Abstract
Empathic decision-making involves making choices on behalf of others in order to maximize their well-being. Examples include the choices that parents make for their children, as well as the decisions of a politician trying to make good choices on behalf of his constituency. We investigated the neurobiological and computational basis of empathic choice using a human fMRI task in which subjects purchased DVDs for themselves with their own money, or DVDs for others with the other's money. We found that empathic choices engage the same regions of ventromedial prefrontal cortex that are known to compute stimulus values, and that these value signals were modulated by activity from a region of inferior parietal lobule (IPL) known to play a critical role in social processes such as empathy. We also found that the stimulus value signals used to make empathic choices were computed using a mixture of self-simulation and other-simulation processes, and that activity in IPL encoded a variable measuring the distance between the other's and self preferences, which provides a hint for how the mixture of self- and other-simulation might be implemented.
Keywords: neuroeconomics, empathy, valuation, decision making, vmPFC, IPL
INTRODUCTION
Humans make different types of decisions. Self-oriented decisions mostly affect ourselves and are guided by the goal of maximizing our own well-being. Examples include what to have for lunch or which clothing to purchase. Pro-social decisions involve trade-offs between our own well-being and the well-being of others. Examples include a donation to charity and purchasing a gift for a friend. Empathic decisions entail decisions made on behalf of other people, with the goal of choosing what is best for them, and without having to sacrifice our own resources. Examples include the myriad of choices that parents make for their children, the decisions of a politician trying to make good choices on behalf of his or her constituents, and economic agents (e.g. in real estate or entertainment) who strive to commit their clients’ time and money to activities the clients prefer. Although a substantial amount of progress has been made in understanding self-oriented (Rangel et al., 2008; Rushworth and Behrens, 2008; Kable and Glimcher, 2009; Rangel and Hare, 2010) and pro-social decisions (Fehr and Camerer, 2007), much less is known about the computational and neurobiological basis of empathic choice.
From a psychological and neurobiological perspective, empathic choice is particularly interesting because it is likely to involve the interaction of two different types of processes: those involved in basic decision-making, such as value computation and comparison, and those involved in social processing, such as empathy and mentalizing.
With respect to basic decision-making, a large body of work has begun to characterize in detail the computations involved in self-oriented decisions. For example, human neuroimaging studies have shown that activity in areas such as ventromedial prefrontal cortex (vmPFC) correlates with the value of stimuli at the time of choice (Kable and Glimcher, 2007; Plassmann et al., 2007, 2010; Tom et al., 2007; Valentin et al., 2007; Hare et al., 2008, 2009; Rolls et al., 2008; Boorman et al., 2009; FitzGerald et al., 2009; Litt et al., 2011). Similar results have been found in non-human primate electrophysiology studies (Wallis and Miller, 2003; Padoa-Schioppa and Assad, 2006, 2008; Kennerley et al., 2009; Kennerley and Wallis, 2009; Padoa-Schioppa, 2009). Activity in vmPFC has also been associated with the computation of stimulus values (SVs) during pro-social choices (Moll et al., 2006; Harbaugh et al., 2007; Tankersley et al., 2007; Hsu et al., 2008; Hare et al., 2010; Tricomi et al., 2010). Importantly, however, none of these previous studies include instances of empathic choice.
With respect to social processing, a separate body of work has begun to characterize the computations involved in social cognition. Empathy is normally defined as the ability to appreciate the emotions and feelings of others as separate from those of the self (Decety, 2010; Shamay-Tsoory, 2011). A significant number of studies, using a wide variety of paradigms, have shown that areas such as the inferior frontal gyrus (IFG), inferior parietal lobule (IPL) and dorsomedial prefrontal cortex (dmPFC) play a critical role in empathy computations (Mitchell, 2009; Zaki et al., 2009; Decety, 2010; Shamay-Tsoory, 2011). Importantly, the previous literature on empathy has also not covered the case of empathic choice, as the tasks used involved the observation and evaluation of other's emotional states, but not decision-making on their behalf. A related literature has studied the neurobiological basis of mentalizing [often called theory of mind (ToM)] and has found that areas such as medial prefrontal cortex (mPFC) and the temporo-parietal junction (TPJ) play a critical role in this process (Saxe and Kanswisher, 2003; Mitchell et al., 2005; Saxe and Wexler, 2005; Mitchell et al., 2006; Saxe, 2006; Mitchell, 2009).
Here, we present the results of a simple human functional magnetic resonance imaging study (fMRI) in which subjects made otherwise identical decisions (purchasing DVDs) in either a self-oriented context, by buying them for themselves with their own funds, or in an empathic context, by buying them for an unknown third party, with this party's funds. This allowed us to investigate two basic questions regarding empathic decision-making.
First, is the same basic neural circuitry involved in making self-oriented and empathic decisions? And, if not, what are the critical differences? Based on the decision and social neuroscience literatures discussed above, we hypothesized that empathic decisions engage the basic elements of the decision-making system, such as the computation of SV signals in vmPFC, but that their computation during empathic choice requires the activation of regions, such as IPL and TPJ, that are known to play a critical role in empathy and mentalizing.
Second, what are the computational properties of the SVs used to make empathic choices? In particular, we were interested in disentangling the extent to which subjects computed the empathic SV signals using self-simulation, other-simulation or other-learning. Under self-simulation, subjects infer the other's DVD values by computing their own value for them. Under other-simulation, subjects use some model of the other individual to infer his value for the DVDs but make no use of their own preferences for them. Under other-learning, subjects learn to compute the other's DVD values by repeatedly observing their behavior. Conceptually, there is an important difference between the last two approaches: other-simulation requires forming a social model of the other person (e.g. gender, nationality, age, etc.), whereas under other-learning, the other's preferences are learned simply by repeated observation and extrapolation. Thus, the other-simulation approach makes heavy use of social models and information, whereas other-learning involves much more basic forms of learning.
METHODS
Subjects
Thirty-two normal-weight, American or Canadian, male subjects participated in the experiment (age: mean = 22.8, s.d. = 3.9). All subjects were right-handed, healthy, had normal or corrected-to-normal vision, had no history of neurological or metabolic illnesses and were not taking any medication that interferes with the performance of fMRI. All subjects were informed about the experiment and gave written consent before participating.
Stimuli
Subjects viewed 100 high-resolution color images of DVD covers of popular films from the last 15 years. They included comedies (e.g. Austin Powers), action films (e.g. Swordfish), dramas (e.g. Magnolia) and thrillers (e.g. Panic Room).
Task
There were two types of subjects in the experiment: one passive subject and 32 active subjects. The role of the passive subject was to be the recipient of the active subjects' decisions.
Active subjects made decisions inside the scanner in two types of trials performed on different days (average lag = 90 days). On the first visit, they participated in an empathic choice task in which they made purchase decisions on behalf of the passive subject (Figure 1A). They were given a budget of $10 that belonged to the passive subject (any unspent funds were returned to him) and were given a summary sheet containing a photograph and some biographic information about the passive subject (see SOMs for detailed instructions). They were then shown images of 100 different DVDs and had to make a decision regarding how much to bid for each one of them on behalf of the subject. Bids were made using a 6-point scale of $0, 2, 4, 6, 8 and 10. After every bid, subjects received feedback equal to the amount by which they had overbid or underbid relative to the passive subject's values (feedback = active subject's bid – passive subject's bid). Active subjects did not receive any form of compensation for making accurate bids. Instead, the instructions simply told them to try to maximize the passive subject's well-being. The mapping of bids to response buttons was counterbalanced across subjects.
Fig. 1.
Behavioral task for (A) empathic choice trials and (B) self-oriented choice trials.
At the conclusion of the experiment, one of the 100 trials was randomly selected and implemented using a Becker–DeGroot–Marschak (BDM) auction. The rules of the auction are as follows. Let b denote the bid made by the subject for a particular item. After the bid is made, a random number n is drawn from a known distribution (in our case, each integer dollar value from $0 to $10 was chosen with equal probability). If b ≥ n, the subject received the DVD and paid a price equal to n. Otherwise, if b < n, the subject did not get the DVD and did not pay anything. The optimal strategy in this type of auction is for the buyer to bid exactly how much he is willing to pay for the item being sold (Becker et al., 1964). The active subjects knew that the outcome of the auction would be implemented and that the person for whom they were bidding would receive any DVD purchased plus any remaining cash from the $10. Note that since only one trial was selected to count, the subjects did not have to worry about spreading the $10 dollars across the different films and could treat every decision as if it were the only one. No deception was used in the experiment. The passive subject actually received DVDs when the subject's decision led to a purchase of the DVD.
During the second day of scanning, active subjects participated in the self-oriented version of the task (Figure 1B). In this case, they performed a similar task, except that now they made purchase decisions for themselves out of a $10 cash endowment that belonged to them. A randomly selected trial was again chosen, and the associated decision implemented at the conclusion of the two sessions. At the end of the second session, subjects were asked to fill out a questionnaire detailing which DVDs they owned or had seen. In order to control for any potential order effects on bidding, the DVDs were shown in the same order as in the first experimental visit.
The passive subject played only the bid-for-self task outside the scanner. His responses were used to compute the feedback signals for the active subjects.
About task order
Given the difficulty in guessing another's film preferences, we were concerned that subjects would exhibit an artificial tendency to use their own preferences to make the purchase decisions for the other. In order to minimize this concern, we decided not to counterbalance the order of the two tasks and to introduce a long, multi-month lag between them. The results described below suggest that we were successful in avoiding a full self-valuation bias during the empathic decisions. However, this raises the natural concern of order confounds. To address this concern we carried out a companion behavioral experiment (see SOMs for details) in which we directly compared the effect of order on bidding behavior. For each individual, we carried out a linear regression of bid-for-other on bid-for-self and other-bid, separately for self-oriented and empathic choice trials. We found no significant differences across the two order conditions (min P = 0.29, t-test), which rules out the order confound.
fMRI data acquisition and preprocessing
The fMRI data were acquired in a 3.0 Tesla Trio MRI Scanner (Siemens). We acquired gradient echo T2-weighted echoplanar (EPI) images with a BOLD contrast in an oblique orientation of 30° to the anterior commissure-posterior commissure line. We also used an eight-channel phased array head coil. Each volume of images had 48 axial slices of 3 mm thickness and 3 mm in-plane resolution with a TR of 3 s. The imaging data were acquired in four separate sessions; the first two, in which subjects bid on behalf of the passive subject, lasted ∼13 min each. The latter two, in which subjects bid for themselves, lasted ∼9 min each. The first two sessions were performed on a separate date than the latter two sessions. Whole-brain high-resolution T1-weighted structural scans (1 × 1 × 1 mm) were acquired for each subject and coregistered to their mean functional EPI images. The structural scans were averaged across subjects to permit anatomical localization of the functional activations at the group level.
Image analysis was performed using Statistical Parametric Mapping software (SPM5; Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). We preprocessed the data in the following way. First, slice-timing correction centered at the middle T2 scan was applied, followed by realignment to the first volume. We then applied spatial normalization to the standard Montreal Neurological Institute (MNI) EPI template with a resampled voxel size of 3 mm2 and performed spatial smoothing using a Gaussian kernel with full width at half maximum of 8 mm. Intensity normalization and high-pass temporal filtering were also applied to the data.
fMRI data analysis
We estimated several models of the BOLD responses to test the various hypotheses.
GLM 1
This general linear model was designed to identify the similarities and differences between empathic and self-oriented choices. It was estimated in three different steps.
First, we estimated a GLM with AR(1) for each individual subject. The model contained the following regressors: R1—indicator function (equal to 1 when the event occurs and 0 otherwise) for bid-for-other screen; R2—indicator function for bid-for-other screen modulated by bid-for-other; R3—indicator function for bid-for-other screen modulated by the absolute value of the difference between bid-for-self and bid-for-other; R4—indicator function for feedback screen; R5—indicator function for feedback screen modulated by the negative absolute value of the feedback error; R6—indicator function for bid-for-self screen; and R7—indicator function for bid-for-self screen modulated by bid-for-self. All regressors were modeled as box–car functions with a duration equal to the subject's reaction time for that trial, except for regressors 4 and 5, which had a duration of 1 s. These regressors were convolved with a canonical hemodynamic response. The model also included motion parameters and session constants as regressors of no interest. Trials with missed responses were not modeled. Second, we computed contrast statistics for all of the tests of interest for each individual subject. Third, we estimated second-level test statistics by computing one-sample t-tests on the single subject contrast coefficients for each contrast of interest.
For inference purposes, all results are reported at P < 0.05 whole brain corrected at the cluster level (using the algorithm by Thomas Nichols; http://www.sph.umich.edu/∼nichols/JohnsGems5.html). The only exception is activity in the vmPFC for which, due to the strong prior hypotheses, we report activity at P < 0.05 small volume cluster corrected (using an anatomical mask of the vmPFC area that included both sides of the medial orbitofrontal cortex and the rectus gyrus).
GLM 2
This model was very similar to GLM 1, except that activity at decision during empathic choices was modulated by two variables: bid-for-self and bid-for-other orthogonalized with respect to bid-for-self. All omitted details are as in GLM 1.
GLM 3
This model was very similar to GLM 1, except that activity at decision during empathic choices was modulated by two variables: bid-for-other and bid-for-self orthogonalized with respect to bid-for-other. All omitted details are as in GLM 1.
GLM 4
This model was very similar to GLM 1, except that activity at decision during empathic choices was modulated by two variables: bid-for-self and a difference signal (given by bid-for-other MINUS bid-for-self). All omitted details are as in GLM 1.
Psychophysiological interactions model
The goal of this analysis was to identify areas exhibiting differential connectivity with vmPFC during empathic and self-oriented choices. The model was estimated in the following steps.
First, we extracted individual average time-series of BOLD activity within an individually defined region of vmPFC, given by a 4 mm sphere surrounding each individual's peak activation for the contrast ‘R2 MINUS baseline’ in GLM-1 within the anatomical mask of the vmPFC shown in Figure 1C. We removed any variance from this time series associated with the motion regressors. The resulting time courses were deconvolved using standard procedures (Gitelman et al., 2003).
Second, we estimated a whole-brain GLM of BOLD responses with AR(1) and the following regressors: R1—interaction between the vmPFC deconvolved time series and an indicator function for bid-for-other screen; R2—interaction between the vmPFC deconvolved time series and an indicator function for bid-for-self screen; R3—indicator function for bid-for-other screen; R4—indicator function for bid-for-self screen; and R5—the vmPFC deconvolved time series. These regressors were convolved with a canonical hemodynamic response. The model also included motion parameters and session constants as regressors of no interest. Note that Regressor 1 identifies areas exhibiting task-related functional connectivity with the vmPFC seed region during empathic choices. Regressor 2 does the same for self-oriented choices.
Third, we calculated the following single subject contrasts: C1—Regressor 1 vs baseline; C2—Regressor 2 vs baseline; and C3—Regressor 1 vs regressor 2.
Fourth, we conducted a second level analysis by calculating a one-sample t-test on the single subject contrast coefficients.
RESULTS
First, we discuss tests designed to investigate if the same basic neural circuitry is involved in making self-oriented and empathic decisions, and to characterize the key differences.
Longer RTs in empathic choice
Mean reaction times when bidding for self were about 500 ms faster than when bidding for other (self: mean = 2.16 s, s.d. = 0.52; other: mean = 2.67 s, s.d. = 0.47; paired t-test P < 0.05). This is consistent with the hypothesis that empathic decisions involve the deployment of extra processes.
Common value coding in vmPFC
We hypothesized that a common area of vmPFC is involved in computing the SVs assigned to DVDs at the time of decision in both the self-oriented and empathic trials. We focused our attention on vmPFC because a large number of studies have found SV signals in this area (see the ‘Introduction’ section). The bids-for-self provide a trial-by-trial measure of the SVs computed in self-oriented trials, whereas the bids-for-other provide a similar measure for empathic decisions.
We tested this hypothesis by estimating a general linear model of BOLD responses (GLM 1) that looked for correlations between the magnitude of the bids placed in each condition and BOLD activity (see the ‘Methods’ section for details). Activity in vmPFC correlated with the bids-for-other during empathic choices (Figure 2A, see Table 1 for a complete list of activations). Activity in the same area of vmPFC also correlated with bids-for-self during self-oriented choices (Figure 2B, see Table 2 for a complete list of activations). A conjunction analysis showed that activity in a common area of vmPFC correlated with SVs in both conditions (Figure 2C), as did activity in areas of the precuneus, middle frontal gyrus and IPL (Supplementary Figure S3).
Fig. 2.
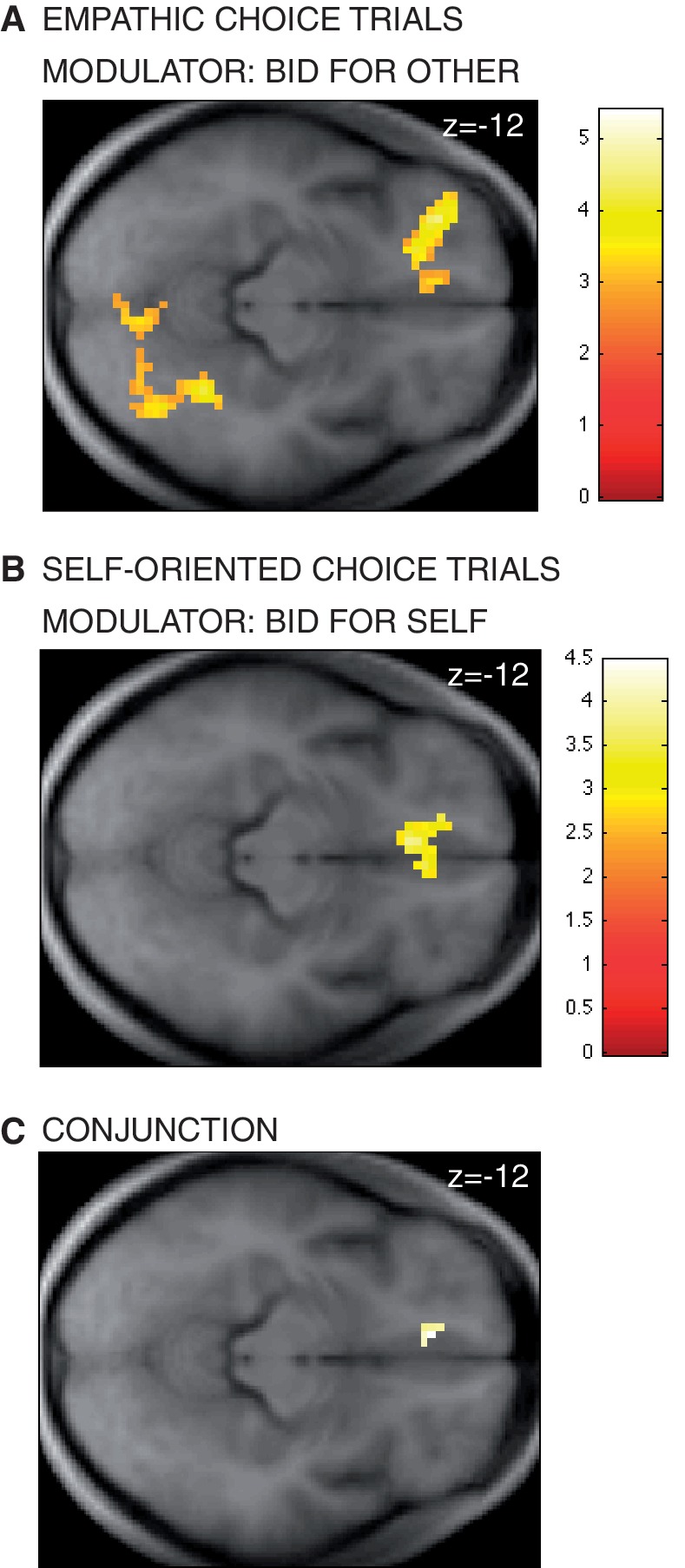
(A) Activity in vmPFC correlated with the bids-for-other during empathic choices (P < 0.05, SVC). (B) Activity in vmPFC also correlated with the bids-for-self during self-oriented choices (P < 0.05, SVC) (C) Conjunction analysis: activity in a common area of vmPFC correlated with the bids placed in both empathic and self-oriented choice trials.
Table 1.
Areas exhibiting a positive correlation with bid-for-other during empathic choice (GLM 1)
| Region | Side | k | T | MNI coordinates |
|---|---|---|---|---|
| x y z | ||||
| Ventral striatum | L/R | 153 | 5.39 | −9 6 −6 |
| Middle frontal gyrus | L | 248 | 4.85 | −27 33 −15 |
| Precuneus/IPL | L | 255 | 4.78 | −39 −57 42 |
| Fusiform/Middle occipital gyrus | R | 632 | 4.52 | 30 −66 0 |
| Posterior cingulate | L | 240 | 4.5 | −6 −42 15 |
| vmPFC* | L | 21 | 3.57 | −9 42 −15 |
Height threshold: T = 2.74, P < 0.05, whole-brain cluster corrected.
Extent threshold: k = 109 voxels, P < 0.005.
*Survives small volume correction at P < 0.05.
Table 2.
Areas exhibiting a positive correlation with bid-for-self during self-oriented choice (GLM 1)
| Region | Side | k | T | MNI coordinates |
|---|---|---|---|---|
| x y z | ||||
| IPL | L | 295 | 4.47 | −45 −36 39 |
| Middle frontal gyrus | L | 617 | 4.43 | −39 36 12 |
| Precuneus | L | 135 | 4.07 | −39 −72 30 |
| vmPFC* | L/R | 105 | 3.79 | −6 27 −12 |
Height threshold: T = 2.74, P < 0.05, whole-brain cluster corrected.
Extent threshold: k = 105 voxels, P < 0.005.
*Survives small volume correction at P < 0.05.
We also looked for differences in the strength of SV coding across the empathic and self-oriented conditions. We carried out this test in two ways. First, using a whole brain analysis and our omnibus threshold, we did not find any regions that exhibited stronger responsivity to bid-for-self during self-oriented choice than to bid-for-other during empathic choices at our omnibus threshold. Second, we carried out an unbiased region-of-interest (ROI) analysis in the area of vmPFC that correlates with SVs in both conditions. A comparison of the average beta values within the ROI for the bid-for-self and bid-for-other regressors revealed no significant differences (P = 0.26, paired t-test).
Differences in the network involved in empathic vs self-oriented choices
We also hypothesized that empathic choice would require the activation of additional regions, such as IPL and TPJ, which are known to be involved in social cognition. We tested this hypothesis in two steps.
First, using GLM 1, we looked for regions that exhibit higher average activity during empathic choices, and areas that exhibit higher average activity during self-oriented choices. A large cluster of regions exhibited stronger activity during empathic choices, including bilateral IPL, bilateral middle frontal gyri, bilateral anterior insula (Supplementary Figure S4A, Table 3). We also found regions exhibiting stronger activity during self-oriented choices, including bilateral supramarginal gyri, middle temporal gyrus, right posterior insula and superior temporal gyrus (Supplementary Figure S4B, Table 3).
Table 3.
Regions exhibiting stronger average (unmodulated) activation in self-oriented vs empathic choice (GLM 1)
| Region | Side | k | T | MNI coordinates |
|---|---|---|---|---|
| x y z | ||||
| Self-oriented > Empathic | ||||
| Inf parietal/Supramarginal gyrus | L | 471 | 12.57 | −51 −54 36 |
| Inf parietal/Supramarginal gyrus | R | 409 | 7.058 | 51 −57 42 |
| Middle temporal gyrus | L | 149 | 5.712 | −63 −33 −9 |
| Middle temporal gyrus | R | 165 | 5.44 | 57 −27 −24 |
| Cingulate gyrus | L | 173 | 5.16 | −9 −18 27 |
| Middle frontal gyrus | R | 218 | 4.714 | 39 12 57 |
| Insula/Superior temporal gyrus | R | 167 | 4.38 | 48 −6 0 |
| Empathic > Self-oriented | ||||
| Middle occipital gyrus/cuneus | L | 11460 | −8.9 | −24 −90 3 |
| Putamen/caudate/thalamus | L | a | −8.63 | −6 9 −3 |
| Middle occipital gyrus/cuneus | R | a | −7.94 | 3 −93 9 |
| Putamen/caudate/thalamus | R | a | −7.2 | 18 −27 3 |
| Inf parietal lobe/Postcentral gyrus | R | a | −7.07 | 36 −27 69 |
| Precentral/middle frontal gyrus | L | a | −7.04 | −18 −75 51 |
| Fusiform/middle temporal gyrus | L | a | −6.51 | −39 −63 −18 |
| Insula/IFG | L | a | −6.23 | −36 33 6 |
| Midbrain | L | a | −6.16 | −18 −24 −3 |
| Precentral/middle frontal gyrus | R | a | −5.45 | 15 −72 54 |
| Midbrain | R | a | −5.36 | 18 −24 3 |
| Inf parietal lobe | L | a | −5.13 | −27 −30 75 |
| Insula/Inf frontal gyrus | R | 180 | −5.04 | 45 21 3 |
Height threshold: T = 2.74, P < 0.05, whole-brain cluster corrected.
Extent threshold: k = 112 voxels, P < 0.005.
aPart of a larger cluster.
Second, we looked for differences in functional connectivity with the vmPFC valuation area between the empathic and self-oriented trials. We did this by estimating a psychophysiological interactions model (PPI) that looks for areas that exhibit increases in functional connectivity at the time of decision separately in self-oriented and empathic trials. The model uses as a seed the area of vmPFC involved in SV coding in both conditions (see ‘Methods’ section for details). We found that activity in bilateral IPL exhibited stronger functional connectivity with vmPFC during empathic choices (Table 4, Figure 3A). In contrast, no regions exhibited stronger functional connectivity with vmPFC during self-oriented choices at our omnibus threshold. Interestingly, the regions of IPL that exhibit stronger functional connectivity with vmPFC overlap with those that exhibit stronger average activity during empathic trials (Figure 3B).
Table 4.
Areas exhibiting positive task related functional connectivity with the vmPFC (PPI analysis)
| Region | Side | k | T | MNI coordinates |
|---|---|---|---|---|
| x y z | ||||
| Self-oriented | ||||
| IFG | R | 164 | 4.1 | 45 9 6 |
| Supramarginal/sup temporal gyrus | R | 169 | 4.3 | 66 −24 36 |
| Inferior parietal lobe | L | 142 | 3.9 | −30 −57 60 |
| Inferior parietal lobe | R | 134 | 4.7 | 45 −39 66 |
| Empathic | ||||
| Middle frontal gyrus | R | 2383 | 6.0 | 45 45 15 |
| Insula/IFG | L | 354 | 4.5 | −36 18 −3 |
| Middle frontal gyrus | L | 493 | 5.3 | −39 33 39 |
| Inferior parietal lobe | L | 2727 | 7.1 | −42 −48 42 |
| Inferior parietal lobe | R | a | 6.9 | 48 −45 54 |
| Empathic > Self-oriented | ||||
| Inferior parietal lobe | L | 5.06 | 145 | −36 −45 42 |
| Inferior parietal lobe | R | 3.92 | 148 | 48 −48 57 |
Height threshold: T = 2.58, P < 0.05, whole-brain cluster corrected.
Extent threshold: k = 102 voxels, P < 0.005.
aPart of a larger cluster.
Fig. 3.
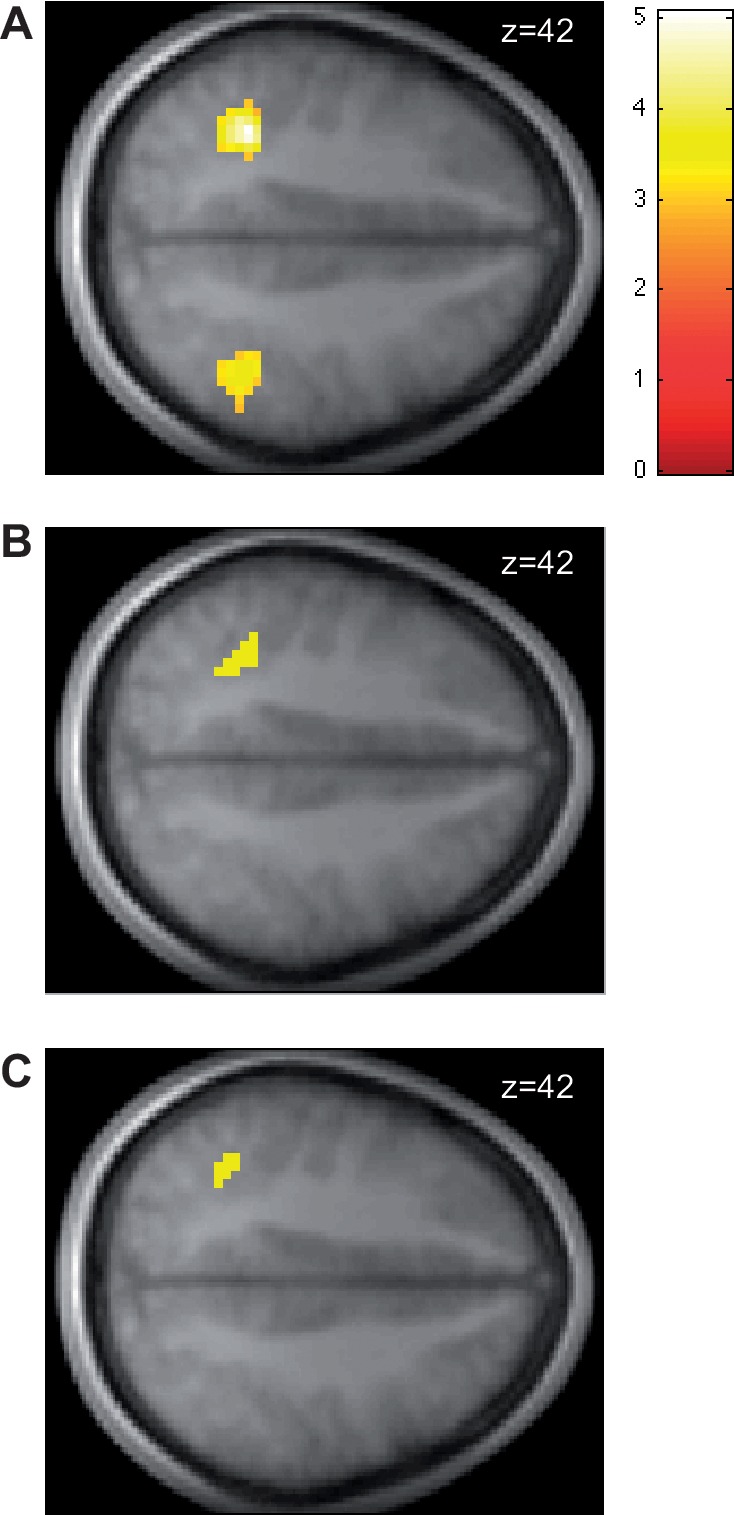
(A) Areas of IPL exhibiting stronger connectivity with the vmPFC valuation region during empathic choices than during self-oriented decisions. (B) Region of IPL exhibiting both stronger functional connectivity with vmPFC and higher average (unmodulated) activity during empathic choices. (C) Region of IPL exhibiting both stronger functional connectivity with vmPFC during empathic choices and a correlation with the difference preference measure. The contrasts are thresholded at P < 0.05, WBC.
Together, these results provide supporting evidence for the hypothesis that empathic choice engages the basic vmPFC valuation system, just as it does in self-oriented choice, but that the computation of these value signals in empathic choice involves the activation of regions of IPL that are known to play a critical role in social cognition.
Next, we investigated the extent to which SV signals are computed using self-simulation, other-simulation, or other-learning, during empathic choices.
No behavioral evidence for other-learning
Under other-learning, the quality of bids-for-other should improve over time. A good measure of the quality of the individual's bids-for-other is given by: correlation(bid-for-other, other-bid) − correlation(bid-for-self, other-bid).
The first term measures the extent to which the subject's bids-for-other correlates with the other's preferences. The second term corrects for the fact that the first term might be artificially large if both individuals tend to like the same movies. The mean quality statistic was 0.06 (s.e. = 0.017, P < 0.0001, t-test). Contrary to the other-learning model, we found no significant difference between the first and second half of trials (P = 0.72, pairwise t-test), which provides evidence against other-learning.
Behavioral bids are consistent with a mixture of self- and other-simulation
A comparison of the differences between the bids that the subjects made for themselves (during self-oriented choice) and those that they made for the other (during empathic choice) provides a behavioral test of the extent to which the SVs were consistent with the self- vs the other-simulation models. The self-simulation model predicts a very high correlation between the bids-for-self and the bids-for-other. In contrast, the other-simulation model predicts a much lower correlation between the two types of bids.
One critical difficulty in carrying out this test is that, regardless of how the bids are computed, they may be correlated because individual preferences are not independent (for example, no one seems to like certain movies). This problem can be circumvented through the following two steps.
First, we estimated a mixed effects linear regression of bid-for-other on two regressors: other-bid and bid-for-self. Importantly, the bid-for-self regressor was orthogonalized with respect to the other-bid. This is important because, then, any variation on bid-for-other that is explained by the bid-for-self regressor cannot be attributed to common preferences. As a result, the relative magnitude of the bid-for-self regressor provides a lower bound on the contribution of self-simulation processes. Both coefficients were statistically significant and of approximately equal magnitude (other-bid: mean = 0.52, s.e. = 0.02, P < 0.0001; bid-for-self: mean = 0.55, s.e. = 0.03, P < 0.0001; t-tests).
Second, we estimated a related regression in which the independent variable was still bid-for-other, but the right-hand-side regressors were bid-for-self and other-bid orthogonalized with respect to bid-for-self. This alternative orthogonalization is useful because now the relative magnitude of the other-bid regression coefficient provides a lower bound on the contribution of other-simulation processes. Both coefficients were again statistically significant (other-bid: mean = 0.24, s.e. = 0.018, P < 0.0001; bid-for-self: mean = 0.81, s.e. = 0.03, P < 0.0001; t-tests).
Together with the previous result, the two regressions suggest that subjects computed SVs during empathic trials using a mixture of self-simulation and other-simulation processes. The relative magnitude of the regressors also suggests that the self-simulation component played a stronger role in our task.
Activity in vmPFC is also consistent with a mixture of self- and other-simulation
We also investigated the extent to which the SV signals computed during empathic choices were consistent with self- or other-simulation. We did this by estimating two new GLMs of BOLD responses. The key difference with the previous models is that activity during empathic choices was now modulated by two variables: bid-for-self and bid-for-other. Importantly, to deal with the problem of preference correlation discussed above, in GLM 2 the bid-for-other was orthogonalized with respect to the bid-for-self, and in GLM 3 the opposite orthogonalization was carried out.
We computed the average regression coefficients for bid-for-self and bid-for-other in both models within the vmPFC region that correlates with SVs in both empathic and self-oriented choice. We found that all regressors were significantly positive (P < 0.0001 in all cases, t-test). For completeness, we carried out similar ROI tests in all of the areas that correlated with SVs in either empathic or self-oriented choices and found similar results. These results provide further neurobiological evidence that SVs during empathic choice are computed using a mixture of the self- and other-simulation processes.
We also carried out an additional post hoc analysis designed to explore the computational role that IPL might play in empathic choice. Based on the results described above, as well as the literature discussed in the ‘Introduction’ section, we speculated that IPL might contribute to the computation of SVs by measuring the extent to which the other's preferences differ from the subject's own preferences. In our task, this signal can be measured by difference = bid-for-other − bid-for-self.
This signal is computationally useful because it would allow subjects to compute their estimate of the value that the other places on the DVDs by computing their own value for it, and then carrying out the additive (and signed) adjustment given by the difference signal.
To test this hypothesis, we estimated a new GLM 4 in which activity during empathic choices is modulated by bid-for-self and the difference signal. Consistent with our post hoc hypothesis, activity in IPL and middle frontal gyrus was significantly correlated with the difference regressor (Table 5). Interestingly, the area of IPL identified in this model overlaps with those exhibiting increased functional connectivity with the vmPFC valuation areas during empathic choices (Figure 3C).
Table 5.
Areas exhibiting a positive correlation with the difference signal during empathic choice (GLM 4)
| Region | Side | k | T | MNI coordinates |
|---|---|---|---|---|
| x y z | ||||
| Inferior parietal lobe/precuneus | L | 242 | 5.22 | −39 −54 42 |
| Middle frontal gyrus | L | 121 | 4.47 | −39 45 −6 |
Height threshold: T = 2.74, P < 0.05, whole-brain cluster corrected.
Extent threshold: k = 112 voxels, P < 0.005.
DISCUSSION
The results presented here provide the following insights about the computational and neurobiological basis of empathic choice. First, empathic choices engage the vmPFC valuation system used in self-oriented decisions, and these value signals seem to be modulated by activity in regions of IPL known to play a critical role in social processes such as empathy. Second, the SVs used to make empathic choices are computed using a mixture of self-simulation and other-simulation. Third, during empathic choices, activity in the IPL encodes a variable measuring the distance between the other's and own preferences. This variable could be used to compute the value of DVDs for other starting from one's own value, which provides a hint for how the mixture of self- and other-simulation is implemented.
The results have implications for various areas of the neural and social sciences. The results extend our growing knowledge of how the brain makes decisions to the case of empathic choice, which had not been previously studied. The results show that empathic decisions involve the combination of two types of processes: the basic valuation circuitry involved in self-oriented decisions and social processes such as empathy. In particular, in contrast to the case of self-oriented choice, in empathic choice, SVs in vmPFC seem to be modulated by a signal from IPL that reflects the difference in preferences between self and other. This result parallels a recent finding in an fMRI study of charitable donations (Hare et al., 2010), which found that the value signals in vmPFC were modulated by an area of posterior superior temporal cortex commonly associated with mentalizing.
The results also extend our understanding of social cognition in several ways. First, they show that the same set of areas that have been shown to play a role in ‘passive’ empathy tasks are also at work during empathic choices. Second, they advance our understanding of the precise computations carried out by IPL (i.e. a measure of the difference between the other's and the self’s preferences) as well as how they affect decision-making (i.e. by modulating activity in the vmPFC valuation circuitry). Third, they advance our understanding of the role of mPFC in social cognition, which has been previously implicated in person-related and not object-related knowledge (Mitchell et al., 2002, 2006; Mitchell, 2009). Our results show that, during empathic choice, mPFC is involved in the computation of SVs. Importantly, the area of mPFC characterized here is significantly more ventral than those identified in previous studies, none of which involved actual empathic choices. Fourth, the statistical influence of own-bids on bids-for-others replicates the ‘false consensus effect’ from social psychology (Ross et al., 1977; Marks and Miller, 1987). The evidence that IPL activity correlates with the difference in the two bids suggests a candidate region for explaining differences in the strength of false consensus across people or context.
The results also have potential practical applications in psychology and economics. They suggest that the ability to make sound empathic decisions might depend on the ability to compute value signals in vmPFC that give sufficient weight to the differences between others and ourselves. It follows that deficits in empathy and general social cognition might impair the ability to make sound empathic decisions, which could interfere with everyday social interaction. Additional evidence for the role of vmPFC in these types of processes comes from lesion studies (Krajbich et al., 2009).
We were surprised to find no other-learning process at work during empathic choice. It is possible that this is due to specific features of the current task that might not generalize to other settings. In particular, the DVD stimuli used here are highly multi-dimensional and complex, which makes it hard to generalize across very different types of films. Thus, we cannot rule out the possibility that other-learning processes might be at work in settings with a simpler stimulus set.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
REFERENCES
- Becker G, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behavioral Science. 1964;9:226–32. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, et al. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62(5):733–43. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Decety J. To what extent is the experience of empathy mediated by shared neural circuits? Emotion Review. 2010;2(3):204–7. [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Sciences. 2007;11(10):419–27. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- FitzGerald THB, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. Journal of Neuroscience. 2009;29(26):8388–95. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage. 2003;19(1):200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–5. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoeple DA, et al. Value computations in VMPFC during charitable decision-making incorporate input from regions involved in social cognition. Journal of Neuroscience. 2010;30:583–90. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vMPFC valuation system. Science. 2009;324:646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, et al. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28(22):5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320(5879):1092–5. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63(6):733–45. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. Journal of Cognitive Neuroscience. 2009;21(6):1162–78. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. European Journal of Neuroscience. 2009;29(10):2061–73. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, et al. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. Journal of Neuroscience. 2009;29(7):2188–92. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision making. Cerebral Cortex. 2011;21:95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Marks G, Miller N. Ten years of research on the false-consensus effect: an empirical and theoretical review. Psychological Bulletin. 1987;102:72–90. [Google Scholar]
- Mitchell JP. Inferences about mental states. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2009;364(1521):1309–16. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, et al. Human fronto-mesolimibic networks guide decisions about charitable donations. PNAS. 2006;103(42):15623–8. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. Journal of Neuroscience. 2009;29(44):14004–14. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–6. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nature Neuroscience. 2008;11(1):95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. Journal of Neuroscience. 2007;27(37):9984–8. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Aversive goal values are negatively encoded in the medial orbitofrontal cortex at the time of decision making. Journal of Neuroscience. 2010;30:10799–808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer CF, Montague PR. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience. 2008;9(7):545–56. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Hare TA. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010;20(2):262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Rolls ET, McCabe C, Redoute J. Expected value, reward outcome, and temporal difference error representations in a probabilistic decision task. Cerebral Cortex. 2008;18:652–63. doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- Ross L, Greene D, House P. The false consensus effect: An egocentric bias in social perception and attribution processes. Journal of Experimental Social Psychology. 1977;13(3):279–301. [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11(4):389–97. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanswisher N. People thinking about thinking people: the role of the temporo-parietal junction in theory of mind. NeuroImage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43(10):1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. The Neuroscientist : A Review Journal Bringing Neurobiology Neurology and Psychiatry. 2011;17(1):18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Tankersley D, Stowe CJ, Huettel SA. Altruism is associate with an increased neural response to agency. Nature Neuroscience. 2007;10(2):150–1. doi: 10.1038/nn1833. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–18. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Rangel A, Camerer CF, O'Doherty J. Neural evidence for inequality-averse social preferences. Nature. 2010;463(7284):1089–91. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O'Doherty J. Determining the neural substrates of goal-directed learning in the human brain. Journal of Neuroscience. 2007;27(15):4019–26. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18(7):2069–81. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11382–7. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



