could affect the decisions of jurors during a trial. Previous neuroimaging studies using faces of members of the general public have identified a pivotal role of the amygdala in perceiving threat. This functional magnetic resonance imaging study used face photographs of male prisoners who had been convicted of first-degree murder (MUR) as threatening facial stimuli. We compared the subjective ratings of MUR faces with those of control (CON) faces and examined how they were related to brain activation, particularly, the modulation of the functional connectivity between the amygdala and other brain regions. The MUR faces were perceived to be more threatening than the CON faces. The bilateral amygdala was shown to respond to both MUR and CON faces, but subtraction analysis revealed no significant difference between the two. Functional connectivity analysis indicated that the extent of connectivity between the left amygdala and the face-related regions (i.e. the superior temporal sulcus, inferior temporal gyrus and fusiform gyrus) was correlated with the subjective threat rating for the faces. We have demonstrated that the functional connectivity is modulated by vigilance for threatening facial features.
Keywords: functional magnetic resonance imaging, amygdala, threat, face recognition, functional connectivity
INTRODUCTION
The perception of facial threat is critical for human survival. We perceive threat from facial expressions of emotions, such as anger and fear [for a review see Staugaard (2010)], and also from alarming facial features that are unrelated to emotions (Winston et al., 2002), such as, mature faces in polar opposition to baby-like faces (Dumas and Testé, 2006). While detecting threatening facial attributes, we make social judgments in daily encounters (Baas et al., 2007). For example, unattractive appearances of defendants can negatively influence juries' decisions (Sigall and Ostrove, 1975; Porter and ten Brinke, 2009). Threatening facial features could be one such form of unattractiveness, eliciting a sense of danger in the observer (Aronoff et al., 1988). Of the two aspects of facial threat, we are concerned with the perception of threatening facial features rather than threatening facial emotions, because threatening facial features make critical influence on social judgement and impression formation in our everyday life (Dumas and Testé, 2006).
Functional magnetic resonance imaging (fMRI) studies have revealed that the amygdala plays a key role in the perception of threatening facial features. For example, a dominating, masculine, angry face radiates a threatening atmosphere, signalling that the person is untrustworthy (Schlicht et al., 2010). To evaluate the extent of threat in terms of untrustworthiness, participants in previous studies have rated the faces of actors (Winston et al., 2002; Engell et al., 2007) and computer-generated faces (Todorov et al., 2008). An increased blood-oxygen-level-dependent (BOLD) response was observed in the bilateral amygdala when participants viewed the faces of university students who were later rated as untrustworthy by an independent group of participants (Winston et al., 2002). Since the participants were not asked to rate the faces for trustworthiness while being scanned, the amygdala activation was interpreted as an ‘automatic’ response to the untrustworthy characteristics of the faces. Engell et al. (2007) further found amygdala activity was correlated more strongly with group ratings than individual ratings of facial trustworthiness, which suggested that the amygdala's automatic response reflected untrustworthy features more precisely than individual conscious judgments. Furthermore, Todorov et al. (2008) deliberately manipulated targeted features of untrustworthiness on computer-generated faces and measured the consequent amygdala activation. The right amygdala showed a negative linear response to increased untrustworthiness, whereas the left amygdala showed more activation in response to faces exhibiting extremes of both trustworthiness and untrustworthiness. All these studies used the faces of the general public or computer-generated faces, and therefore, the authenticity or extremeness of untrustworthy facial features, not to mention potential facial threat, is questionable.
Amygdala activation has also been reported in response to full-blown facial expressions of threatening emotions, such as anger (Straube et al., 2005) and fear (Bishop et al., 2004; Williams et al., 2008). These findings are consistent with a long-held view on the vital role of the amygdala in fear conditioning with rodents, non-human primates and humans (Morrison and Salzman, 2010; Salzman and Fusi, 2010). However, a recent meta-analysis (Ball et al., 2009) indicates amygdala activation in response to positive, negative and neutral emotional valence. Contemporary reviews (Adolphs, 2010; Morrison and Salzman, 2010; Pessoa and Adolphs, 2010) also agree that amygdala activation is related to emotional arousal and intensity, regardless of positive or negative emotional valence.
The studies that we have reviewed so far examined amygdala activation independently. Simultaneous activation of the amygdala and other regions provides a broader perspective of neural processes for threat perception. For instance, threatening emotional faces simultaneously activate both the amygdala and other regions, such as the insula and the fusiform gyrus in social phobics (Straube et al., 2005) and the insula and the superior temporal sulcus in a sample of healthy young adults (Williams et al., 2008). However, co-activation of these different brain regions does not necessarily indicate that they are functionally connected. It is functional connectivity studies that allow researchers to identify how different parts of the brain interact and coordinate with each other based on temporal correlations between spatially remote neurophysiological events (Li et al., 2009).
Functional connectivity between the amygdala and other regions varies in the degree of connectivity and in the pattern of neural network. In healthy participants, the fusiform gyrus showed positive covariation with the right amygdala and negative covariation with the left amygdala in response to viewing fearful faces (Das et al., 2005). Increased functional coupling between the amygdala and the fusiform gyrus has also been reported when the participants observed facial expressions of disgust and happiness, as well as fear (Fairhall and Ishai, 2007; Amting et al., 2010).
When clinical populations were compared to nonclinical controls, reduced functional connectivity between the amygdala and other cortical structures (e.g. cingulate cortex, the insula, prefrontal cortex and orbitofrontal cortex) has been revealed in women with posttraumatic stress disorder (Fonzo et al., 2010), individuals with a social anxiety disorder (Hahn et al., 2011) and postnatally depressed mothers (Moses-Kolko et al., 2010), while they were viewing threatening facial expressions of emotions. The reduced connectivity in the clinical groups was associated with hypervigilance, that is characteristic to these clinical conditions, and with the diminished regulation between the amygdala and the functionally coupled regions. Given hypervigilance to identifying threat or enhanced ability to identify threat in depressed individuals (Watters and Williams, 2011), social anxiety disorder (Mueller et al, 2009) and posttraumatic stress disorder (Morey et al., 2009), those healthy people who are better at discriminating threatening facial features may exhibit the functional connectivity pattern similar to that of the clinical populations with respect to the degree of connectivity and the pattern of neural network.
The present study was designed to look for brain activity in response to perceived threat in the faces of individuals convicted of first-degree murder compared with faces of the general public members, and to examine how individual differences among young healthy adults in perceiving threatening facial features interact with functional connectivity between the amygdala and other brain regions. Our hypotheses were that the amygdala activation would be stronger in response to prisoners' faces than control faces, that the functional connectivity between the amygdala and the other regions related to facial processing (e.g. the fusiform gyrus and the superior temporal sulcus) and emotional processing (e.g. cingulate cortex, the insula, prefrontal cortex and orbitofrontal cortex) would differ between the prisoner and control conditions, and that the extent of connectivity between the regions would be correlated with the subjective rating of facial threat.
METHODS
Stimuli preparation
We prepared 60 colour photographs of male faces: one-half were images of the faces of prisoners convicted of first-degree murder (MUR), which were obtained from US prison websites; the other half were control faces with neutral expressions (CON), which were selected from the Centre for Vital Longevity Face Database (Minear and Park, 2004) and were roughly matched with respect to race and age. We used American faces because the status of the prisoners was likely to be unknown to the Japanese participants.
We manipulated the photographs using commercial software (Adobe Photoshop; Adobe Systems, Inc., San Jose, CA, USA). After standardizing the size, resolution and luminance, the photographs were cropped to produce an ellipse-shaped image that contained only the eyebrows, eyes, nose and mouth of the individual on a black background.
Prior to the fMRI study, we conducted a behavioural study to verify the differential degrees of perceived threat in the faces of the MUR and the CON groups (Supplementary Methods and Supplementary Fig. S1).
Participants
The fMRI study tested 18 healthy native Japanese university students (11 males and seven females; all right-handed; mean age ± s.d. = 20.8 ± 1.5 years) who had not taken part in the behavioural study. The participants were recruited from a student agency that referred research participants. All of the participants gave their written informed consent after reading, understanding and agreeing to the study protocol, which was approved by the ethics committee of the National Institute for Physiological Sciences. Prior to the fMRI experiment, the physical and mental conditions of the participants were carefully screened to ensure that they were free from any past or current history of neuropsychiatric illness.
Experimental procedure
We employed an event-related design, consisting of 60 trials per session. Images of the faces of the convicted murderers were presented (the MUR condition) in one-half of the trials, and images of the control faces were presented in the other half (the CON condition). For each trial, a face image was displayed for 2000 ms after the exposure of a red fixation cross for 500 ms. Trials were presented in a randomized order with respect to the 60 photographs and were separated by a period of exposure to a white fixation cross, which was presented in a jittered manner, ranging from 7.3 to 10.7 s (average duration = 9.0 s). The stimuli were projected onto a transparent screen that was suspended from the bore of a magnet at a distance of 75 cm from the participants' eyes. The participants viewed the stimuli through a tilted mirror attached to the one-channel head coil of the head-only scanner and responded using a magnetic compatible button box held in the right hand. Tight but comfortable padding was placed around each participant's head to minimize head movement. We controlled the stimuli and measured the participants' responses, using Presentation software (Neurobehavioral Systems, Inc., Albany, CA, USA).
Participants inside the MRI scanner were asked to press a button with their right index finger as soon as they saw a face image and to pay as much attention as possible to each image so that they could answer a question about the face once they exited the scanner. At this stage, the participants were not informed that they would be specifically asked to evaluate the extent of dangerousness. All participants repeated the same task session twice, and each task session consisted of the presentation of 60 images of faces in different randomized orders.
After the scan, each participant entered the MRI control room and viewed the same face images on the computer screen in a differently randomized order. For each trial, a face image was displayed for 2000 ms after the exposure of a red fixation cross for 500 ms, and the trials were separated by a 3000 ms period of exposure to a white fixation cross. The participants rated the extent to which they perceived each face to be dangerous on a four-point Likert scale where 1 indicated ‘not at all’, 2 indicated ‘a little’, 3 indicated ‘somewhat’ and 4 indicated ‘very’. Presentation software (Neurobehavioral Systems, Inc.) was used for both stimulus presentation and response recording.
Image acquisition and pre-processing
We obtained functional images of the whole brain in an axial-oblique position, using a 3-Tesla MRI scanner (Allegra, Siemens, Erlangen, Germany). The scanner was equipped with a single-shot echo-planar imaging (EPI) sequence [repetition time (TR) = 2.0 s, echo time (TE) = 30 ms; flip angle (FA) = 80°; 64 × 64 matrix and 34 slices; voxel size = 3 × 3 × 3 mm] which was sensitive to the BOLD contrast. In total, 351 images were obtained during each session. Since we set the amygdala for a region of interest, and because of the amygdala's proximity to an air–tissue interface where signal could drop out, we ensured that we obtained adequate amygdala coverage from each participant before data analysis. After discarding the first four images due to unsteady magnetization, the remaining successive images in each run were analyzed. A high-resolution anatomical T1-weighted image was also acquired (MPRAGE; TR = 2.5 s; TE = 4.38 ms; FA = 8°; 256 × 256 matrix and 192 slices; voxel size = 0.75 × 0.75 × 1 mm) for each participant. We analyzed the data using the SPM8 software (Wellcome Department of Imaging Neuroscience, London, UK). Initially, we temporally realigned the signal in each slice to that obtained in the middle slice by using a sinc interpolation. All of the volumes were then realigned spatially to the first volume. We next normalized all the volumes to the standard Montreal Neurological Institute (MNI) space by using a transformation matrix obtained from the normalization process of the mean EPI image of each individual participant to the EPI template image. The normalized images were spatially smoothed with an 8 mm Gaussian kernel.
Statistical analysis
First-level analysis
After pre-processing, we conducted a statistical analysis of the data from each participant using the general linear model. In the first level (a fixed-effects model), each event was modelled as a haemodynamic response function (HRF). High-pass frequency filters (128 s) were applied to the time-series data. The images were scaled to a grand mean of 100 over all of the voxels and scans within a session. The MUR and CON conditions were modelled separately. The data from all trials, including the trials in which participants failed to press the button, were included in the same regressor, because the number of failed trails over the 60 trials was too small (mean ± s.d. = 0.94 ± 1.76) to construct separate regressors for each. In addition, the six movement parameters obtained during the realignment were entered as regressors. Parameter estimates for the MUR and CON conditions, and for the difference between the conditions, were computed from the least mean-squares fit of the model to the time-series data. Images of the parameter estimates, representing the event-related activity at each voxel, were created for each condition and each participant. In the parametric modulation analysis, the danger rating for each face (for both the MUR and the CON conditions) obtained after the scanning session was entered as a covariate. This analysis investigated the brain region where the signal was parametrically (positively or negatively) modulated by the danger score for each face stimulus.
Second-level analysis
We analysed the individual contrast images that pertained to the difference in signal between the MUR and CON conditions, and the main effect contrast that involved both the MUR and CON conditions, using one-sample t-tests. Parametric modulation analysis was used to investigate the brain regions that had a positive or negative relationship with the danger rating for each face. The statistical threshold was set at P = 0.001, uncorrected for height, and at k > 10 voxels for spatial extent.
Psychophysiological interaction analysis
Psychophysiological interaction (PPI) analysis was used to investigate the functional relationship between the regions involved in the processing of threatening facial features (Friston et al., 1997). PPI analysis was designed to explain the neural responses in one brain area in terms of the interactions with the influences of another brain region (e.g. the amygdala) and a task condition. Here, we examined whether signal coupling between the amygdala and other regions differed between the MUR and CON conditions. Our specific hypothesis was that the functional connectivity between the amygdala and the face-related regions would differ between the conditions and that the extent of the connectivity between the regions would correlate with the subjective ratings of dangerousness. To test this hypothesis, we initially calculated the difference in danger scores for each participant by subtracting the mean score for the CON condition from the mean score for the MUR condition (i.e. MUR–CON). The difference in danger score was referred to as the ‘vigilance index’ (i.e. vigilance for threatening facial features of MUR faces) and represented an individual's ability to discriminate MUR faces from CON faces. We investigated the relationship between the extent of the functional connectivity identified in the PPI analysis and the vigilance index in the following analysis.
Initially, we conducted the PPI analysis separately for the left and right amygdala as source regions. The analysis comprised three regressors: the psychological variable, representing the task condition; the physiological variable, representing the signal response in the amygdala; and the interaction term of these two variables. The psychological variable was a vector coding for the task condition (MUR CON) = (1–1) convolved with the HRF. We determined the amygdala source region by using the group analysis involving both the MUR and the CON conditions. This analysis identified the bilateral amygdala as the source region (P = 0.05, family-wise error rate (FWE) corrected for height, and k = 20 voxels for spatial extent; right, x = 26, y = 4, z = –20, left, x = –26, y = 0, z = –18). We obtained the individual time-series data for the amygdala by extracting the first principal component from a sphere (r = 4 mm) centred on the peak voxel. These time-series data were mean-corrected and high-pass filtered to remove low-frequency signal drift. The physiological factor was then multiplied by the psychological factor, which constituted the interaction term. The PPI analysis was then carried out for each participant, which involved the creation of a design matrix with the interaction term, the psychological factor and the physiological factor as regressors (Neufang et al., 2008; Cremers et al., 2010). The data from the two sessions were included in a single design matrix for each participant. The PPI analysis generated a contrast representing regions that had stronger functional connectivity with the amygdala for MUR faces than for CON faces.
Subsequently, the contrast images for the interaction term from the PPI analysis were subjected to a second-level group analysis. We conducted the group analysis separately, using either the left or the right amygdala as a source region. To examine how the extent of the functional connectivity between the amygdala and other regions correlated with the difference in danger score for the MUR and the CON conditions, we conducted a regression analysis, convolving the vigilance index as a variable. The search volume was restricted to the left hemisphere for the analysis with the left amygdala as a source region and to the right hemisphere for the analysis with the right amygdala as a source region, using the WFU_PickAtlas software (version 2.4; http://fmri.wfubmc.edu/cms/software#PickAtlas). The statistical analysis was conducted at P = 0.001, uncorrected for the height and at k > 10 voxels for the spatial extent. Small-volume correction for multiple comparisons was also applied for regions that we had predicted a priori that is the fusiform gyrus and superior temporal sulcus, using a sphere with a 10 mm radius (20 mm diameter). A sphere was centred around a coordinate within the left fusiform gyrus (x = −42, y = −58, z = −18) and the left superior temporal sulcus (x = −54, y = −48, z = 4) as defined by a prior neuroimaing study that searched brain regions involved in face perception, regardless of stimulus format, emotional valence or task demands (Ishai et al., 2005). Corrected P values were based on this small-volume correction procedure. The parameter estimates of the PPI were extracted from a spherical region of interest (ROI; r = 4 mm) placed at a peak voxel using the MarsBaR software (http://marsbar.sourceforge.net) and plotted against the vigilance index. The time-series data in each of the regions identified were extracted from a sphere (r = 4 mm) centred on the peak voxel for two typical participants' data and plotted against the corresponding time points in the time-series data extracted from the left amygdala separately for the MUR and CON conditions. The extracted time-series data were mean-corrected and high-pass filtered to remove low-frequency signals.
RESULTS
Behavioural results
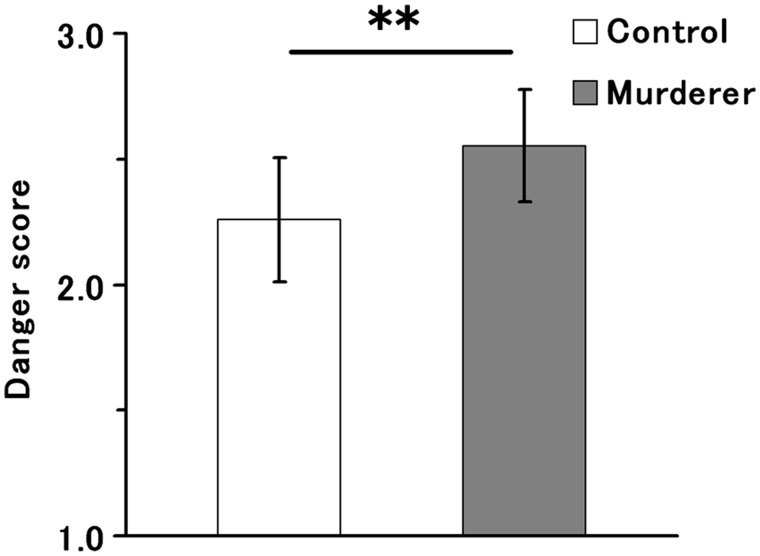
The mean ± s.d. danger scores for the MUR and CON conditions were 2.55 ± 0.22 and 2.26 ± 0.25, respectively (Figure 1). A one-way analysis of variance (ANOVA) showed a significant difference in the mean score between the conditions (F1,34 = 13.63, P < 0.001). Individual mean vigilance indexes (MUR–CON) ranged from −0.10 to 0.57, with 17 out of 18 people's individual mean vigilance indexes (MUR–CON) being positive, leaving only one participant's mean index negative (−0.10). The mean ± s.d. reaction times for the MUR and CON conditions were 1167 ± 200 ms and 1197 ± 205 ms, respectively. A one-way ANOVA did not show a significant difference in the mean reaction time between the conditions (F1,34 = 0.19, P = 0.66).
Fig. 1.
Mean danger scores for the control faces (white) and the prisoners' faces (grey). Bars indicate one s.d. The double asterisk denotes a significant difference in the mean danger score between the faces (P < 0.01).
Subtraction analysis and parametric modulation analysis
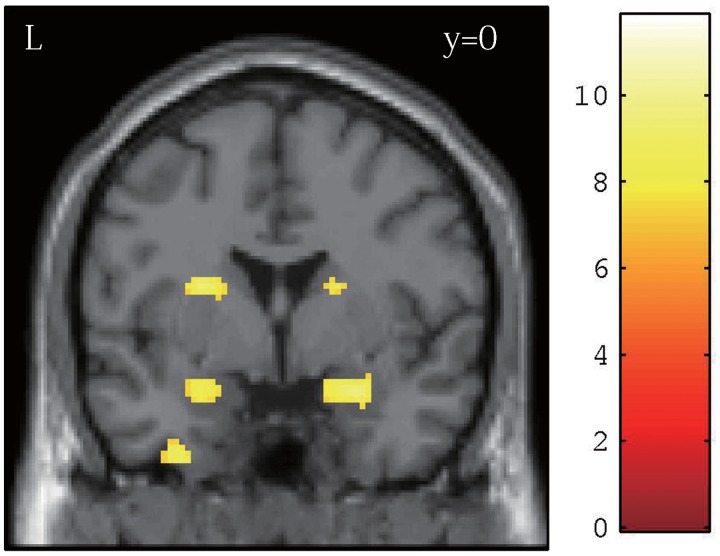
The activation in the amygdala region did not differ significantly between the MUR and CON conditions. However, the group analysis involving both the MUR and CON conditions revealed significant activation in the bilateral amygdala (Figure 2). In the parametric modulation analysis, the amygdala activity did not show a significant (positive or negative) association with the danger ratings.
Fig. 2.
Significant activation in the bilateral amygdala in the group analysis involving both the MUR and the CON conditions superimposed on the SPM8 canonical image. The statistical threshold was set at P = 0.05, FWE corrected for height, and at k = 20 voxels for spatial extent. The amygdala region in the left and right hemispheres was used as a source region for the PPI analysis.
PPI analysis
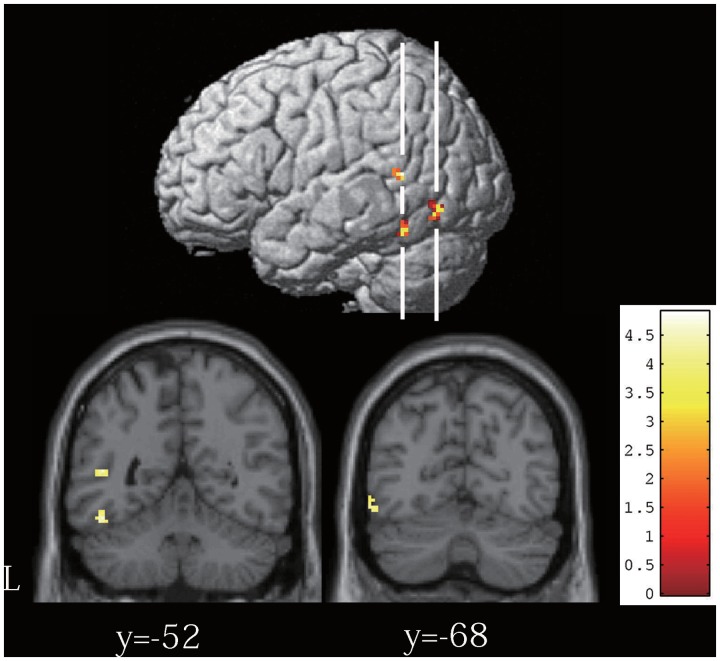
The subtraction of danger ratings MUR–CON (i.e. vigilance index) was significantly correlated with the extent of functional connectivity between the left amygdala and the posterior part of the left temporal lobe (Figure 3). These clusters were located in the superior temporal sulcus, fusiform gyrus and inferior temporal gyrus (Table 1).
Fig. 3.
Top: significant results for the PPI analysis using the left amygdala as a source region rendered on the SPM8 template image. The coordinates, T-value and voxel size of three clusters in the left posterior temporal lobe are listed in Table 1. White lines in the brain template indicate the location at y = –52 mm (anterior) and y = –68 mm (posterior) in the coronal section shown in the bottom of the figure. Bottom: the image on the left shows two clusters in the superior temporal sulcus (superior) and the fusiform gyrus (inferior). The image on the right shows a cluster in the inferior temporal gyrus.
Table 1.
Results of psychophysiological interaction analysis
| Region name | L/R | BA | x, y, z | T | Voxels |
|---|---|---|---|---|---|
| Superior temporal sulcus* | L | 22 | −48, −50, 10 | 4.89 | 22 |
| Fusiform gyrus* | L | 37 | −46, −52, −18 | 4.59 | 19 |
| Inferior temporal gyrus | L | 37 | −56, −68, −10 | 4.22 | 26 |
L/R, left/right, BA, Brodmann's area.
*P < 0.05 corrected for multiple comparisons after small-volume correction.
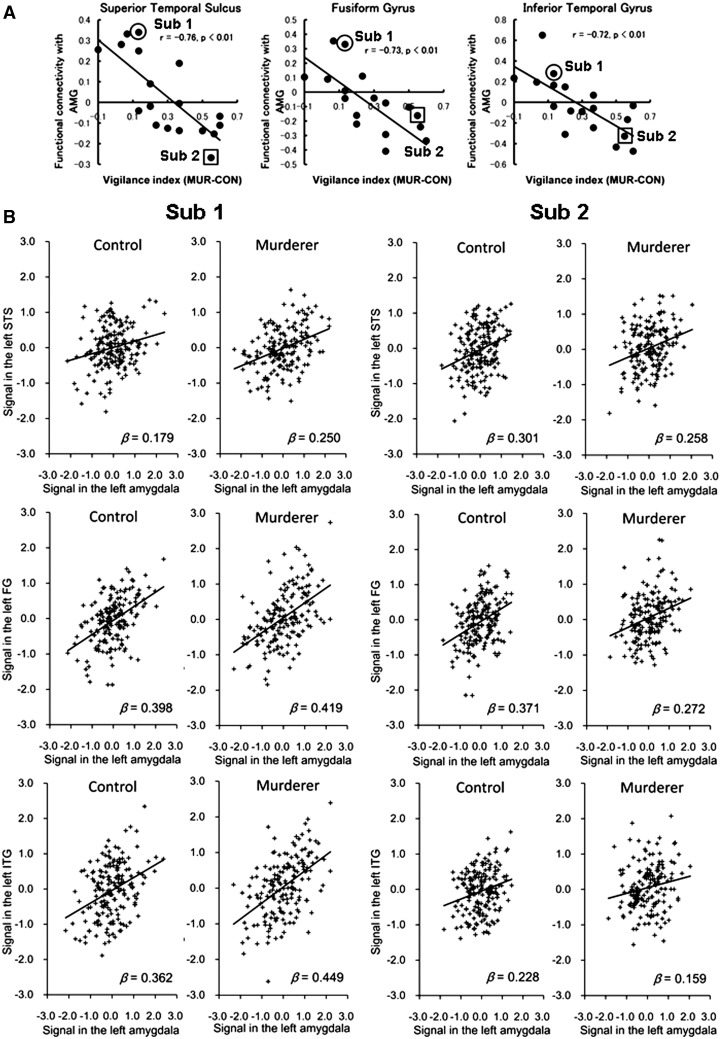
The data on the extent of functional connectivity extracted from these clusters were plotted against the vigilance index (Figure 4A). Positive values on the vertical axis of the correlation scatter plots indicate that the connectivity between the regions was positive for the MUR condition and negative for the CON condition. In contrast, negative values on the vertical axis indicate that the connectivity between the regions was negative for the MUR condition and positive for the CON condition. The negative correlations for all three brain regions suggest that those participants who rated the prisoners' faces as more dangerous than the control faces showed lower functional connectivity between the amygdala and the face-related regions for the MUR condition compared with the CON condition. The plots for two typical participants are shown in Figure 4B. These data showed weakened coupling of activity in the face-related regions with activity in the amygdala under the CON condition compared with the MUR condition for Participant 1, and under the MUR condition compared with the CON condition for Participant 2.
Fig. 4.
Results of the PPI analysis. (A) Correlation plots (left, superior temporal sulcus; middle, fusiform gyrus; and right, inferior temporal gyrus) showing negative correlations between the extent of functional connectivity and the subjective rating of facial dangerousness (i.e. the vigilance index). The vertical axis indicates the extent of functional connectivity between the amygdala and each of the face-related regions. The horizontal axis indicates the difference in the danger scores between the prisoners' faces and the control faces. Each dot represents a participant. The regression line, correlation coefficient and P-value are shown. A negative value on the vertical axis implies that the connectivity between the amygdala and each region is lower for the prisoners' faces than for the control faces. A positive value on the horizontal axis indicates that the participants rated the prisoners' faces as more dangerous than the control faces. (B) Representative plots for two typical participants. Time points of the time-series data extracted from the face-related regions compared with those from the amygdala. Each dot represents a pair of corresponding time points. β indicates the correlation coefficient and a smaller value represents weaker coupling of activity of the region with the amygdala.
We found no significant relationship between the extent of the functional connectivity and the vigilance index when using the right amygdala source.
DISCUSSION
The present study investigated brain activity in response to perceived threatening facial features in two sets of face photographs, murderers (MUR) and controls (CON), and the relationship with subjective ratings of threat. Our first novel finding was that the extent of functional connectivity between the amygdala and the face-related regions (i.e. the superior temporal sulcus, fusiform gyrus and inferior temporal gyrus) was negatively correlated with the subtraction of subjective rating score (MUR–CON) (i.e. the vigilance index) representing vigilance for threatening facial features. In other words, those vigilant participants who rated MUR faces more dangerous than CON faces showed decreased connectivity while viewing MUR faces, as compared to those who were not so vigilant.
This finding resembles the diminished connectivity that clinical samples exhibited compared to non-clinical samples, but in a different neural network (i.e. the cingulate cortex, the insula, prefrontal cortex, orbitofrontal cortex) with the amygdala (Fonzo et al., 2010; Moses-Kolko et al., 2010; Hahn et al., 2011). In terms of the network with the amygdala, our results are more consistent with the previous studies that showed simultaneous activation of the amygdala and the fusiform gyrus (Das et al., 2005; Straube et al., 2005; Fairhall and Ishai, 2007; Amting et al., 2010), and the amygdala and the superior temporal sulcus (Williams et al., 2008). Where there are differences between our results and the findings from the foregoing studies, these may be due to a combination of stimulus (threatening facial features vs threatening facial emotions) and sample (nonclinical vs clinical) factors. While this hypothesis can be only verified with future experiments with different combinations of the two factors, we would like to make a tentative interpretation of the decreased connectivity between the amygdala and the facial areas in those participants who are more vigilant for threatening facial features.
Mather et al. (2010) considered weaker connectivity, though in the different network from ours, to represent social withdrawal and stronger connectivity to represent support-seeking behaviour. The activation patterns of our results may also be construed as a withdrawal response from MUR faces and an approach response to CON faces. Withdrawal in this context is seen as a freeze response to threat (Bracha et al., 2004); an adaptive response in humans that takes place before flight and fight responses or when escape is impossible or fighting would be pointless. Future research should include explicit measures of freezing behaviour and confirm that the functional connectivity represents a freeze response.
Reduced connectivity between the posterior superior temporal sulcus, the fusiform gyrus and the amygdala has been linked to the pathophysiology of social perception deficits in autism (e.g. Pelphrey and Carter, 2008). Using the same prisoners' faces as stimuli, we found that individuals with very high-functioning autism could distinguish prisoners' faces from control faces equally well as neurotypical individuals who were matched for non-verbal ability (Miyahara et al., 2010). In our previous study, however, a pair of MUR and CON faces was presented on a sheet of paper in order for the participants to compare their perceived dangerousness. It would be interesting to examine whether the connectivity in the neural network differed between the neurotypical individuals and those with autism when viewing facial stimuli presented in the same method as the current study.
Our results demonstrated functional connectivity only in the left hemisphere in response to an implicit instruction to view faces with potentially threatening features. In a systematic review and meta-analysis, Baas et al. (2004) found that the left amygdala tended to be activated more often than the right amygdala in response to threat. Moreover, systematic reviews and meta-analyses by both Baas et al. (2004) and Wager et al. (2003) consistently highlighted the difficulties in determining the hemispheric specialization of the amygdala for emotions, due to the limited number of studies available and the use of various research protocols. Some recent studies (e.g. Habel et al., 2007; Williams et al., 2008) have referred to laterality of amygdala activation; however, they have also used different research paradigms and not all have investigated functional connectivity. We are, therefore, currently unable to explain our finding of significant functional connectivity only in the left hemisphere. Nonetheless, we believe that our results make a significant contribution to the accumulating body of data that will eventually clarify the laterality of amygdala activation through future research.
Our initial hypothesis that there would be differential amygdala activation in response to MUR and CON faces was not supported by our fMRI data. In contrast, our behavioural data showed that the participants rated the MUR faces as more dangerous than the CON faces after the scan. In other words, it was likely that the participants might be more threatened by MUR faces than CON faces in the scan, but the amygdala did not seem to care. This finding appears contradictory to the notion of the amygdala's automatic (Winston et al., 2002) and unconscious (Engell et al., 2007) processing of untrustworthy faces. In a seminal review, Adolphs (2010) concluded that such an old view should be revised in favour of a new one holding that the amygdala is not essential in the automatic and unconscious process; it would mostly play a modulatory role in the network along with the temporal cortex. Our data on functional connectivity between the amygdala and other temporal regions are consistent with this new view of the amygdala's role in threat perception.
There is still a possibility that the implicit experimental task used in our study might have lowered the sensitivity to detecting minor differences in facial threat. Previously, both implicit and explicit tasks were reported to facilitate increased amygdala activity. Two studies employed implicit tasks and asked participants to first view faces in the fMRI scanner and to rate arousal (Straube et al., 2005) and trustworthiness (Todorov et al., 2008) afterwards. An explicit task of facial affect recognition (Williams et al., 2008) also increased amygdala activity significantly when fearful faces were present. One study directly examined the difference in bilateral amygdala activation for the identification of facial affect and revealed greater activation in the explicit condition than in the implicit condition (Habel et al., 2007). These studies differed from our study that investigated threatening facial features and used the implicit task in the scanner to later rate dangerousness. However, the facial expressions of emotions included threatening emotions (i.e. fear and anger) (Straube et al., 2005; Habel et al., 2007; Williams et al., 2008) and the explicit condition to identify facial expressions of emotions facilitated greater bilateral amygdala activation than the implicit condition (Habel et al., 2007). A future study, using an explicit task to rate facial threat in the scan, might produce a differential amygdala activation between MUR and CON conditions.
It is also possible that the murderers' faces, which did not portray full-blown angry or fearful expressions, were not of sufficient intensity to lead to a heightened amygdala response. Alternatively, the race of the faces might have led to an equivalent amygdala response whether the faces were MUR or CON. The Japanese participants might have perceived all the Americans' neutral faces as similarly arousing, if not threatening, due to the unfamiliarity in their daily life.
Finally, expecting a necessary increase in amygdala activity in the MUR condition relative to the CON condition may be problematic because the activity in the amygdala on its own may not be as important as the amygdala's role as a modulator (Adolphs, 2010; Pessoa and Adolphs, 2010). Indeed, a meta analysis by Wager et al. (2003) indicates that even when a particular brain area is identified as central to processing of a stimulus, only 30–65% of imaging studies result in increased activation in that area. It seems likely that the amygdala's activation communicated to other brain areas, as measured in the functional connectivity analyses, will often be most important, possibly helping to ready an individual for an appropriate response.
In conclusion, the present study revealed that the extent of functional connectivity between the amygdala and the face-related regions was correlated with individuals' subjective perceptions of the threat in faces. Further research is needed in which functional connectivity analysis is applied to examine (i) the difference between implicit and explicit tasks; (ii) any differences between clinical and nonclinical samples, in the connectivity between the amygdala and the other regions, in response to the same facial stimuli.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We would like to thank Masatsugu Tsujii and Iori Tani for assisting us in the data collection for the pilot behavioural study. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas, ‘Face perception and recognition’ (#20119004 to T.I.), by a Grant-in-Aid for Scientific Research (S#21220005 to N.S.) from the Japan Society for the Promotion of Science, and by a Grant-in-Aid for Academic Frontier Project for Private Universities and ‘Development of biomarker candidates for social behaviour’ carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- Adolphs R. What does the amygdala contribute to social cognition? Annals of The New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amting JM, Greening SG, Mitchell DG. Multiple mechanisms of consciousness: the neural correlates of emotional awareness. Journal of Neuroscience. 2010;30:10039–47. doi: 10.1523/JNEUROSCI.6434-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff J, Barclay AM, Stevenson LA. The recognition of threatening facial stimuli. Journal of Personality and Social Psychology. 1988;54(4):647–55. doi: 10.1037//0022-3514.54.4.647. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Reviews. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baas D, van't Wout M, Aleman A, Kahn RS. Social judgment in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychological Medicine. 2007;38:747–54. doi: 10.1017/S0033291707001729. [DOI] [PubMed] [Google Scholar]
- Ball T, Derix J, Wentlandt J, et al. Anatomical specificity of functional amygdala imaging of responses to stimuli with positive and negative emotional valence. Journal of Neuroscience Methods. 2009;180(1):57–70. doi: 10.1016/j.jneumeth.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety; controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7(2):184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bracha HS, Ralston TC, Matsukawa JM, Williams AE, Bracha AS. Does "fight or flight" need updating? Psychosomatics. 2004;45(5):448–9. doi: 10.1176/appi.psy.45.5.448. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49(1):963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–8. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Dumas R, Testé B. The influence of criminal facial stereotypes on juridical judgments. Swiss Journal of Psychology. 2006;65(4):237–44. [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17:2400–6. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Habel U, Windischberger C, Derntl B, et al. Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45:2369–77. doi: 10.1016/j.neuropsychologia.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56(3):881–9. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Research Bulletin. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Li K, Guo L, Nie J, Li G, Liu T. Review of methods for functional brain connectivity detection using fMRI. Computerized Medical Imaging and Graphics. 2009;33(2):131–9. doi: 10.1016/j.compmedimag.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Lighthall NR, Nga L, Gorlick MA. Sex differences in how stress affects brain activity during face viewing. NueroReport. 2010;21:933–7. doi: 10.1097/WNR.0b013e32833ddd92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, and Computers. 2004;36:630–3. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Ruffman T, Fujita C, Tsujii M. How well can young people with Asperger's disorder recognize threat and learn about affect in faces? A pilot study. Research in Autism Spectrum Disorders. 2010;4:242–8. [Google Scholar]
- Morey RA, Dolcos F, Petty CM, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatric Research. 2009;43(8):809–17. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman DC. Re-valuing the amygdala. Current Opinion in Neurobiology. 2010;20:221–30. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. American Journal of Psychiatry. 2010;167(11):1373–80. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine. 2009;39(7):1141–52. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Fink GR, Herpertz-Dahlmann B, Willmes K, Konrad K. Developmental changes in neural activation and psychophysiological interaction patterns of brain regions associated with interference control and time perception. Neuroimage. 2008;43(2):399–409. doi: 10.1016/j.neuroimage.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Charting the typical and atypical development of the social brain. Developmental Psychopathology. 2008;20(4):1081–102. doi: 10.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11(11):773–782. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S, ten Brinke L. Dangerous decisions: a theoretical framework for understanding how judges assess credibility in the courtroom. Legal and Criminological Psychology. 2009;14:119–134. [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht EJ, Shimojo S, Camerer CF, Battaglia P, Nakayama K. Human wagering behavior depends on opponents' faces. PLoS ONE. 2010;5(7):e11663. doi: 10.1371/journal.pone.0011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigall H, Ostrove N. Beautiful but dangerous: effects of offender attractiveness and nature of the crime on juridic judgment. Journal of Personality and Social Psychology. 1975;31(3):410–4. [Google Scholar]
- Staugaard SR. Threatening faces and social anxiety: a literature review. Clinical Psychology Review. 2010;30:669–90. doi: 10.1016/j.cpr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H, Miltner WHR. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–8. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience. 2008;3:119–127. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watters AJ, Williams LM. Negative biases and risk for depression; integrating self-report and emotion task markers. Depression and Anxiety. 2011;28(8):703–18. doi: 10.1002/da.20854. [DOI] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, Mattingley JB. Stimulus-driven and strategic neural responses to fearful and happy facial expressions in humans. European Journal of Neuroscience. 2008;27:3074–82. doi: 10.1111/j.1460-9568.2008.06264.x. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.