Abstract
Background
Medication discrepancies at care transitions are common and lead to patient harm. Medication reconciliation is a strategy to reduce this risk.
Objectives
To summarize available evidence on medication reconciliation interventions in the hospital setting and identify the most effective practices.
Data Sources
Medline (1966 through February 2012) and hand search of article bibliographies.
Study Selection
26 controlled studies.
Data Extraction
Data were extracted on study design, setting, participants, inclusion/exclusion criteria, intervention components, timing, comparison group, outcomes, and results.
Data Synthesis
Studies were grouped by type of medication reconciliation intervention: pharmacist-related, information technology (IT), or other, and assigned quality ratings utilizing U.S. Preventative Services Task Force criteria.
Results
15 of 26 studies reported on pharmacist-related interventions, 6 evaluated IT interventions, and 5 studied other interventions. 6 studies were classified as good quality. The comparison group for all studies was usual care, with no direct comparisons of different types of interventions. Studies consistently demonstrated a reduction in medication discrepancies (17/17 studies), potential adverse drug events (5/6 studies), and adverse drug events (2/3 studies), but showed inconsistent reduction in post-discharge healthcare utilization (improvement in 2/8 studies). Key aspects of successful interventions included intensive pharmacy staff involvement and targeting the intervention to a ‘high-risk’ patient population.
Conclusions
There is a paucity of rigorously designed studies comparing different inpatient medication reconciliation practices and their effects on clinical outcomes. Available evidence supports medication reconciliation interventions that heavily utilize pharmacy staff and focus on patients at high-risk for adverse events. Higher quality studies are needed to determine the most effective approaches to inpatient medication reconciliation.
Introduction
Adverse drug events (ADEs), defined as patient injuries related to a drug,1 are an epidemic patient safety issue, occurring in 5–40% of hospitalized patients and in 12–17% of patients post-discharge.2–3 Transitions of care, such as hospital admission and discharge, contribute to ADEs in part due to medication discrepancies, or unexplained differences in documented medication regimens across different sites of care.4–5 Medication discrepancies are common, occurring in up to 70% of patients at admission or discharge,6–10 with nearly one-third of these having potential to cause patient harm (i.e., potential adverse drug events, or PADEs).10 ADEs associated with medication discrepancies can prolong hospital stays and, in the post-discharge period, may lead to emergency room visits, hospital readmissions, and utilization of other healthcare resources.11–12
Medication reconciliation is a strategy to reduce the occurrence of medication discrepancies that may lead to ADEs or PADEs. Medication reconciliation is the “process of identifying the most accurate list of all medications a patient is taking…and using this list to provide correct medications for patients anywhere within the health care system.”13 Recognizing the potential impact of properly reconciling medications during care transitions, in 2005 The Joint Commission added medication reconciliation to its list of National Patient Safety Goals.14
During the past decade, various medication reconciliation interventions have been described, but the specific elements important to successful efforts have not been fully appreciated. We performed a systematic review of the literature to summarize the available evidence on medication reconciliation in the hospital setting and to identify the most effective practices.
Methods
Data Sources and Searches
We performed a systematic search of English language articles published from 1966 through February 2012 on medication reconciliation during patient hospitalization. Using Medline, we searched a combination of Medical Subject Headings (MeSH) and keywords including “medication reconciliation,” “medication errors/prevention and control,” “patient discharge,” “medication systems, hospital,” “medical records systems, computerized,” “medication list,” and “medication record.” We performed a second electronic search adding the keyword “patient admission” in combination with the above list to update the search and to incorporate interventions that took place on admission and not just at discharge. We hand-searched the reference lists of relevant articles.
Study Selection
Controlled intervention studies that met the following criteria were eligible for inclusion: English language, medication reconciliation was the primary focus of the intervention, the comparison group was defined, the intervention was clearly described, the intervention took place in the hospital setting during the period of hospitalization and/or transition into or out of the hospital, and quantitative results were provided. One reviewer (SM or KS) performed initial independent assessments of titles for relevance and subsequent examination of abstracts and articles for inclusion, which was then verified by a second reviewer (SM or KS). Discrepancies were resolved by a third reviewer (JS or SK).
Data Extraction
One reviewer (SM) extracted relevant data from included articles, which was then verified by 2 other reviewers (KS and JS). Information was obtained regarding study design, setting, number of participants, components of the intervention, timing of the intervention related to hospital course, comparison group, outcome measures, type of outcome, and results (data extraction tool shown in Appendix A).
Data Synthesis and Analysis
Studies were first grouped into the following 3 categories, based on the primary component of the intervention: (1) pharmacist-related, (2) information technology (IT), or (3) other type. Two authors (SM and JS) then collectively determined 4 common types of reported outcomes, including: (1) medication discrepancies, defined as unexplained differences in documented medication regimens across different sites of care, (2) potential adverse drug events (PADEs), defined as medication discrepancies with potential to cause patient harm, (3) adverse drug events (ADEs), defined as patient injuries related to a drug, and (4) healthcare utilization, defined as post-discharge emergency room visits, hospital readmissions, and/or utilization of other healthcare resources. Meta-analysis was not feasible due to heterogeneity in methods, interventions, and reported outcomes.
Studies were also rated on quality and categorized as “good”, “fair”, or “poor”, utilizing the U.S. Preventive Services Task Force (USPSTF) standardized criteria for assessing internal validity of individual studies,15 adapting the criteria for pre-post studies where needed. Two authors (SM and SK) graded each study individually and then resolved any differences by consensus.
Observational (Non-Controlled) Studies
Observational studies that met the same inclusion criteria as described were examined and data extracted in the same manner as reported above. Although not included in this manuscript, information on these studies can be found in the e-appendix.
Results
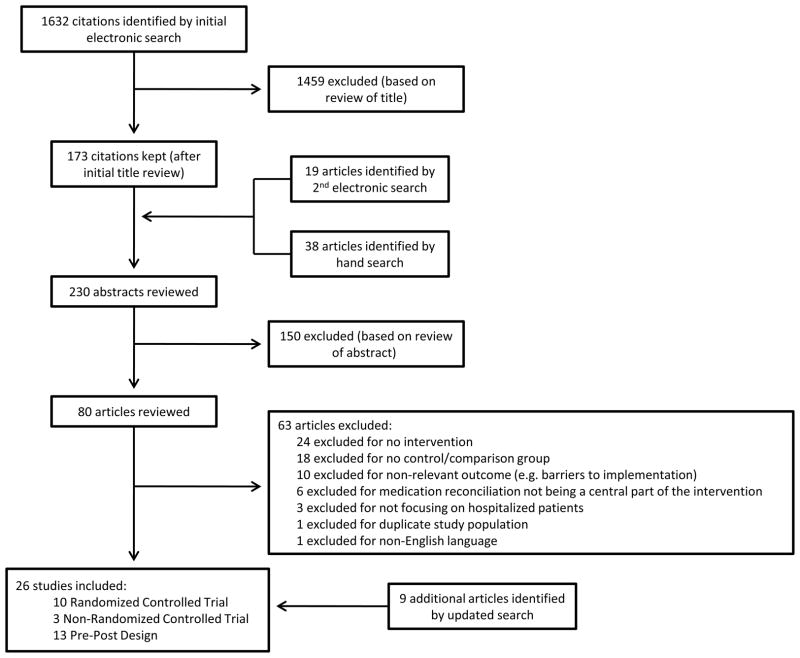
Of the 1632 articles initially identified via electronic search, 173 abstracts were reviewed. A second electronic search and hand search of references yielded an additional 57 abstracts. Of the 230 abstracts reviewed, 80 publications warranted full review. Of these, 17 articles met criteria for inclusion. An updated search identified 9 additional articles meeting criteria for inclusion, resulting in 26 total included articles, including 10 randomized controlled trials, 3 non-randomized trial with a concurrent control group, and 13 pre-post studies (Figure 1). Of the 26 included studies, 14 were conducted in countries other than the United States including Canada,16–17 Australia,18–19 New Zealand,20 Northern Ireland,21 United Kingdom,22 Belgium,23 Denmark,24 Netherlands,25 and Sweden.26–29
Figure 1.
Fifteen studies reported on pharmacist-related interventions,16–19, 21–22, 24–26, 28–33 6 studies reported on IT-focused interventions,34–39 and 5 studies reported on other types of interventions including educating staff about medication reconciliation20, 40 and use of a standardized medication reconciliation tool.23, 27, 41 The majority of studies were classified as poor quality (15 of 26)18–23, 27, 29–30, 34–35, 37–38, 40–41 with 6 studies classified as good quality,24–25, 28, 31, 36, 39 and the remaining 5 studies classified as fair quality.16–17, 26, 32–33 A summary of the timing and components of the interventions and quality ratings of the studies is shown in Table 1, and results of the studies are summarized in Table 2. Comparison groups for all included studies was “usual care,” as defined in Table 1.
Table 1.
Timing and Components of Interventions
| First Author, Yr (Study Design) | N | Timing of Intervention | Components of Intervention | Control Group | 1 USPSTF Quality Rating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Admission | Admission | During Hospitalization | Discharge | Post-Discharge | Medication History Taking | Medication Reconciliation | Patient Counseling | Communication with Outpatient Providers | Review Appropriateness of Medications | Post-discharge Communication with Patient | ||||
| PHARMACIST-RELATED INTERVENTIONS | ||||||||||||||
| Michels, 2003 (Pre-Post) | NR | √ | √ | √ | √ | Usual care prior to intervention (nurse or physician recorded home medication list which was used for admission orders) | POOR | |||||||
| Bolas, 2004 (RCT) | 162 | √ | √ | √ | √ | √ | √ | √ | Usual care (standard clinical pharmacy service which did not routinely perform discharge counseling) | POOR | ||||
| Nickerson, 2005 (RCT) | 253 | √ | √ | √ | √ | √ | Usual care (nurse performed discharge counseling and transcribed discharge note from medical record) | FAIR | ||||||
| Schnipper, 2006 (RCT) | 176 | √ | √ | √ | √ | √ | Usual care (ward-based pharmacist performed routine review of medication orders, nurse performed discharge counseling) | GOOD | ||||||
| Kwan, 2007 (RCT) | 464 | √ | √ | √ | Usual care (nurse conducted medication history and surgeon generated post-operative medication orders) | FAIR | ||||||||
| Bergkvist, 2009 (Pre-Post) | 115 | √ | √ | √ | √ | √ | √ | Usual care prior to intervention (standard care without pharmacist involvement in reconciliation or review of medications on admission or discharge) | FAIR | |||||
| Gillespie, 2009 (RCT) | 400 | √ | √ | √ | √ | √ | √ | √ | √ | √ | Usual care (standard care without direct involvement of pharmacists at the ward level) | GOOD | ||
| Koehler, 2009 (RCT) | 41 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | Usual care (floor nursing staff performed medication reconciliation and medication education) | FAIR | |
| Vasileff, 2009 (2 Non-RCT) | 74 | √ | √ | √ | Usual care (physician obtained medication history from patient and generated orders) | POOR | ||||||||
| Walker, 2009 (2 Non-RCT) | 724 | √ | √ | √ | √ | √ | √ | √ | Usual care (nurses provided patients with printed list of medications and instructions at discharge; Medicare beneficiaries received phone call 72 hours post-discharge) | FAIR | ||||
| Eggink, 2010 (RCT) | 85 | √ | √ | √ | √ | Usual care (nurses provided verbal and written instructions at discharge, physician provided patient with medication list to give to their primary care physician) | GOOD | |||||||
| Lisby, 2010 (RCT) | 99 | √ | √ | √ | √ | Usual care (medication review by junior physician on admission, and by senior physician within 24 hours of admission) | GOOD | |||||||
| Mills, 2010 (Pre-Post) | 100 | √ | √ | √ | √ | Usual care (Admitting junior physician obtained medication history and reconciled medications when patient arrived on ward from the ER) | POOR | |||||||
| Hellstrom, 2011 (Pre-Post) | 210 | √ | √ | √ | √ | √ | Usual care (standard care without pharmacist involvement in medication reconciliation on admission or during hospitalization, standard physician performed medication reconciliation on discharge) | POOR | ||||||
| 3 Marotti, 2011 (RCT) | 357 | √ | √ | √ | √ | Usual care (medication history taking and prescribing performed by physician on admission) | POOR | |||||||
| IT INTERVENTIONS | Components | |||||||||||||
| Poole, 2006 (Pre-Post) | 100 | √ | Formation of a medication list from pre-existing electronic sources Reconciliation of discharge medications with this list |
Usual care prior to intervention (patients discharged without use of a discharge medication worksheet) | POOR | |||||||||
| Agrawal, 2009 (Pre-Post) | NR | √ | Formation of a medication list from pre-existing electronic sources Reconciliation of admission orders with this list |
3 Usual care during pilot phase (standard care without use of electronic medication reconciliation system) | POOR | |||||||||
| Murphy, 2009 (Pre-Post) | NR | √ | √ | Pharmacist performed medication history and reconciliation on admission Formation of a medication list from pre-existing electronic sources Reconciliation of discharge medications with this list |
Usual care prior to intervention (standard care without direct involvement of pharmacist on ward level and without electronic reconciliation) | POOR | ||||||||
| Schnipper, 2009 (RCT) | 322 | √ | √ | Formation of a medication list from pre-existing electronic sources Reconciliation of admission orders and discharge medications with this list Pharmacist confirmation of reconciliation at admission |
Usual care (ward-based pharmacist performed routine review of medication orders, nurse performed discharge counseling) | GOOD | ||||||||
| Boockvar, 2011 (2 Non-RCT) | 795 | √ | Formation of a medication list from pre-existing electronic sources Reconciliation of admission orders with this list |
Usual care (no computerized availability of recent VA outpatient medication use) | POOR | |||||||||
| Showalter, 2011 (Pre-Post) | 34088 | √ | Formation of a medication list from pre-existing electronic sources Reconciliation of discharge medications with this list |
Usual care prior to intervention (manual completion of a printed medication reconciliation document) | GOOD | |||||||||
| OTHER INTERVENTIONS | Components | |||||||||||||
| Varkey, 2007 (Pre-Post) | 102 | √ | √ | √ | Multidisciplinary medication reconciliation with use of reconciliation form on admission and discharge Education of staff on medication reconciliation including real-time feedback on detected medication discrepancies |
Usual care during “Phase I” (nurses, pharmacists and physicians used a medication reconciliation form to collect and reconcile medications at admission and discharge, but no feedback was given) | POOR | |||||||
| Midlov, 2008 (Pre-Post) | 427 | √ | Use of a physician generated medication report to next provider of care at time of discharge that includes details of medication changes made during hospital course | Usual care prior to intervention (no structured way that medication changes were communicated to outpatient providers) | POOR | |||||||||
| Chan, 2010 (Pre-Post) | 407 | √ | Multidisciplinary medication history and reconciliation on admission Education of health care providers on importance of medication reconciliation via lectures, posters around hospital, and reminder notes in patient charts |
Usual care prior to intervention (pharmacist performed medication history on small number of patients; this did not change during the study) | POOR | |||||||||
| Tessier, 2010 (Pre-Post) | 100 | √ | Nursing performed medication reconciliation with use of a 6-step instructional pamphlet | Usual care prior to intervention (not described) | POOR | |||||||||
| De Winter, 2011 (Pre-Post) | 260 | √ | ED physician performed medication history taking and reconciliation with use of a standardized “limited questions list” questionnaire | Usual care (admitting physician performed medication history taking and reconciliation without use of a standardized tool) | POOR | |||||||||
Abbreviations: IT = Information Technology; RCT = Randomized Controlled Trial; Non-RCT = Non-Randomized Controlled Trial; NR = Not Reported
USPSTF = U.S. Preventive Services Task Force, utilizing set criteria for assessing internal validity of individual studies. Please email corresponding author for further details on how quality ratings were assigned.
Non-RCT had a concurrent control group, but the sample was a convenience sample as opposed to a randomized sample
Given poor compliance during pilot phase, comparison group was reflective of usual care prior to intervention
Table 2.
Study Outcomes
| First Author, Yr (Study Design) | * Outcomes Examined | Results | P value or OR [95% CI] | |||
|---|---|---|---|---|---|---|
| Medication Discrepancies | Potential Adverse Drug Events (PADEs) | Adverse Drug Events (ADEs) | Healthcare Utilization | |||
| PHARMACIST-RELATED INTERVENTIONS | ||||||
| Michels, 2003 (Pre-Post) | + |
|
<0.001 | |||
|
<0.001 | |||||
| Bolas, 2004 (RCT) | + | ~ |
|
0.005 | ||
| Decrease in drug frequency mismatch at 10–14 days post-discharge | 0.004 | |||||
|
>0.05 | |||||
| Nickerson, 2005 (RCT) | + |
|
NR | |||
| Schnipper, 2006 (RCT) | + | ~ |
|
0.01 | ||
|
>0.05 | |||||
| Kwan, 2007 (RCT) | + | + |
|
<0.001 | ||
|
<0.001 | |||||
| Bergkvist, 2009 (Pre-Post) | + |
|
0.012 | |||
| Gillespie, 2009 (RCT) | + |
|
0.84 [0.72–0.99] | |||
|
0.53 [0.37–0.75] | |||||
|
0.2 [0.1–0.41] | |||||
|
>0.05 | |||||
| Koehler, 2009 (RCT) | + |
|
0.04 | |||
|
>0.05 | |||||
|
0.05 | |||||
| Vasileff, 2009 (Non-RCT) | + | + |
|
<0.05 | ||
|
IRR < 0.8, except for one possible pairing (not specified) | |||||
| Walker, 2009 (Non-RCT) | + | ~ |
|
<0.001 | ||
|
>0.05 | |||||
| Eggink, 2010 (RCT) | + | ~ |
|
0.57 [0.37, 0.88] | ||
|
NR (stated in text “non-significant) | |||||
| Lisby, 2010 (RCT) | ~ | ~ |
|
>0.05 | ||
| Mills, 2010 (Pre-Post) | + |
|
>0.05 | |||
| Hellstrom, 2011 (Pre-Post) | ~ |
|
0.138 | |||
| Marotti, 2011 (RCT) | + |
|
<0.001 | |||
| IT INTERVENTIONS | ||||||
| Poole, 2006 (Pre-Post) | + |
|
<0.001 | |||
| Agrawal, 2009 (Pre-Post) | + |
|
NR | |||
| Murphy, 2009 (Pre-Post) | + |
|
0.001 | |||
| Schnipper, 2009 (RCT) | + |
|
0.72 [0.52–0.99] | |||
| Boockvar, 2011 (Non-RCT) | + |
|
0.57 [0.33, 0.98] | |||
|
1.04 [0.68, 1.61] | |||||
| Showalter, 2011 (Pre-Post) | ~/− |
|
0.17 | |||
|
0.02 | |||||
| OTHER INTERVENTIONS | ||||||
| Varkey, 2007 (Pre-Post) | + |
|
0.018 | |||
|
0.003 | |||||
| Midlov, 2008 (Pre-Post) | + |
|
0.049 | |||
| Chan, 2010 (Pre-Post) | + | + |
|
<0.001 | ||
|
0.023 | |||||
| Tessier, 2010 (Pre-Post) | + |
|
0.03 | |||
| De Winter, 2011 (Pre-Post) | + |
|
<0.001 | |||
Abbreviations: LOS = length of stay; IRR = Inter-rater reliability; IT = information technology; ED = Emergency Department; PCP = Primary Care Physician; RCT = randomized controlled trial
Outcomes examined intervention versus “usual care” as the comparison group (detailed in Table 1) for all studies
indicates statistically significant improvement with intervention versus control in at least one outcome in this category
indicates no statistically significant difference between intervention and control in at least one outcome in this category
indicates statistically significant worsening with intervention versus control in at least one outcome in this category
The 15 studies involving pharmacist-related interventions included diverse roles of the pharmacy staff in the medication reconciliation process, as well as varied timing of pharmacy staff involvement during the patient’s hospitalization. Four of the 15 studies were rated as good quality24–25, 28, 31 (Table 1). The majority of these studies involved licensed pharmacists, although pharmacy residents32 and pharmacy technicians30 were also utilized. These interventions reduced medication discrepancies (10/10 studies)16–19, 21–22, 25–26, 30, 33 and potential adverse drug events (improvement in 2 of 3 studies),16, 18, 25 but showed mixed effects on preventable adverse drug events (improvement in 1 of 2 studies)24, 31 and healthcare utilization (improvement in 2 of 7 studies)21, 24, 28–29, 31–33 (Table 2). In the larger of these last 2 studies, Gillespie et al. utilized a pharmacist to perform medication histories and reconciliation on admission and discharge, patient and provider medication counseling during hospitalization, communication with the primary care physician on discharge, and communication with the patient 2 months after discharge. This intervention reduced the odds of all hospital visits by 16% (OR 0.84, 95% CI 0.72–0.99), including a 47% reduction in emergency department (ED) visits and an 80% reduction in drug-related readmissions in the 12 months following hospital discharge. No difference was seen in all-cause hospital readmission or overall mortality.28 Koehler et al. reported on a similar intensive intervention, but utilized pharmacy residents instead of licensed pharmacists. This intervention decreased 30-day ED visits/readmissions (10% in the intervention group versus 38.1% in the control group, p=0.04).32 Common themes of these 2 successful studies included (1) limiting the intervention to elderly patients (age ≥ 80 and ≥ 70 years, respectively); (2) intensive pharmacy staff involvement including medication history-taking on admission and medication reconciliation on admission, during hospitalization, and at hospital discharge; (3) communication with the primary care physician via direct communication or use of a template; and (4) telephone follow-up after discharge. The 5 studies that demonstrated no effect on healthcare utilization had more limited roles for the intervention pharmacist21, 29, 31 or utilized them for a more limited time during hospitalization (e.g., admission or discharge only).24, 31, 33
The 6 studies that reported IT-focused medication reconciliation interventions all improved access to electronically available sources of preadmission medication information such as ambulatory electronic medical records.34–39 These interventions leveraged data to create a preadmission medication list and facilitated comparison of this list with admission and/or discharge orders to help with the medication reconciliation process. Two of the 6 studies were rated as good quality.36, 39 IT-related interventions reduced medication discrepancies (3/3 studies),34–35, 37 potential adverse drug events (1/1 study),36 and adverse drug events (1/1 study),38 but demonstrated no improvement/slightly increased healthcare utilization (within 1 study).39 Through implementation of an electronic medication reconciliation tool and process redesign, Schnipper et al. decreased the incidence of potential adverse drug events, with an average of 1.05 PADEs per patient in the intervention arm versus 1.44 in the control arm (RR 0.72, 95% CI 0.52–0.99).36 However, Showalter et al. demonstrated that implementation of an automated medication reconciliation tool on discharge, that also included autopopulation of other discharge instructions, resulted in no difference in composite 30-day healthcare utilization, and was associated with an increase in 30-day hospital readmission (11% post-intervention versus 10.2% pre-intervention, p=0.02). The authors hypothesized that improving the discharge instructions to inform patients of worrisome symptoms may have led to higher rates of subsequent (appropriate) readmissions.39
Among the 5 studies that described other types of interventions, 2 provided education/feedback for the staff about medication reconciliation,20, 40 and 3 used a standardized medication reconciliation tool. The standardized tools included a discharge report that provided a brief hospital summary detailing all medication changes that occurred during hospitalization,27 a six-step standardized nursing approach to medication history taking and reconciliation on admission,41 and a standard questionnaire used by emergency room physicians on admission.23 None of these studies were rated as good quality. These studies demonstrated improvement in medication discrepancies (4/4 studies)20, 23, 40–41 and in potential adverse drug events (2/2 studies).20, 27 For example, Midlov et al. described use of a physician-generated medication report for post-discharge providers that included a brief summary of hospitalization, medications on discharge, and detailed medication changes made during hospitalization and reasons for those changes, which resulted in decreased PADE from 8.9% pre-intervention to 4.4% post-intervention (p=0.049).27 The intervention was limited to elderly patients admitted from/returning to a nursing home.
Of all 26 studies, 13 focused the intervention on a “high risk” sub-group of patients. This “high risk” category was most commonly defined as older patients, with age threshold from 55 to 80 years.18, 20–21, 24, 26–29, 32, 37 Other definitions of “high risk” included polypharmacy with thresholds ranging from greater than 4 to 13 medications18, 20–21, 25, 32–33, 36 and having greater than 3 co-morbid conditions.18, 32 Several studies included a combination of these criteria to define the intervention cohort.18, 20–21, 32
Observational studies described similar types of interventions as the comparative studies, with pharmacist-led interventions again the most commonly reported (see e-Appendix).
Discussion
This systematic review of hospital-based medication reconciliation practices found that various interventions including those involving pharmacy staff, IT, and other types of interventions successfully decreased medication discrepancies and potential adverse drug events, but demonstrated inconsistent benefit on adverse drug events and healthcare utilization, compared to usual care.
Most studies reported on pharmacist-related interventions (15/26 studies), which included a number of articles that evaluated clinical outcomes such as preventable adverse drug events24, 31 and healthcare utilization,21, 24, 28–29, 31–33 rather than solely examining process measures such as medication discrepancies. Further, this category of intervention had the greatest number studies rated as good quality, with 4 of 15 studies rated in this category.24–25, 28, 31 In the two studies that demonstrated improvement in healthcare utilization,28, 32 the pharmacy staff was heavily involved, performing a comprehensive medication history at admission, medication reconciliation at admission and discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient.
Notably, the majority of reported pharmacist-related interventions also included an the taking of an accurate medication history at time of admission, as noted in Table 1. Errors in obtaining an accurate preadmission medication history have great potential for harm, as they can propagate throughout a patient’s hospitalization and after discharge. They are also the most common reason for PADEs caused by medication discrepancies.8 Although it is difficult to distinguish the impact of an accurate medication history from the impact of successful medication reconciliation when both are included in the intervention, in reality these two process steps are both necessary components of the overall medication reconciliation process. It is therefore unrealistic to consider a successful medication reconciliation program that does not also include an initial accurate medication list from which to begin the reconciliation process.
Other common elements of the successful pharmacist-related medication reconciliation efforts included communication with post-discharge providers regarding the discharge medication regimen, including how and why the regimen differed from prior to admission,17, 21, 28, 32–33 and patient education and follow-up.17, 21, 26, 28, 31–33
It is worth noting that the pharmacist-related interventions were comprised of studies that utilized licensed pharmacists as well as studies that used less resource intensive pharmacy staff, such as pharmacy residents32 and pharmacist technicians,30 demonstrating the viability of using other personnel in this role.
Despite these demonstrations of successful implementation of pharmacist-related interventions, the comparison group in all of these studies was “usual care,” and therefore the evidence does not definitively support pharmacist-led medication reconciliation as superior to other reported interventions.
In review of all pharmacist and non-pharmacist-related interventions, common elements of successful interventions was the targeting of a “high risk” sub-group,18, 26–28, 32, 36–37 evidence of institutional support,28, 36 and performing the intervention in a defined population, e.g. patients to/from a nursing home27 or in the setting of an elective surgical admission.19
Despite these reports of successful medication reconciliation interventions, this review highlights the paucity of rigorously designed studies on inpatient medication reconciliation. Only 26 studies met inclusion criteria for our review, and of these, only 10 are randomized controlled trials,16–17, 19, 21, 24–25, 28, 31–32, 36 only 1 of which was conducted at more than 1 site.36 On quality review, only 6 of 26 studies met criteria to be classified as good quality.24–25, 28, 31, 36, 39 Further, comparison groups in all studies were “usual care,” rather than alternative interventions. This is understandable given the state of medication reconciliation efforts prior to 2005, but it limits our ability to draw conclusions on the most effective practices of medication reconciliation. Also, “usual care” relating to medication reconciliation efforts has likely improved since it was first mandated by The Joint Commission, making it difficult to compare the efficacy of certain interventions in older versus newer studies. Additionally, most studies investigated process measures alone, such as presence of medication discrepancies with potential for harm, rather than clinical outcomes, which were reported in only 9 of the 26 studies.21, 24, 28–29, 31–33, 38–39 While process measures are easily studied, pertinent to the issue of medication safety, and responsive to change, it is important to distinguish between these and actual patient outcomes.
There are many reasons why it has been difficult to rigorously examine medication reconciliation efforts despite its recognized importance to patient safety. As noted in the Society of Hospital Medicine’s 2010 Consensus Statement,42 medication reconciliation efforts are often resource-intensive (e.g., HIT, pharmacists) and need to overcome several challenges, including the disjointed nature of the American healthcare system, the need to maintain up-to-date and accurate medication lists across different venues of patient care, and difficulty with identifying and maintaining role responsibility in the process. Further, electronic medication reconciliation solutions are often part of larger electronic medical record systems, making it difficult to study them in isolation. Therefore, studies comparing two different interventions are logistically difficult, and it may be more feasible to expect comparisons of one intervention currently in use to that intervention plus the addition of another one.
There are several limitations of this review. Along with the lack of rigorous study design in most included studies, as discussed above, it is possible (and in fact likely) that other medication reconciliation interventions have been implemented and studied, found to be unsuccessful, and never published. Second, there were a large number of included studies from outside the United States, which potentially limits generalizability in U.S. healthcare settings. Differences in patient safety culture and/or better access to medication information (e.g., through nationalized health records) may make implementation efforts more successful in other countries than in the United States. Third, this review is intentionally limited to medication reconciliation practices within, or in transition to/from the hospital setting, and therefore does not include the broader scope of all medical settings, including primary care or other clinic venues.
Conclusions
In summary, there are limited data on most effective practices of inpatient medication reconciliation, and a lack of rigorously designed controlled studies comparing different approaches to medication reconciliation to each other.
In the context of these limitations, existing evidence supports pharmacist-related interventions compared to usual care in producing the best patient outcomes, with high degree of pharmacist or pharmacy staff involvement in all medication reconciliation-related processes producing the best patient outcomes. Targeting interventions to a subset of patients considered at greatest risk of an adverse drug event, such as elderly patients, patients taking many medications, and/or patients with many co-morbid conditions, may be of highest yield. This evidence also suggests that taking an accurate medication history and communicating with post-discharge providers are important steps, especially for achieving reduction in post-discharge healthcare utilization.
Future research should include randomized controlled trials when possible (and interrupted time series or ‘stepped wedge’ designs when not possible), utilizing rigorous outcome assessment that includes clinical as well as process outcomes. Studies should also compare interventions to each other or evaluate the incremental benefits of adding a second intervention to one already in use, ensuring standardized and consistent measurement methods and detailed descriptions of usual care. Additionally, the SHM consensus statement on medication reconciliation recommends a set of key action items for addressing identified barriers to implementation and reporting;42 these should also be utilized in future research and quality improvement efforts. Despite the aforementioned difficulties in performing these types of rigorous studies, it should be emphasized that it is because of the resources required for successful medication reconciliation efforts that precise estimates of impact, based on rigorously conducted studies, are required.
This review should help inform the development of future interventions, both for research and for institutions wishing to improve medication safety during transitions in care.
Acknowledgments
Dr. Mueller was supported by an Institutional National Research Service Award #T32- HP10251 and by the Division of General Medicine at Brigham and Women’s Hospital.
Dr. Sponsler - None
Dr. Kripalani was supported in part by R01 HL089755 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Dr. Schnipper was supported in part by R01 HL089755 from the National Heart, Lung, and Blood Institute and by 1 R18 HS019598-01 from the Agency for Healthcare Research and Quality (AHRQ). He had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures:
Dr. Stephanie Mueller – None
Dr. Kelly Cunningham Sponsler – None
Dr. Sunil Kripalani – Dr. Kripalani is a consultant to and holds equity in PictureRx, LLC. The terms of this agreement were reviewed and approved by Vanderbilt University in accordance with its conflict of interest policies. PictureRx did not provide materials or support for this review.
Dr. Jeffrey Schnipper – Dr. Schnipper is a consultant to the Society of Hospital Medicine in its Glycemic Control Mentored Implementation project. The terms of this agreement were reviewed and approved by Brigham and Women’s Hospital and Harvard Medical School in accordance with its conflict of interest policies.
References
- 1.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Jama. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 2.Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998 Apr 15;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005 Sep 12;165(16):1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD, Coleman EA, Min SJ. A new tool for identifying discrepancies in postacute medications for community-dwelling older adults. Am J Geriatr Pharmacother. 2004 Jun;2(2):141–147. doi: 10.1016/s1543-5946(04)90019-0. [DOI] [PubMed] [Google Scholar]
- 6.Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005 Feb 28;165(4):424–429. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 7.Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm. 2004 Aug 15;61(16):1689–1695. doi: 10.1093/ajhp/61.16.1689. [DOI] [PubMed] [Google Scholar]
- 8.Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008 Sep;23(9):1414–1422. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Cmaj. 2005 Aug 30;173(5):510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008 Oct;42(10):1373–1379. doi: 10.1345/aph.1L190. [DOI] [PubMed] [Google Scholar]
- 11.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005 Apr;20(4):317–323. doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995 Oct 9;155(18):1949–1956. [PubMed] [Google Scholar]
- 13.Institute for Healthcare Improvement. [Accessed January 7, 2010];Medication Reconciliation Review. 2007 http://www.ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/Medication+Reconciliation+Review.htm.
- 14.JOINT COMMISSION 2005 NATIONAL PATIENT SAFETY GOALS. [Accessed December 7, 2010];Medication Reconciliation. http://www.fojp.com/Focus_2005_1.pdf.
- 15.U.S. Preventive Services Task Force. [Accessed February 28, 2012];Criteria for Assessing Internal Validity of Individual Articles. http://www.uspreventiveservicestaskforce.org/uspstf08/methods/procmanualap7.htm.
- 16.Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007 May 28;167(10):1034–1040. doi: 10.1001/archinte.167.10.1034. [DOI] [PubMed] [Google Scholar]
- 17.Nickerson A, MacKinnon NJ, Roberts N, Saulnier L. Drug-therapy problems, inconsistencies and omissions identified during a medication reconciliation and seamless care service. Healthc Q. 2005;8(Spec No):65–72. doi: 10.12927/hcq..17667. [DOI] [PubMed] [Google Scholar]
- 18.Vasileff HM, Whitten LE, Pink JA, Goldsworthy SJ, Angley MT. The effect on medication errors of pharmacists charting medication in an emergency department. Pharm World Sci. 2009 Jun;31(3):373–379. doi: 10.1007/s11096-008-9271-y. [DOI] [PubMed] [Google Scholar]
- 19.Marotti SB, Kerridge RK, Grimer MD. A randomised controlled trial of pharmacist medication histories and supplementary prescribing on medication errors in postoperative medications. Anaesth Intensive Care. 2011 Nov;39(6):1064–1070. doi: 10.1177/0310057X1103900613. [DOI] [PubMed] [Google Scholar]
- 20.Chan AH, Garratt E, Lawrence B, Turnbull N, Pratapsingh P, Black PN. Effect of education on the recording of medicines on admission to hospital. J Gen Intern Med. 2010 Jun;25(6):537–542. doi: 10.1007/s11606-010-1317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharm World Sci. 2004 Apr;26(2):114–120. doi: 10.1023/b:phar.0000018601.11248.89. [DOI] [PubMed] [Google Scholar]
- 22.Mills PR, McGuffie AC. Formal medicine reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010 Dec;27(12):911–915. doi: 10.1136/emj.2009.082255. [DOI] [PubMed] [Google Scholar]
- 23.De Winter S, Vanbrabant P, Spriet I, et al. A simple tool to improve medication reconciliation at the emergency department. Eur J Intern Med. 2011 Aug;22(4):382–385. doi: 10.1016/j.ejim.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Lisby M, Thomsen A, Nielsen LP, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol. 2010 May;106(5):422–427. doi: 10.1111/j.1742-7843.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 25.Eggink RN, Lenderink AW, Widdershoven JW, van den Bemt PM. The effect of a clinical pharmacist discharge service on medication discrepancies in patients with heart failure. Pharm World Sci. 2010 Dec;32(6):759–766. doi: 10.1007/s11096-010-9433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergkvist A, Midlov P, Hoglund P, Larsson L, Bondesson A, Eriksson T. Improved quality in the hospital discharge summary reduces medication errors--LIMM: Landskrona Integrated Medicines Management. Eur J Clin Pharmacol. 2009 Oct;65(10):1037–1046. doi: 10.1007/s00228-009-0680-1. [DOI] [PubMed] [Google Scholar]
- 27.Midlov P, Deierborg E, Holmdahl L, Hoglund P, Eriksson T. Clinical outcomes from the use of Medication Report when elderly patients are discharged from hospital. Pharm World Sci. 2008 Dec;30(6):840–845. doi: 10.1007/s11096-008-9236-1. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009 May 11;169(9):894–900. doi: 10.1001/archinternmed.2009.71. [DOI] [PubMed] [Google Scholar]
- 29.Hellstrom LM, Bondesson A, Hoglund P, et al. Impact of the Lund Integrated Medicines Management (LIMM) model on medication appropriateness and drug-related hospital revisits. Eur J Clin Pharmacol. 2011 Jul;67(7):741–752. doi: 10.1007/s00228-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 30.Michels RD, Meisel SB. Program using pharmacy technicians to obtain medication histories. Am J Health Syst Pharm. 2003 Oct 1;60(19):1982–1986. doi: 10.1093/ajhp/60.19.1982. [DOI] [PubMed] [Google Scholar]
- 31.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 32.Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009 Apr;4(4):211–218. doi: 10.1002/jhm.427. [DOI] [PubMed] [Google Scholar]
- 33.Walker PC, Bernstein SJ, Jones JN, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009 Nov 23;169(21):2003–2010. doi: 10.1001/archinternmed.2009.398. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal A, Wu WY. Reducing medication errors and improving systems reliability using an electronic medication reconciliation system. Jt Comm J Qual Patient Saf. 2009 Feb;35(2):106–114. doi: 10.1016/s1553-7250(09)35014-x. [DOI] [PubMed] [Google Scholar]
- 35.Murphy EM, Oxencis CJ, Klauck JA, Meyer DA, Zimmerman JM. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm. 2009 Dec 1;66(23):2126–2131. doi: 10.2146/ajhp080552. [DOI] [PubMed] [Google Scholar]
- 36.Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009 Apr 27;169(8):771–780. doi: 10.1001/archinternmed.2009.51. [DOI] [PubMed] [Google Scholar]
- 37.Poole DL, Chainakul JN, Pearson M, Graham L. Medication reconciliation: a necessity in promoting a safe hospital discharge. J Healthc Qual. 2006 May-Jun;28(3):12–19. doi: 10.1111/j.1945-1474.2006.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 38.Boockvar KS, Blum S, Kugler A, et al. Effect of admission medication reconciliation on adverse drug events from admission medication changes. Arch Intern Med. 2011 May 9;171(9):860–861. doi: 10.1001/archinternmed.2011.163. [DOI] [PubMed] [Google Scholar]
- 39.Showalter JW, Rafferty CM, Swallow NA, Dasilva KO, Chuang CH. Effect of standardized electronic discharge instructions on post-discharge hospital utilization. J Gen Intern Med. 2011 Jul;26(7):718–723. doi: 10.1007/s11606-011-1712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varkey P, Cunningham J, O’Meara J, Bonacci R, Desai N, Sheeler R. Multidisciplinary approach to inpatient medication reconciliation in an academic setting. Am J Health Syst Pharm. 2007 Apr 15;64(8):850–854. doi: 10.2146/ajhp060314. [DOI] [PubMed] [Google Scholar]
- 41.Tessier EG, Henneman EA, Nathanson B, Plotkin K, Heelon M. Pharmacy-nursing intervention to improve accuracy andcompleteness of medication histories. Am J Health Syst Pharm. 2010 Apr 15;67(8):607–611. doi: 10.2146/ajhp090104. [DOI] [PubMed] [Google Scholar]
- 42.Greenwald JL, Halasyamani L, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010 Oct;5(8):477–485. doi: 10.1002/jhm.849. [DOI] [PubMed] [Google Scholar]