Abstract
Objectives
To evaluate the effect of a freeze–thaw cycle on β-trace protein (βTP) and β2-microglobulin (β2M).
Design and methods
We compared βTP and β2M concentrations before and after a single freeze–thaw cycle in long-term stored samples from 172 participants of the Third National Health and Nutrition Examination Survey (NHANES III).
Results
Measurements of βTP and β2M before and after freeze–thaw were highly correlated with Spearman’s coefficients of 0.90 and 0.99, respectively. Serum concentrations of βTP were slightly lower after freeze–thaw (−0.05 mg/L, P=0.006). Measurements of β2M did not differ before and after freeze–thaw (P=0.35).
Conclusions
βTP and β2M measurements were robust to a single freeze–thaw cycle, although β2M appeared more stable than βTP. These results have implications for future studies of these biomarkers.
Keywords: β-trace protein (βTP), β2-microglobulin, National Health and Nutrition Examination Survey, Freeze–thaw, Kidney filtration marker
Introduction
β-trace protein (βTP) and β2-microglobulin (β2M) are low molecular weight serum proteins freely filtered by the glomerulus and are increasingly being viewed as useful markers of glomerular filtration rate. βTP, a 168 amino acid glycoprotein weighing 23–29 kDa [1], originates from the choroid plexus of the central nervous system, while β2M, a subunit of the major histocompatibility (MHC) class I molecule weighing 12 kDa, is produced by all nucleated cells [2,3]. In clinical and epidemiological studies, researchers often rely on stored serum samples for the measurement of numerous biomarkers; however, previous studies have not described the potential effects of freeze–thaw cycles on βTP or β2M. Freeze–thaw cycles have been shown to denature some serum proteins and cause dissociation into inactive dimers [4–6] although many are robust to the freeze–thaw process [7]. The purpose of this study was to determine the influence of a single freeze–thaw cycle on concentrations of βTP and β2M measured in stored serum samples.
Materials and methods
We selected a random sample of 200 specimens obtained from persons 60 years or older who participated in the Third National Health and Nutrition Examination Survey (NHANES III), a large cross-sectional survey of the civilian non-institutionalized population conducted in 1988–1994 [8]. This freeze–thaw study was conducted as part of a larger project to measure markers of kidney function in stored serum samples in NHANES III [9,10]. Informed consent was obtained from all participants. Protocols for the conduct and implementation of this study were approved by the Institutional Review Boards of both the National Center for Health Statistics and the Johns Hopkins Bloomberg School of Public Health.
In 2009 the specimens, which had been frozen immediately after collection, were thawed and βTP and β2M were measured. These samples were refrozen and subsequently thawed for second βTP and β2M measurements 4 to 13 months later. Samples were stored at −70 °C until the time of βTP and β2M measurement. βTP and β2M were measured at the University of Minnesota using particle-enhanced immunonephelometric assays (N Latex β-trace protein assay and N Latex β2-microglobulin assay, Siemens Diagnostics, IL). The inter-assay coefficients of variation for the βTP and β2M assays were 5.7% (mean 0.594 mg/L) and 2.7% (mean 1.757 mg/L), respectively. Assays were performed from September 2009 through July 2010 and entailed 2 reagent lots and 2 calibrators for βTP, and 3 reagent lots and 2 calibrators for β2M. To monitor reagent performance, monthly means were calculated for both βTP and β2M, and controls were assayed twice daily during all assay runs. There were no progressive shifts in assay means for βTP or β2M.
For the two biomarkers, we excluded extreme outliers (difference>3 standard deviations from the mean; equivalent to 0.3% of observations from a normal distribution). We compared measurements before and after freeze–thaw using paired t-tests, Pearson’s and Spearman’s correlation coefficients, and the intra-class correlation coefficient using one-way analysis of variance (ANOVA). We also calculated the coefficient of variation of the difference between the two measurements (CVd), using the following equation:
CVd= standard deviation/mean
where x is the value after freeze–thaw, y is the value before freeze–thaw, and n is the total number of pairs.
Confidence intervals for the CVd were obtained by bootstrapping 1000 replications with a correction for bias. We used scatterplots and Bland–Altman plots to visually compare the βTP and β2M measurements before and after freeze–thaw. We fit linear regression models using Deming regression to correct for measurement error.
Results
After excluding 6 outliers, there were 172 samples with sufficient volume and valid measurements of βTP and β2M after the first and after the second freeze–thaw. The mean age of individuals in the study sample was 70 years (SD, 7). Forty eight percent were male, 58% were non-Hispanic white, 24% were non-Hispanic black, 16% were Mexican American, and 3% were of other race/ethnicity.
Summary statistics are shown in Table 1. Values of βTP before and after the freeze–thaw cycle were highly correlated with a Pearson’s correlation of 0.94. However, the values of βTP were slightly lower on average after the single freeze–thaw cycle (mean difference: −0.05 mg/L, 95% CI: −0.06, −0.04; P=0.006). The CVd was 13.2% (95% CI: 11.8, 14.6). Consistent with the slightly lower values of βTP after freeze–thaw, the slope from the Deming regression model was significantly less than 1.0 (slope=0.91; 95% CI: 0.85, 0.97) (Fig. 1A). The Bland–Altman plot shows that the difference for βTP was relatively consistent across the range of the data (Fig. 1C).
Table 1.
Summary statistics for serum measurements of β-trace protein and β2 microglobulin before and after a single freeze–thaw cycle, N=172 pairs.
| β-Trace protein (mg/L) | β2 Microglobulin (mg/L) | |
|---|---|---|
| Before freeze–thaw, reference rangea | 0.40 to 1.36 | 1.50 to 4.56 |
| After freeze–thaw, reference rangea | 0.43 to 1.45 | 1.51 to 4.43 |
| Before freeze–thaw, mean (SD) | 0.75 (0.23) | 2.42 (0.72) |
| After freeze–thaw, mean (SD) | 0.70 (0.22) | 2.42 (0.74) |
| Mean difference (before–after) (95% CI) | −0.05 (−0.06 to −0.04) | 0.01 (−0.01 to 0.02) |
| P-valueb | 0.006 | 0.35 |
| CVDifference, (95% CI)c | 13.2 (11.8 to 14.6) | 3.85 (3.25 to 4.44) |
| Pearson’s correlation, r | 0.94 | 0.99 |
| Intra-class correlation coefficient (95% CI) | 0.91 (0.86 to 0.97) | 0.98 (0.98 to 0.99) |
| Deming regression coefficient, β1 (SE) | 0.91 (0.03) | 1.03 (0.01) |
| Deming regression constant, βo (SE) | 0.02 (0.02) | −0.06 (0.03) |
| Linear regression with no constant, β (SE) | 0.93 (0.01) | 1.00 (0.003) |
The reference range represents the 2.5th to 97.5th percentiles.
Paired t-test of the hypothesis that the means are equal.
Confidence intervals generated from 1000 bootstrap replications.
Fig. 1.
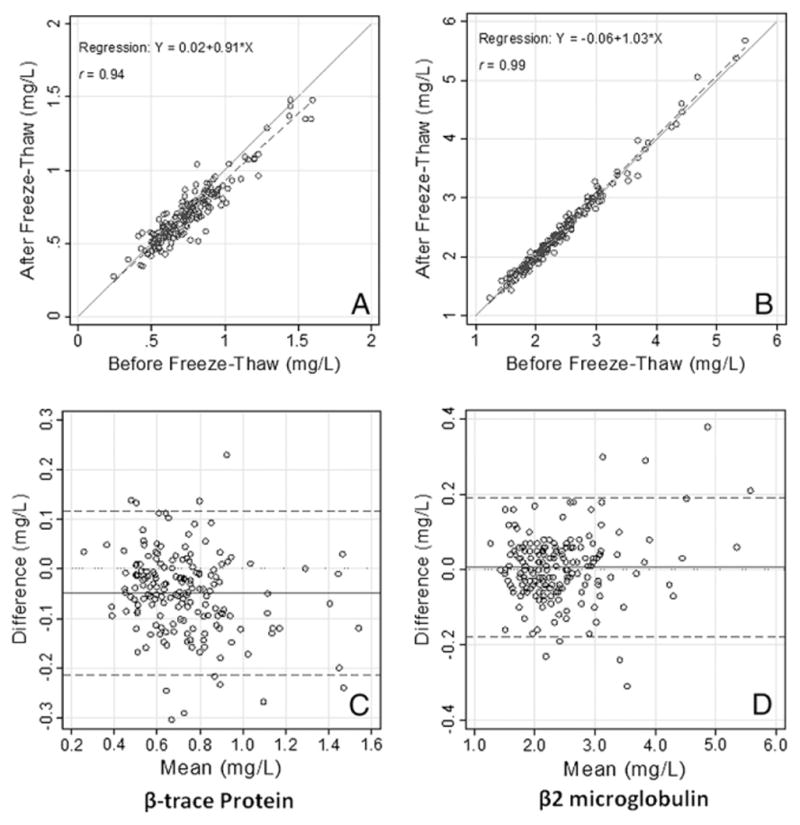
(A and B) Scatterplots comparing concentrations of βTP or β2M before and after a single freeze–thaw cycle. The solid line is the 45° line of identity (y=x). The dashed line is the Deming regression line. (C and D) Bland–Altman plots of the differences in concentrations of βTP or β2M before and after freeze–thaw versus the mean. The solid line is the mean difference, the dashed lines represent ±2 standard deviations.
Values of β2M were highly correlated with a Pearson’s correlation of 0.99 but there was no significant difference in values before and after the freeze–thaw cycle (mean difference: 0.01, 95% CI: −0.01, 0.02; P=0.35) (Table 1). Variability between the measurements was lower than for βTP, with a CVd of 3.85% (95% CI: 3.25, 4.44). The slope of the Deming regression line was slightly greater than the identity line (Fig. 1B) and was almost significantly different from 1.0 (slope=1.03; 95% CI: 1.00, 1.06). However, this positive slope was accompanied by a negative constant of −0.06, and linear regression with no constant negated most of the observed increase in slope (slope=1.0). The Bland–Altman plot shows that the differences appeared to be randomly distributed across the range of β2M values.
Discussion
In this study we evaluated the effects of a single freeze–thaw cycle on serum concentrations of βTP and β2M measured in long-term stored samples. We found that both of markers were robust to freeze–thaw with high correlations between the two measurements. We observed some evidence of a minor systematic difference in βTP after freeze–thaw, suggesting possible degradation. This small (6.7%) reduction in βTP after the additional freeze–thaw was slightly greater than the expected range of error for the assay (inter-assay CV of 5.7%). However, the correlation between βTP values before and after freeze–thaw was extremely high, such that the relative rankings of individuals would be unchanged. This would result in minimal to no effects on relative measures of association in epidemiologic studies such as relative risks or odds ratios.
For all tests of reliability, β2M performed better than βTP in this study. β2M had a lower CVd, higher correlation coefficients, and no significant difference in values before and after the freeze–thaw cycle. Our results suggest that β2M is more robust to freeze–thaw cycles compared to βTP. This difference may also reflect higher precision of the β2M assay (inter-assay CV of 2.7% for β2M vs 5.7% for βTP).
This study has several limitations that should be considered in the interpretation of the results. First, serum specimens were originally collected from NHANES participants in 1988–1994. Samples were stored for approximately 20 years prior to our first measurement. Thus, the results from this study may not be generalizable to fresh serum samples. Second, we only examined the impact of a single freeze–thaw cycle and there were 4–13 months between the two measurements.
Ultimately, our results suggest that measurements of βTP and β2M in long-term stored serum samples are relatively unaffected by a single freeze–thaw cycle. Our findings are consistent with prior research, suggesting limited effects of freeze–thaw cycles on a large number of different serum proteins stored at −70 °C [7]. These findings have important implications for clinical and epidemiologic studies that utilize previously thawed samples for the measurement of βTP or β2M.
Acknowledgments
ES, JHE, and GPR have no relevant financial relationships to disclose. JC has consulted for Amgen and Merck and has an investigator-initiated grant from Amgen. LAS has an investigator grant from Pharmalink and Gilead Inc. This project was partially funded by Siemens and by the National Institute of Diabetes and Digestive and Kidney Diseases grant U01 DK067651. SPJ is supported in part by the NIH/NHLBI Cardiovascular Epidemiology Training Grant T32HL007024. LAS is supported in part by the NIH/NIDDK grant K23DK081017. Siemens had no role in the study design, data collection, analysis, interpretation of data, or report writing.
Abbreviations
- βTP
β-trace protein
- β2M
β2-microglobulin
- NHANES III
Third National Health and Nutrition Examination Survey
- ANOVA
one-way analysis of variance
- CV
coefficient of variation
References
- 1.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. 1997;7:499–506. doi: 10.1093/glycob/7.4.499. [DOI] [PubMed] [Google Scholar]
- 2.Ploegh HL, Orr HT, Strominger JL. Major histocompatibility antigens: the human (HLA-A -B -C) and murine (H-2K H-2D) class I molecules. Cell. 1981;24:287–99. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- 3.Berggård I, Bearn AG. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968;243:4095–103. [PubMed] [Google Scholar]
- 4.Wei Y, Huang C, Chou W, Lee H. α-Crystallin protects human arginosuccinate lyase activity under freeze–thaw conditions. Biochimie. 2011 doi: 10.1016/j.bioci.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Privalov PL. Cold denaturation of proteins. Crit Rev Biochem Mol Biol. 1990;25:281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 6.Franks F. Protein destabilization at low temperatures. Adv Protein Chem. 1995;46:105–39. doi: 10.1016/s0065-3233(08)60333-2. [DOI] [PubMed] [Google Scholar]
- 7.Comstock GW, Burke AE, Norkus EP, Gordon GB, Hoffman SC, Helzlsouer KJ. Effects of repeated freeze–thaw cycles on concentrations of cholesterol, micro-nutrients, and hormones in human plasma and serum. Clin Chem. 2001;47:139–42. [PubMed] [Google Scholar]
- 8.Centers for Disease Control. [Accessed November 22, 2011.];NHANES–NHANES III—reports and reference manuals. Available at: http://www.cdc.gov/nchs/nhanes/nh3rrm.htm1988.
- 9.Köttgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation. 2008;51:385–94. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.NHANES III data documentation. [Accessed November 22, 2011.];Laboratory assessment: cystatin C (NHANES III surplus sera) Available at ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/nhanes/nhanes3/27a/SSCYSTAT.pdf2007.


