Abstract
Recent evidence indicates that a sexually dimorphic feature of humans, the facial width-to-height ratio (FWHR), is positively correlated with reactive aggression, particularly in men. Also, predictions about the aggressive tendencies of others faithfully map onto FWHR in the absence of explicit awareness of this metric. Here, we provide the first evidence that amygdala reactivity to social signals of interpersonal challenge may underlie the link between aggression and the FWHR. Specifically, amygdala reactivity to angry faces was positively correlated with aggression, but only among men with relatively large FWHRs. The patterns of association were specific to angry facial expressions and unique to men. These links may reflect the common influence of pubertal testosterone on craniofacial growth and development of neural circuitry underlying aggression. Amygdala reactivity may also represent a plausible pathway through which FWHR may have evolved to represent an honest indicator of conspecific threat, namely by reflecting the responsiveness of neural circuitry mediating aggressive behavior.
Keywords: aggression, facial width-to-height ratio, amygdala reactivity, fMRI
INTRODUCTION
Interpersonal aggression is a major societal concern accounting for approximately $37 billion in medical costs and loss of productivity (Corso et al., 2007). Although considerable effort and progress has been made in understanding extreme forms of aggression such as intermittent explosive disorder and psychopathy (Blair, 2010; Coccaro et al., 2011), the majority of aggression, and that having the greatest negative impact on health and well-being, is expressed more variably within the general population. Thus, identifying basic mechanisms underlying normal variability in aggression is critical for advancing programs aimed at curtailing and preventing such destructive behavior.
Animal models suggest that aggression is largely supported by a neural circuitry comprising the periaqueductal gray matter (PAG), hypothalamus and amygdala, which triggers activity in the other two regions in response to provocation (Siegel et al., 2007). Consistent with this research, human neuroimaging studies have found that individuals at risk for engaging in provoked or reactive aggression have relatively increased amygdala reactivity to angry facial expressions (Coccaro et al., 2007; Beaver et al., 2008; Manuck et al., 2010). Collectively, these findings suggest that the amygdala plays a key role in mediating aggression and that relatively increased amygdala reactivity to social signals of interpersonal threat may represent a neural correlate of one's propensity for reactive aggression (Carré et al., 2011).
A parallel line of research has revealed that a body size independent sexually dimorphic feature of humans (Weston et al., 2007), the facial width-to-height ratio (FWHR), is positively correlated with reactive aggression (Carré and McCormick, 2008), non-reciprocation of trust (Stirrat and Perrett, 2010) and cheating behavior (Haselhuhn and Wong, 2011) particularly in men. Moreover, predictions about the aggressive tendencies of others faithfully map onto the FWHR in the absence of explicit awareness of this metric (Carré et al., 2009, 2010). This suggests that the FWHR, like facial expressions of emotion, may represent an honest signal that may serve to guide adaptive behavioral responses to conspecific challenge (Darwin, 1872). Given that the FWHR becomes sexually dimorphic around puberty (Weston et al., 2007), we have speculated that its association with aggression may reflect the common influence of pubertal testosterone on craniofacial growth and the development of neural circuitry underlying aggression (Carré and McCormick, 2008). Indeed, there is some evidence that testosterone directly modulates craniofacial growth in humans (Verdonck et al., 1999), and animal models indicate that pubertal testosterone shapes the development of neural structures implicated in aggressive behavior (Sisk and Zehr, 2005; Ahmed et al., 2008).
In the current study, we tested the above proposal using functional magnetic resonance imaging in a sample of healthy young adults. Given previous associations between amygdala reactivity to angry faces and aggressive behavior (Coccaro et al., 2007; Beaver et al., 2008), we focused our analyses on this structure. In accordance with this work, we predicted that amygdala reactivity to signals of interpersonal provocation, namely angry facial expressions, would be specifically linked to aggressive behavior. Moreover, given the previously observed association between FWHR and aggression (Carré and McCormick, 2008), we predicted that the association between amygdala reactivity to threat and aggression would be particularly pronounced among individuals with large FWHRs. Finally, previous studies indicate that correlations between FWHR and behavior are found in men but not women (Carré and McCormick, 2008; Stirrat and Perrett, 2010; Haselhuhn and Wong, 2011). Thus, we predicted that associations between FWHR, brain activation and variation in self-reported aggression would be observed only in men.
METHOD
Participants
Sixty-four participants (28 men, 19.39 ± 1.35 years old) were recruited from the Duke Neurogenetic Study (DNS), an ongoing protocol investigating neurogenetic pathways of variation in human behavior. As assessed through clinical interviews, all participants were in good general health, free of current or past psychopathology and were not taking psychotropic medication. Informed consent to participate in this study was provided according to Duke University guidelines.
Measures
FWHR
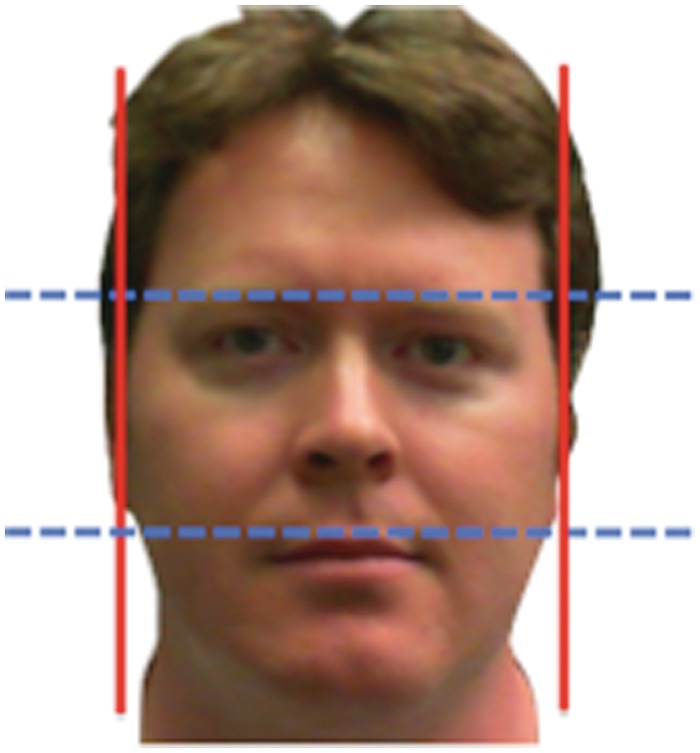
In accordance with previous work (Weston et al., 2007; Carré and McCormick, 2008), the FWHR (Figure 1) was determined by dividing the distance between the left and right zygion (width) by that between the brow and upper lip (height). NIH IMAGEJ software was used to measure the FWHR of digitized images. Inter-rater (J.M.C. and K.R.M.) reliabilities were high for all measures (width: r = 0.99; height: r = 0.99; ratio: r = 0.95).
Fig. 1.
The FWHR was determined by dividing the distance between the left and right zygion (width, vertical lines) by that between the brow and upper lip (height, horizontal lines).
Self-reported physical aggression
The Buss-Perry Aggression Questionnaire (BPAQ) was used to assess variation in aggressive behavior (Buss and Perry, 1992). Given that the FWHR has been linked to overt physical aggression, we focused our analyses on the physical aggression scale of the BPAQ. Reliability of this scale in our sample was good (Cronbach's α = 0.83).
Amygdala reactivity paradigm
Our fMRI challenge paradigm has been used extensively to elicit a robust and replicable amygdala response across an array of experimental protocols and sample populations (Hariri et al., 2002, 2005; Fisher et al., 2006; 2009; Zhou et al., 2008). In the paradigm, there are four blocks of a perceptual face-matching task interleaved with five blocks of a sensorimotor control task. During face-matching blocks, participants view a trio of faces and select one of two faces (on the bottom) identical to a target face (on the top). Each face-matching block consists of six different trios, balanced for gender, all of which were derived from a standard set of pictures of facial affect (Ekman and Friesen, 1976). Thus, in each block, participants see 18 faces (six trials × three faces of the same expression). The DNS version of this paradigm consists of one block each of fearful, angry, surprised and neutral facial expressions presented in a pseudorandom order across participants. During the sensorimotor control blocks, participants view a trio of simple geometric shapes (circles and vertical and horizontal ellipses) and select one of two shapes (bottom) that are identical to a target shape (top). Each sensorimotor control block consists of six different shape trios. All blocks are preceded by a brief instruction (‘Match Faces’ or ‘Match Shapes’) that lasts 2 s. In the task blocks, each of the six face trios is presented for 4 s with a variable inter-stimulus interval (ISI) of 2–6 s (mean = 4 s), for a total block length of 48 s. A variable ISI is used to minimize expectancy effects and resulting habituation and maximize amygdala reactivity throughout the paradigm. In the control blocks, each of the six shape trios is presented for 4 s with a fixed ISI of 2 s, for a total block length of 36 s. Total task time is 390 s.
BOLD fMRI data acquisition
Each participant was scanned using a research dedicated GE MR750 3T scanner at the Duke-UNC Brain Imaging and Analysis Center (BIAC). This scanner is equipped with high-power, high-duty cycle 50-mT/m gradients at 200 T/m/s slew rate and an eight-channel head coil for parallel imaging at high bandwidth up to 1 MHz. A semi-automated high-order shimming program was used to ensure global field homogeneity. A series of 34 interleaved axial functional slices aligned with the anterior commissure–posterior commissure (AC–PC) plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifact (TR/TE/flip angle = 2000 ms/30 ms/60; FOV = 240 mm; 3.75 × 3.75 × 4 mm voxels; interslice skip = 0). Four initial RF excitations were performed (and discarded) to achieve steady-state equilibrium. To allow for spatial registration of each participant's data to a standard coordinate system, high-resolution three-dimensional structural images were acquired in 34 axial slices co-planar with the functional scans (TR/TE/flip angle = 7.7 s/3.0 ms/12; voxel size = 0.9 × 0.9 × 4 mm; FOV = 240 mm, interslice skip = 0).
BOLD fMRI data preprocessing
Preprocessing of all BOLD fMRI data was conducted using SPM8 (www.fil.ion.ucl.ac.uk/spm). Images for each participant were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model (final resolution of functional images = 2 mm isotropic voxels) and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter set at 6-mm full-width at half-maximum. Voxelwise signal intensities were ratio-normalized to the whole-brain global mean.
After preprocessing, linear contrasts using canonical hemodynamic response functions were used to estimate expression-specific (e.g. Angry > Shapes) BOLD contrast images for each participant. These individual contrast images (i.e. weighted sum of the β images) were then used in second-level random-effects models to determine mean expression-specific amygdala reactivity using one-sample t-tests with a voxel-level statistical threshold of P < 0.05, FWE corrected for multiple comparisons across the entire search volume. Contrast estimates were then extracted from functional clusters exhibiting a main effect of task using the above threshold within anatomically defined amygdala regions of interest (ROIs). The bilateral amygdala ROIs were derived from the MNI-based automatic anatomic labeling (aal) atlas in the WFU PickAtlas v. 2.4 (Maldjian et al., 2003).
Analytical strategy
To facilitate regression analyses, BOLD parameter estimates from amygdala clusters exhibiting main effects of task (e.g. angry faces > shapes, fearful faces > shapes, surprise faces > shapes, neutral faces > shapes, angry faces > neutral faces, fearful faces > neutral faces, surprise faces > neutral faces) were extracted using the VOI tool in SPM8. For each expression-specific functional cluster, BOLD parameter estimates were extracted from the peak activation voxel. In addition to producing the necessary values for our regression models, extracting parameter estimates from functional clusters activated by our fMRI paradigm rather than clusters specifically correlated with our independent variables of interest precludes the possibility of any correlation coefficient inflation that may result when an explanatory covariate is used to select a region of interest (Viviani, 2010). We have successfully used this more conservative analytic strategy in recent studies (Hyde et al., 2011; Carré et al., in press).
Hierarchical multiple regression analyses were computed to examine associations between FWHR, expression-specific amygdala reactivity, gender and physical aggression scores. For all analyses, physical aggression was used as the dependent variable. FWHR, amygdala reactivity values and gender were entered as predictors on Step 1 of the model; all two-way interactions were entered on Step 2 of the model; and all three-way interactions were entered on Step 3 of the model.
RESULTS
Gender differences in FWHR and physical aggression
Preliminary analyses revealed one male participant with a FWHR ( = 2.35) > 3.5 s.d.'s from the mean. This participant was removed from all subsequent analyses. Although not significantly different (t61 = 1.69, P = 0.097), men had larger FWHRs (M = 1.81, s.d. = 0.12) compared to women (M = 1.75, s.d. = 0.12). Men scored significantly higher (t61 = 2.97, P = 0.004) on the physical aggression scale of the BPAQ (M = 18.7, s.d. = 5.46) compared to women (M = 14.92, s.d. = 4.65).
Amygdala reactivity, FWHR and physical aggression
Angry faces > shapes contrast
Three-way interactions emerged indicating that the relationship between amygdala reactivity and physical aggression was moderated by FWHR and gender (gender × right amygdala reactivity × FWHR, R2change = 5.9%, P = 0.03; gender × left amygdala reactivity × FWHR, R2change = 6.2%, P = 0.04). To interpret these interactions, separate analyses were computed for men and women.
Right amygdala reactivity
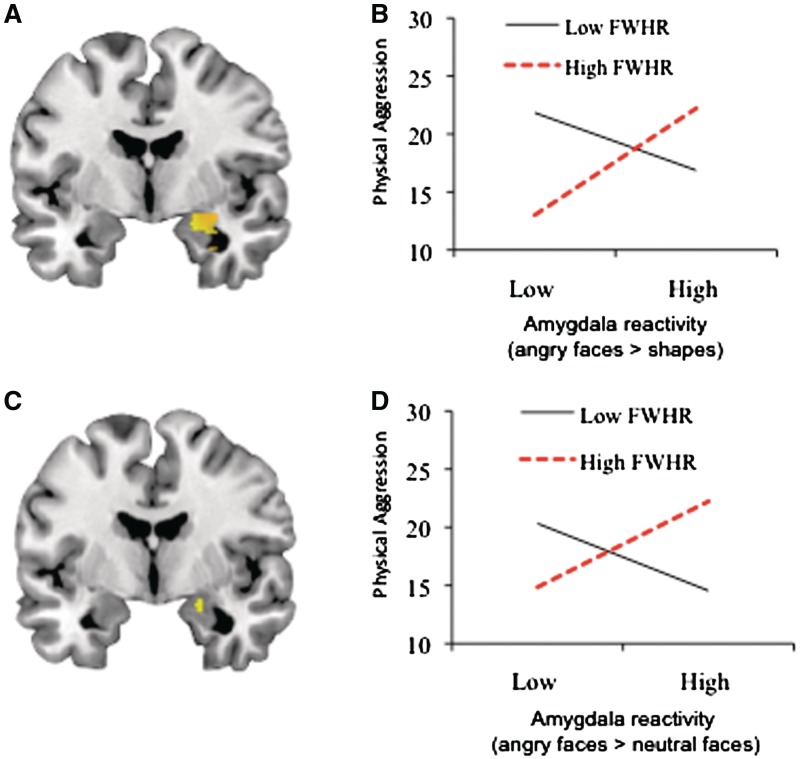
For men, the first step of the regression model (including right amygdala reactivity and FWHR) did not reach statistical significance (R2 = 17%, P = 0.091). Nevertheless, right amygdala reactivity was positively correlated with physical aggression scores (partial r = 0.40, P = 0.042), whereas FWHR was not associated with physical aggression scores (partial r = −0.08, P = 0.71). Importantly, the interaction between right amygdala reactivity and FWHR was significant (R2change = 30.8%, P = 0.001). Simple slopes analyses (Figure 2B) indicated that right amygdala reactivity was positively correlated with physical aggression scores among men with relatively high (unstandardized β = 8.34, p = 0.0002) but not low FWHRs (unstandardized β = −4.06, p = 0.13). For women, the first step of the regression model (including right amygdala and FWHR) did not predict physical aggression scores (R2 = 0.1%, P = 0.99). Furthermore, the right amygdala × FWHR interaction did not predict variation in physical aggression scores (R2change = 0.3%, P = 0.74).
Fig. 2.
FWHR moderates the relationship between amygdala reactivity to angry faces and physical aggression scores among men. (A) Mean amygdala reactivity to angry faces > shapes (x = 20, y = −4, z = −16; 210 voxels, t = 9.39, P < 0.05, corrected). (B) FWHR moderates the relationship between amygdala reactivity from (A) and physical aggression scores. Positive correlation between amygdala reactivity and physical aggression scores for men with high FWHRs (b = 8.34, P = 0.0002) but not low FWHRs (b = −4.06, P = 0.13). (C) Mean amygdala reactivity to angry faces > neutral faces (x = 20, −4, −16, 5 voxels, t = 3.17, P < 0.05, corrected). (D) FWHR moderates the relationship between amygdala reactivity from (B) and physical aggression scores. Positive correlation between amygdala reactivity and physical aggression for men with high FWHRs (b = 3.52, P = 0.028) but not low FWHRs (b = −2.49, P = 0.10). Note: For visualization purposes, high and low amygdala reactivity and FWHR values represent ±1 standard deviation from the mean on each variable.
Left amygdala reactivity
For men, the first step of the regression model (including left amygdala reactivity and FWHR) did not predict variation in physical aggression scores (R2 = 4.4%, P = 0.58). Also, the left amygdala reactivity × FWHR interaction was not statistically significant (R2change = 10.2%, P = 0.11). For women, the first step of the regression model (including left amygdala and FWHR) did not predict physical aggression scores (R2 < 0.1%, P = 0.99). Furthermore, the left amygdala × FWHR interaction did not predict variation in physical aggression scores (R2change = 4.3%, P = 0.24).
All other contrasts (fearful faces > shapes; surprise faces > shapes; neutral faces > shapes)
For all other expression-specific contrasts, there were no significant main effects of FWHR or amygdala reactivity on physical aggression scores (all P's > 0.49) and the two- and three-way interactions did not account for a significant proportion of variance in physical aggression scores (all P's > 0.14).
Differential reactivity to emotional expressions
In addition to contrasting expression-specific amygdala activation with low-level baseline (i.e. shapes), we also examined differential expression-specific amygdala activation by comparing each emotional expression directly against neutral faces. For the fearful faces > neutral faces and surprise faces > neutral faces contrasts, there were no significantly activated voxels in the amygdala that survived correction for multiple comparisons (P < 0.05, FWE-corrected), and thus, we did not extract BOLD parameter estimates for these contrasts. For the angry faces > neutral faces contrast, two clusters survived our FWE-corrected threshold (right amygdala, k = 5 voxels, t = 3.17, x = 20, y = −4, z = −16, Figure 2C; left amygdala, k = 3 voxels, t = 3.36, x = −24, y = −8, z = −12). Given the gender-specific patterns of associations observed using the low-level baseline (i.e. angry faces > shapes), we again split our analyses by gender.
Right amygdala reactivity
For men, the first step of the regression model (including right amygdala reactivity and FWHR) did not predict variation in physical aggression scores (R2 = 0.8%, P = 0.91). However, similar to the results obtained using the low-level baseline contrast (i.e. angry faces > shapes), physical aggression scores among men were predicted by a right amygdala × FWHR interaction (R2change = 22.8%, P = 0.015). Simple slopes analyses (Figure 2D) indicated that right amygdala reactivity was positively correlated with physical aggression scores among men with relatively high (unstandardized β = 3.52, p = 0.028) but not low FWHRs (unstandardized β = −2.49, p = 0.10). For women, the first step of the regression model (including right amygdala reactivity and FWHR) did not predict variation in physical aggression scores (R2 = 0.5%, P = 0.92). Furthermore, the right amygdala × FWHR interaction did not predict variation in physical aggression scores (R2change = 0.3%, P = 0.75).
Left amygdala reactivity
For men, the first step of the regression model (including left amygdala reactivity and FWHR) did not predict variation in physical aggression scores (R2 = 8.6%, P = 0.34). Also, the left amygdala reactivity × FWHR interaction did not predict variation in physical aggression scores (R2change = 0.1%, P = 0.87). For women, the first step of the regression model (including left amygdala and FWHR) did not predict physical aggression scores (R2 = 2.2%, P = 0.69). Furthermore, the left amygdala × FWHR interaction did not predict variation in physical aggression scores (R2change = 6.9%, P = 0.13).
DISCUSSION
Our data indicate that for men with relatively high FWHRs, heightened amygdala reactivity to social signals of interpersonal threat (i.e. angry facial expressions) predicts individual differences in physical aggression. In contrast, this amygdala reactivity is unrelated to physical aggression among men with relatively low FWHRs. These patterns were both specific to angry facial expressions and unique to men.
Previous work indicates that patients with intermittent explosive disorder, who more readily and frequently engage in reactive aggression, have relatively increased amygdala reactivity to angry but not sad, fearful, happy, surprised, disgusted or neutral facial expressions (Coccaro et al., 2007). Consistent with this work, we found that only the relationship between amygdala reactivity to angry and not fearful, surprised or neutral facial expressions was positively correlated with normal variability in physical aggression in healthy men. Collectively, these findings suggest that propensity for engaging in aggression is specifically associated with the reactivity of the amygdala (and likely downstream effects on the hypothalamus and PAG) to social signals of interpersonal challenge or threat.
The current findings also indicate that the modulatory effect of FWHR on the relationship between amygdala reactivity and aggressive behavior is specific to men. This finding is consistent with behavioral data indicating that correlations between FWHR and behavior are more robust in men than women (Carré and McCormick, 2008; Stirrat and Perrett, 2010; Haselhuhn and Wong, 2011). Thus, it appears that amygdala reactivity to angry facial expressions may not represent a neural correlate for aggressive behavior among women. Unfortunately, previous imaging studies that have reported heightened amygdala reactivity to angry facial expressions among aggression-prone individuals were conducted with relatively small samples (e.g. N = 20–22), precluding the analysis of gender-dependent effects (Coccaro et al., 2007; Beaver et al., 2008). Thus, future work will be needed to further validate the gender-specific patterns of findings observed in the current study.
Unlike our previous report (Carré and McCormick, 2008), we found no bivariate association between FWHR and aggressive behavior. One potential explanation for this null finding is that while our previous study used behavioral measures of aggression (i.e. a laboratory task and number of penalty minutes during a hockey season), the current study used a self-report measure. Indeed, the extant research in neuroendocrinology indicates that testosterone–aggression associations are typically more robust when behavioral measures are acquired (see Carré et al., 2011; for review).
In summary, these data are the first to identify a putative neural mechanism linking variation in facial structure to individual differences in human aggression. Specifically, our data indicate that heightened amygdala reactivity to social signals of interpersonal challenge represents a mechanism linking large FWHRs to human aggression. Associations between individual differences in amygdala reactivity, facial structure and aggressive behavior may emerge as a function of androgenic mechanisms acting during puberty to shape craniofacial growth and the development of neural circuits underlying aggression. Future work in which both testosterone concentrations and facial structure are assessed repeatedly during puberty will be critical to substantiate our hypothesis that testosterone is the common biological factor shaping variation in facial structure and brain function. Our findings further represent a plausible pathway through which FWHR may have evolved to represent an honest indicator of conspecific threat, namely by reflecting the responsiveness of neural circuitry mediating aggressive behavior.
Conflict of Interest
None declared.
Acknowledgments
This study was supported by Duke University and a grant from the Harry Frank Guggenheim Foundation.
References
- Ahmed EI, Zehr JL, Schulz KM, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature Neuroscience. 2008;11:995–7. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Passamonti L, Calder AJ. Appetitive motivation predicts the neural response to facial signals of aggression. Journal of Neuroscience. 2008;28:2719–25. doi: 10.1523/JNEUROSCI.0033-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Neuroimaging of psychopathy and antisocial behavior: a targeted review. Current Psychiatric Reports. 2010;12:76–82. doi: 10.1007/s11920-009-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry MP. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;78:772–90. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Carré JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactive to angry facial expression in men but not women. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, McCormick CM. In your face: facial metrics predict aggressive behaviour in the laboratory and in varsity and professional hockey players. Proceeding of the Royal Society of London: Biological Sciences. 2008;275:2651–6. doi: 10.1098/rspb.2008.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, McCormick CM, Hariri AR. The social neuroendocrinology of human aggression. Psychoneuroendocrinology. 2011;36:935–44. doi: 10.1016/j.psyneuen.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM, Mondloch CJ. Facial structure is a reliable cue of aggressive behavior. Psychological Science. 2009;20:1194–8. doi: 10.1111/j.1467-9280.2009.02423.x. [DOI] [PubMed] [Google Scholar]
- Carré JM, Morrissey MD, Mondloch CJ, McCormick CM. Estimating aggression from emotionally neutral faces: which facial cues are diagnostic? Perception. 2010;39:356–77. doi: 10.1068/p6543. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biological Psychiatry. 2011;69:1153–9. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Corso PS, Mercy JA, Simon TR, Finkelstein EA, Miller TR. Medical costs and productivity losses due to interpersonal and self-directed violence in the United States. American Journal of Preventative Medicine. 2007;32:474–82. doi: 10.1016/j.amepre.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animal. London: Murray; 1872. [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, et al. Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cerebral Cortex. 2009;19:2499–507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, et al. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nature Neuroscience. 2006;9:1362–3. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Haselhuhn MP, Wong EM. Bad to the bone: facial structure predicts unethical behaviour. Proceedings of the Royal Society of London: Biological Sciences. in press;279:571–6. doi: 10.1098/rspb.2011.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–6. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, et al. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor predict amygdala reactivity in men. Psychoneuroendocrinology. 2010;35:94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological bases for development of pharmacological treatments of aggressive disorders. Current Neuropharmacology. 2007;5:135–47. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Stirrat M, Perrett DI. Valid facial cues to cooperation and trust: male facial width and trustworthiness. Psychological Science. 2010;21:349–54. doi: 10.1177/0956797610362647. [DOI] [PubMed] [Google Scholar]
- Verdonck A, Gaethofs M, Carels C, de Zegher F. Effects of low-dose testosterone treatment on craniofacial growth in boys with delayed puberty. European Journal of Orthodontics. 1999;21:137–43. doi: 10.1093/ejo/21.2.137. [DOI] [PubMed] [Google Scholar]
- Viviani R. Unbiased ROI selection in neuroimaging studies of individual differences. Neuroimage. 2010;50:184–9. doi: 10.1016/j.neuroimage.2009.10.085. [DOI] [PubMed] [Google Scholar]
- Weston EM, Friday AE, Lio P. Biometric evidence that sexual selection has shaped the hominin face. PLoS ONE. 2007;2:e710. doi: 10.1371/journal.pone.0000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]