Abstract
Both HIV infection and high levels of early life stress (ELS) have been related to abnormalities in frontal-subcortical structures, yet the combined effects of HIV and ELS on brain structure and function have not been previously investigated. In this study we assessed 49 non-demented HIV-seropositive (HIV+) and 47 age-matched HIV-seronegative healthy control (HC) adults. Levels of ELS exposure were quantified and used to define four HIV-ELS groups: HC Low-ELS (N = 20); HC High-ELS (N = 27); HIV+ Low-ELS (N = 24); HIV+ High-ELS (N = 25). An automated segmentation tool measured volumes of brain structures known to show HIV-related or ELS-related effects; a brief neurocognitive battery was administered. A significant HIV-ELS interaction was observed for amygdala volumes, which was driven by enlargements in HIV+ High-ELS participants. The HIV+ High-ELS group also demonstrated significant reductions in psychomotor/processing speed compared with HC Low-ELS. Regression analyses in the HIV+ group revealed that amygdala enlargements were associated with higher ELS, lower nadir CD4 counts, and reduced psychomotor/processing speed. Our results suggest that HIV infection and high ELS interact to increase amygdala volume, which is associated with neurocognitive dysfunction in HIV+ patients. These findings highlight the lasting neuropathological influence of ELS and suggest that high ELS may be a significant risk factor for neurocognitive impairment in HIV-infected individuals.
Keywords: HIV, Stress, Amygdala, Neuroimaging, Cognition
INTRODUCTION
HIV infection can have significant neuropathological consequences (e.g., Jernigan et al., 2011). Several brain regions, including the basal ganglia, subcortical regions, and frontal cortices, are known to be particularly susceptible to HIV (Wiley et al., 1999). Consistent with these findings, neuro-imaging studies have commonly revealed volumetric, functional, and metabolic abnormalities within frontal-subcortical regions (Ances et al., 2006; Aylward et al., 1993; Becker et al., 2011; Cohen, Harezlak, Gongvatana, et al., 2010; Jernigan et al., 1993; Paul, Cohen, Navia, & Tashima, 2002; Towgood et al., 2012), which have in turn been linked to increased neurocognitive difficulty in HIV+ patients (Castelo, Sherman, Courtney, Melrose, & Stern, 2006; Cohen, Harezlak, Schifitto, et al., 2010; Harezlak et al., 2011; Melrose, Tinaz, Castelo, Courtney, & Stern, 2008; Moore et al., 2006; Paul, Ernst, et al., 2008).
HIV-associated neurocognitive disorders (HAND) represent a significant public health challenge. Up to 50% of individuals with HIV develop some form of cognitive impairment during the course of their disease (Heaton et al., 2010, 2011), suggesting that some patients might be at greater risk for developing neurocognitive impairment than others. In an era in which HIV-infected individuals are surviving into old age due to advances in combination antiretroviral therapies (cART), it is vital that we understand the various etiological factors associated with HAND to better prevent and treat these disorders (Clark & Cohen, 2010). Several factors have been associated with increased risk for neurocognitive impairment in HIV+ patients, including comorbid medical conditions [e.g., hepatitis C virus (HCV) (Clifford, Evans, Yang, & Gulick, 2005; Devlin et al., 2012; Martin-Thormeyer & Paul, 2009)] and psychosocial comorbidities [e.g., alcoholism (Fama, Rosenbloom, Nichols, Pfefferbaum, & Sullivan, 2009; Schulte, Mueller-Oehring, Rosenbloom, Pfefferbaum, & Sullivan, 2005), drug abuse (Rippeth et al., 2004)].
Early life stress (ELS) is a potentially important psychosocial factor that is likely associated with increased risk of HAND, yet it has received little attention with respect to HIV comorbidity. The HIV epidemic in the United States occurs largely within a context of considerable social inequality (Adimora et al., 2006; CDC, 2005; DHHS, 2001; Eaton & Muntaner, 1999; Hader, Smith, Moore, & Holmberg, 2001; Krueger, Wood, Diehr, & Maxwell, 1990; St Louis et al., 1991; Wenzel & Tucker, 2005). Consequently, substantial numbers of HIV-infected individuals are exposed to environmental conditions associated with high levels of ELS. There is strong evidence that significant exposure to ELS can have pathological effects on the brain (Andersen et al., 2008; Cohen, Grieve, et al., 2006; Mehta et al., 2009; Paul, Henry, et al., 2008; Seckfort et al., 2008; Stein, Koverola, Hanna, Torchia, & McClarty, 1997; Tottenham et al., 2009). It is, therefore, critical for neuroAIDS research to develop a better understanding of how ELS affects neuropathology in HIV+ patients, as it is possible that some of the observed HIV-related neural abnormalities may be partially attributable to, or even compounded by, the neuropathological effects associated with experiencing high levels of ELS.
Exposure to substantial amounts of ELS in otherwise healthy individuals has been associated with structural abnormalities in frontal-subcortical regions, including decreased anterior cingulate cortex (ACC) (Cohen, Grieve, et al., 2006), caudate (Cohen, Grieve, et al., 2006), and hippocampal (Andersen et al., 2008; Stein et al., 1997) volumes, as well as increased amygdala volumes (Mehta et al., 2009; Tottenham et al., 2009). There is thus significant overlap between the neuropathology of HIV and ELS, with both involving similar structures in frontal-subcortical networks. Yet, to our knowledge no study has examined the brain effects of ELS in HIV-infected individuals. In the current study we investigated whether high levels of ELS modulate HIV-related structural brain abnormalities and neurocognitive impairment. We examined the effects of HIV-serostatus and ELS exposure on brain structure and neurocognitive function in HIV-seropositive and HIV-seronegative individuals. Volumes of brain regions known to show HIV-related or ELS-related effects [i.e., caudate (Ances et al., 2006; Becker et al., 2011; Cohen, Grieve, et al., 2006; Cohen, Harezlak, Schifitto, et al., 2010), putamen (Becker et al., 2011), hippocampus (Andersen et al., 2008; Stein et al., 1997), ACC (Cohen, Grieve, et al., 2006), amygdala (Mehta et al., 2009; Tottenham et al., 2009)] were measured using magnetic resonance imaging (MRI). We administered a battery of neurocognitive measures and assessed the relation between brain structure and neurocognitive performance.
We predicted that the HIV+ group would demonstrate volumetric abnormalities in the caudate, putamen, and hippocampus (Becker et al., 2011; Cohen, Harezlak, Schifitto, et al., 2010; Hall et al., 1996; Jernigan et al., 1993; Paul, Ernst, et al., 2008; Petito, Roberts, Cantando, Rabinstein, & Duncan, 2001; Sa et al., 2004), and that individuals with high ELS exposure would display volumetric abnormalities in the ACC, caudate, hippocampus, and amygdala. We further predicted that HIV+ patients with high ELS would display the greatest volumetric effects. Lastly, we expected that both degree of ELS exposure (Tottenham et al., 2009) and markers of HIV-disease severity (e.g., nadir CD4 lymphocyte count) would correlate with degree of structural abnormality, and that greater volumetric abnormality would be associated with greater cognitive impairment in HIV-infected individuals (Cardenas et al., 2009; Childs et al., 1999; Cohen, Harezlak, Schifitto, et al., 2010; Jernigan et al., 2011; Valcour et al., 2006).
METHOD
Participants
We included 49 non-demented HIV-seropositive participants (HIV+) and 47 HIV-seronegative healthy control participants (HC). HIV+ participants were recruited from The Miriam Hospital Immunology Clinic. HC participants were acquaintances of HIV+ participants and individuals recruited from the community. All scored above 23 on the Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) and were fluent in English. We excluded participation on the basis of reported history of developmental or learning disability; major psychiatric illness (e.g., schizophrenia, bipolar disorder); positive urine toxicology (cocaine, methamphetamine, opiates); neurological illness affecting the central nervous system; and traumatic head injury with a loss of consciousness of 10 + min. Substance use exclusion criteria were current alcohol dependence; use of heroin/opiates/intravenous drugs within the past 12 months; and use of cocaine within the past 3 months. This research was approved by The Miriam Hospital’s Institutional Review Board. All individuals gave their informed consent and were financially compensated for their time.
Demographic Measures
Disease duration in HIV+ participants was obtained by self-report and verified against the medical record. History of cART use, nadir CD4 levels (i.e., the lowest ever CD4 count), current CD4 levels, and viral load (i.e., plasma HIV RNA levels) were obtained from the medical record. Forty-one of 49 HIV+ participants were on cART. Nadir CD4 levels ranged from 0 to 845 cells/μL. Current CD4 levels ranged from 105 to 1491 cells/μL. HIV+ participants were categorized in terms of viral load as undetectable (<75 copies/mL) or detectable (≥75 copies/mL); these data were unavailable for two participants. All HIV+ and HC participants were assessed for active HCV infection, defined as positive HCV antibody, determined by ELISA, and positive qualitative HCV RNA measured by PCR.
The Wechsler Test of Adult Reading (WTAR) estimated premorbid levels of intellectual function (Wechsler, 2001); scaled scores were derived using published normative data. The Kreek-McHugh-Schluger-Kellogg scale (KMSK) assessed history of alcohol and drug use, and provided three subscales that characterized lifetime consumption of alcohol (KMSK-A), cocaine (KMSK-C), and opiates (KMSK-O) (Kellogg et al., 2003). The Center for Epidemiological Studies-Depression Scale (CESD) (Radloff, 1977), Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983), and Posttraumatic Stress Disorder Checklist—Civilian (PCLC) (Weathers, Litz, Herman, Huska, & Keane, 1993) evaluated current levels of depression, stress, and posttraumatic stress disorder (PTSD) symptoms, respectively.
Early Life Stress Quantification
Participants completed the Early Life Stress Questionnaire (ELSQ) (Cohen, Paul, et al., 2006), which assessed the occurrence of 17 adverse life-events (e.g., family conflict, abuse, bullying, neglect) before age 18 years. The ELSQ has been used in several studies examining consequences of ELS exposure (Cohen, Grieve, et al., 2006; Cohen, Paul, et al., 2006; Paul, Henry, et al., 2008; Seckfort et al., 2008). This scale was selected based on its documented sensitivity in detecting neural abnormalities in relation to ELS history in adults (Cohen, Grieve, et al., 2006; Paul, Henry, et al., 2008; Seckfort et al., 2008).
HIV+ and HC groups were divided into High-ELS and Low-ELS exposure groups based on their ELSQ responses. A cut-point of ≥3 adverse events has been shown to provide the greatest discrimination on the basis of ELS for both behavioral and neural measures (Cohen, Grieve, et al., 2006; Cohen, Paul, et al., 2006; Seckfort et al., 2008). High-ELS was thus defined as endorsement of 3 or more ELS events, and Low-ELS was classified as endorsement of fewer than 3 events (Seckfort et al., 2008). Accordingly, HIV+ and HC participants were grouped as follows: HC Low-ELS (N = 20), HC High-ELS (N = 27), HIV+ Low-ELS (N = 24), and HIV+ High-ELS (N = 25). Table 1 details participant group characteristics.
Table 1.
Demographic characteristics of the participant groups
| Variable | HC Low-ELS (M/F = 10/10)
|
HC High-ELS (M/F = 14/13)
|
HIV+ Low-ELS (M/F = 13/11)
|
HIV+ High-ELS (M/F = 19/6)
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 45.95 | 12.78 | 41.96 | 10.34 | 46.92 | 9.19 | 44.24 | 10.34 |
| Education (years) | 14.45a | 3.41 | 12.59 | 2.93 | 12.33a | 1.69 | 12.80 | 2.16 |
| Ethnic composition | ||||||||
| % Caucasian American | 80.0 | 70.4 | 62.5 | 52.0 | ||||
| % African American | 10.0 | 22.2 | 16.7 | 32.0 | ||||
| % Hispanic American | 0.0 | 3.7 | 8.3 | 12.0 | ||||
| % Biracial or “other” | 10.0 | 3.7 | 12.5 | 4.0 | ||||
| % Male | 50.0 | 51.9 | 54.2 | 76.0 | ||||
| MMSE (/30) | 28.25 | 1.07 | 28.56 | 1.31 | 28.46 | 1.06 | 28.68 | 0.80 |
| WTAR (SS) | 104.25 | 16.11 | 101.81 | 14.28 | 95.17 | 15.40 | 96.24 | 16.60 |
| Number of ELS events | 1.20a,b | 0.77 | 5.30a,c | 2.23 | 1.42c,d | 0.78 | 4.68b,d | 1.70 |
| CESD (/60) | 10.50a | 8.17 | 17.76 | 15.38 | 16.42 | 12.58 | 24.12a | 14.19 |
| PSS (/70) | 20.75a | 8.52 | 22.19 | 8.86 | 19.21b | 7.27 | 26.83a,b | 7.59 |
| PCLC (/85) | 30.45 | 14.34 | 35.04 | 17.05 | 35.38 | 15.00 | 42.08 | 17.21 |
| KMSK-Alcohol (/13) | 8.00 | 3.96 | 10.07 | 3.54 | 9.33 | 4.05 | 9.17 | 4.40 |
| KMSK-Cocaine (/16) | 3.00a | 5.11 | 5.67 | 6.42 | 6.88 | 6.47 | 9.63a | 6.54 |
| KMSK-Opiate (/13) | 0.15 | 0.49 | 1.63 | 3.35 | 1.58 | 3.54 | 2.96 | 4.70 |
| HCV-infected (%) | 0.0* | 14.8 | 20.8 | 44.0** | ||||
| Length of infection (years) | 14.46 | 6.60 | 13.04 | 7.98 | ||||
| Number of patients on cART | 21 | 20 | ||||||
| Nadir CD4 (cells/μl) | 167.35 | 129.59 | 203.68 | 191.47 | ||||
| Current CD4 (cells/μl) | 560.96 | 313.51 | 460.92 | 257.35 | ||||
| Viral load, Number of patients | ||||||||
| Undetectable (<75 copies/mL) | 16 | 20 | ||||||
| Low (<400 copies/mL) | 1 | 0 | ||||||
| High (>400 copies/mL) | 6 | 4 | ||||||
Note. HC = Healthy Control; ELS = early life stress; M/F = Male-Female; MMSE = Mini-Mental State Exam; WTAR = Wechsler Test of Adult Reading; SS = Standard Score; CESD = Center for Epidemiologic Studies Depression Scale; PSS = The Perceived Stress Scale; PCLC = PTSD Checklist—Civilian; KMSK = Kreek-McHugh-Schluger-Kellogg scale; HCV = hepatitis C virus; cART = combination antiretroviral therapy. Across rows, means with the same superscript are significantly different at the p <.05 level. Asterisks indicate that the reported value significantly deviates from expected values as determined by the chi-square test,
p ≤.05,
p ≤.01.
Magnetic Resonance Imaging
Structural images of the brain were obtained using a 3-Tesla scanner (Siemens TIM Trio; Siemens, New York, NY). High-resolution T1-weighted MPRAGE images were acquired in the sagittal plane (resolution = 0.86 mm × 0.86 mm × 0.86 mm; TR = 2250 ms; TE = 3.06 ms; TI = 900 ms; flip angle = 9°; FOV = 220 mm).
Brain volumes were measured using the Individual Brain Atlases using Statistical Parametric Mapping Software (IBASPM) (Alemán-Gómez, Melie-García, & Valdés-Hernández, 2006) toolbox version 1.0 for Statistical Parametric Mapping 5 (SPM5; Wellcome Department of Imaging Science; www.fil.ion.ucl.ac.uk/spm/). Individual brain volumes were segmented into gray matter, white matter, and cerebrospinal fluid and normalized via nonlinear registration to the MNI152 template. Volume estimates were calculated for each parcellation as well as for total intracranial volume. Grey matter voxels were then aligned to a map of 116 anatomically labeled structures (Tzourio-Mazoyer et al., 2002), and inverse-transformed into their native spaces, producing volume measurements for individual brain structures in original space based on an MNI anatomical atlas. Brain segmentations were visually inspected for accuracy; none were discarded. Volumetric data were extracted for five regions of interest (ROI) in each hemisphere: ACC, caudate, putamen, hippocampus, and amygdala. We assessed unilateral rather than bilateral ROI volumes given the evidence that ELS may have lateralized effects (Maheu et al., 2010; Stein et al., 1997). All ROI volumes were corrected for total intracranial volume (by dividing each ROI volume by total intracranial volume) before analysis.
Neurocognitive Measures
A brief battery of neuropsychological tests sensitive in detecting HIV-related cognitive impairments (Heaton et al., 1995) was administered to examine abilities in four cognitive domains:
Psychomotor/Processing Speed: Trail Making Test, Part A (Reitan & Davison, 1974); Wechsler Adult Intelligence Scale III (WAIS-III) Symbol Search and Digit-Symbol Coding (Wechsler, 1997)
Executive Functioning: Trail Making Test, Part B (Reitan & Davison, 1974); verbal fluency (FAS) (Borkowski, Benton, & Spreen, 1967); WAIS-III Letter-Number Sequencing (Wechsler, 1997)
Fine-motor Dexterity: Grooved Pegboard Test, dominant and non-dominant hand performance (Klove, 1963)
Verbal Memory: Hopkins Verbal Learning Test – Revised, total trials 1–3 and delayed recall (Benedict, Schretlen, Groninger, & Brandt, 1998; Brandt & Benedict, 2001).
Raw scores were computed to Z-scores based on published normative data (Brandt & Benedict, 2001; Spreen & Strauss, 1998; Tombaugh, 2004; Wechsler, 1997). Z-scores for tests in each cognitive domain were averaged to compute a domain-specific composite score for each participant (Castelo, Courtney, Melrose, & Stern, 2007).
Statistical Analyses
One-way analyses of variance (ANOVAs) and χ2 tests were used to assess differences in demographic variables between the four groups. Post hoc comparisons were conducted using a Sidak correction for multiple comparisons. Two-way ANOVAs with factors of HIV-status and ELS-status were used to compare the four groups’ ROI volumes and cognitive composite scores, and to examine the interaction between HIV-status and ELS-status. Demographic variables that differed significantly between groups were entered as covariates. Post hoc comparisons were conducted using independent-samples t tests.
Linear regression analyses were conducted to assess the extent to which HIV-disease factors and ELS exposure affected the volumes of those ROIs that differed significantly between groups. Because our aim was to understand the effects of ELS on brain pathology in HIV+ patients, regression analyses were restricted to the HIV+ group. In each model, ROI volumes were entered as the dependent variable, and the independent variables included viral load group (undetectable/detectable), current CD4 count, nadir CD4 count, and ELSQ score. Separate regression analyses were implemented to examine the association between ROI volumes and cognitive functions for those cognitive composites on which significant group differences were observed. We restricted these analyses to the HIV+ group, as our goal was to understand the relation between brain volumes and neurocognitive impairment in HIV+ patients specifically. In these analyses, ROI volumes were entered as independent variables and composite scores were entered as the dependent variable.
RESULTS
Participant Demographics
The four groups were well matched in terms of age (p = .37), general cognitive status (MMSE; p = .60), estimated pre-morbid intelligence (WTAR; p = .16), proportion of Caucasian to non-Caucasian participants (χ2 = 4.26; p = .23) and male:female ratio (χ2 = 4.42; p = .22) (Table 1). Groups did not differ significantly on PTSD symptoms (PCLC; p = .11). Mean scores on the PTSD scale were below clinically significant levels (<44) for all groups.
Significant group differences were observed on years of education (F[3,92]= 2.87; p <.05), current stress levels (F[3,91]= 4.12; p <.01), current depression symptoms (F[3,90]= 3.93; p <.01), history of cocaine use (F[3,91]= 4.32; p <.01), and proportion of HCV-infected individuals (χ2 = 13.99; p <.01). Post hoc analyses indicated that HC Low-ELS reported higher education levels compared with HIV+ Low-ELS (p = .05). HIV+ High-ELS reported greater current stress levels than HIV+ Low-ELS (p <.01). Depression levels were higher in HIV+ High-ELS compared with HC Low-ELS (p <.01); mean CESD scores were above the cut-off score (>15), indicating significant depression levels, in all groups except HC Low-ELS. Lifetime history of cocaine use was higher in HIV+ High-ELS compared with HC Low-ELS (p <.01); however, mean KMSK-C scores were below clinical dependence levels (KMSK-C >10) for all four groups, as were mean scores for alcohol and opiate use (KMSK-A >10; KMSK-O >8). HC Low-ELS contained a significantly low number of HCV-infected individuals (Z = −2.0, p = .05), and HIV+ High-ELS contained a significantly high number of HCV-infected individuals (Z = 2.5; p = .01).
HIV+ High-ELS and HIV+ Low-ELS were not significantly different with respect to years diagnosed, current CD4 counts, nadir CD4 levels (all t’s <1.22, p’s >.23), proportion of individuals with detectable viral loads (χ2 = .59; p = .44), or percent of participants on cART (Fisher’s exact test; p = .70).
MRI Volumetrics
Mean ROI volumes for each group are shown in Table 2. Intracranial volumes did not differ significantly between groups (F[3,92]= .13; p = .94), permitting valid group comparisons using intracranial-corrected ROI volumes. Significant group differences were observed in the right and left amygdala (described below). Analyses in all additional ROIs were non-significant (p >.05).
Table 2.
Mean ROI volumes (cm3) for each group
| Brain region | HC Low-ELS
|
HC High-ELS
|
HIV+Low-ELS
|
HIV+High-ELS
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Right amygdala | 1.65 | 0.17 | 1.62a | 0.22 | 1.67b | 0.26 | 1.70a,b | 0.17 |
| Left amygdala | 1.38a | 0.13 | 1.43b | 0.19 | 1.44c | 0.20 | 1.47a,b,c | 0.15 |
| Right ACC | 3.91 | 0.62 | 4.10 | 0.98 | 3.66 | 0.69 | 3.68 | 0.67 |
| Left ACC | 4.30 | 0.80 | 4.68 | 1.18 | 4.35 | 0.97 | 4.24 | 0.73 |
| Right hippocampus | 4.17 | 0.50 | 4.21 | 0.80 | 4.34 | 0.75 | 4.36 | 0.56 |
| Left hippocampus | 4.39 | 0.59 | 4.43 | 0.83 | 4.60 | 0.88 | 4.50 | 0.62 |
| Right caudate | 4.16 | 0.70 | 4.13 | 0.67 | 4.36 | 0.86 | 4.27 | 0.70 |
| Left caudate | 4.03 | 0.65 | 3.91 | 0.58 | 4.03 | 0.76 | 4.06 | 0.85 |
| Right putamen | 3.73 | 0.58 | 3.82 | 0.74 | 3.95 | 0.66 | 3.96 | 0.54 |
| Left putamen | 3.94 | 0.60 | 4.05 | 0.78 | 4.15 | 0.75 | 4.17 | 0.66 |
Note. ROI = region of interest; HC = healthy control; ELS = early life stress; ACC = anterior cingulate cortex. Across rows, means with the same superscript differ significantly (p <.05) after correcting for total intracranial volume.
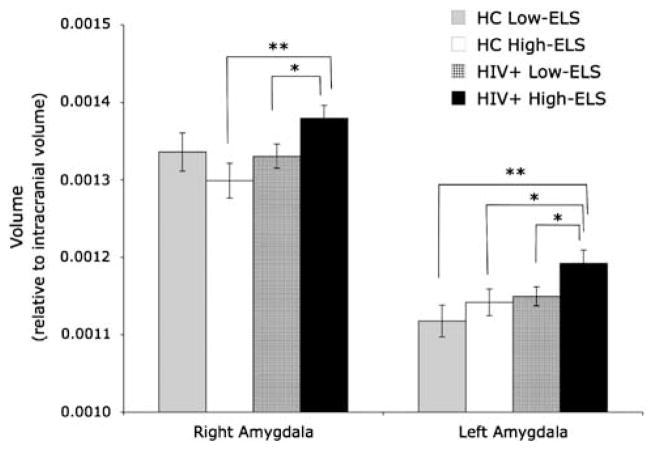
In the right amygdala (F[3,92] = 2.94; p = .04), we observed a significant interaction between HIV and ELS (F[1,92]= 4.53; p = .04), and a marginally significant main effect of HIV (F[1,93]= 3.92; p = .07), indicating that right amygdala volumes in HIV+ were significantly larger than those in HC (t[83.9]= 2.00; p = .05). These effects were significant (HIV: F[1,83]= 7.42; p <.01; HIV-ELS interaction: F[1,83]= 5.04; p = .03) even after controlling for group differences in education, current stress, depression, cocaine use history, and HCV status. To investigate the source of the HIV-ELS interaction, we conducted follow-up analyses, which revealed that the interaction was driven by higher volumes in HIV+ High-ELS compared with HIV+ Low-ELS (t[47] = 2.13; p = .04; r = .30) and HC High-ELS (t[50]= 2.84; p <.01, r = .37) (Figure 1).
Fig. 1.
Mean (+SEM) amygdala volume measures (corrected for intracranial volume) for each group. Note: *p ≤.05, **p <.01.
In the left amygdala (F[3,92]= 3.27; p = .02), we observed significant main effects of HIV (F[1,92]= 5.88; p = .02) and ELS (F[1,92]= 3.90; p = .05), indicating that left amygdala volumes for HIV+ were significantly larger than those of HC, and that left amygdala volumes in the High-ELS group were larger than those in the Low-ELS group. These effects were maintained (HIV: F[1,83]= 8.42; p <.01; ELS: F[1,83]= 5.56; p = .02) even after controlling for group differences in education, current stress, depression, cocaine use history, and HCV status. To further clarify the main effects, independent-samples t tests were conducted, which indicated that the group effects were driven by significant left amygdala enlargement in HIV+ High-ELS compared with HIV+ Low-ELS (t[47]= 2.00; p = .05; r = .28), HC High-ELS (t[50]= 2.05; p = .04; r = .28), and HC Low-ELS (t[43] = 2.81; p <.01; r = .39) (Figure 1).
Neurocognitive Measures
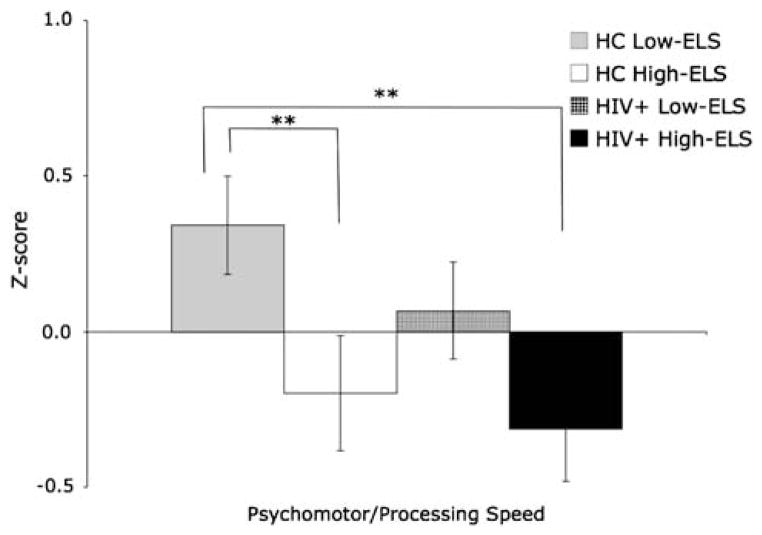
Analyses of the groups’ performances on the psychomotor/processing speed composite measure (F[3,91]= 3.47; p = .02) revealed a significant main effect of ELS (F[1,91]= 9.06; p <.01). This effect was maintained (F[1,82]= 3.36; p = .07) even after controlling for group differences in education, current stress, depression, cocaine use history, and HCV status. To further delineate the effect of ELS we conducted post hoc analyses, which revealed that participants in the HIV+ High-ELS and HC High-ELS groups were significantly slower on these measures compared with HC Low-ELS (t[43]= 2.89; p <.01; r = .40; t[45]= 2.58; p = .01; r = .36, respectively) (Figure 2). No additional significant group differences in composite scores were observed.
Fig. 2.
Mean (+SEM) group scores on the psychomotor/processing speed composite index. Note: **p ≤.01.
Relation of HIV-Disease Factors and ELS Exposure to ROI Volumes
The relation of amygdala volumes to HIV-disease factors (i.e., viral load, current CD4, nadir CD4) and ELS exposure (ELSQ score) in HIV+ was examined using linear regression. In the right amygdala we observed a trend level effect for ELS scores [β= .28 (t = 1.85; p = .07)], indicating that greater ELS exposure was associated with larger right amygdala volumes. In the left amygdala, enlargement was significantly associated with higher ELS scores [β= .39 (t = 2.59; p = .01)] and lower nadir CD4 counts (i.e., poorer historical immunological health) [β= −.40 (t = −2.24; p = .03)]. No additional variables were significant.
Relation of ROI Volumes to Neurocognitive Functions
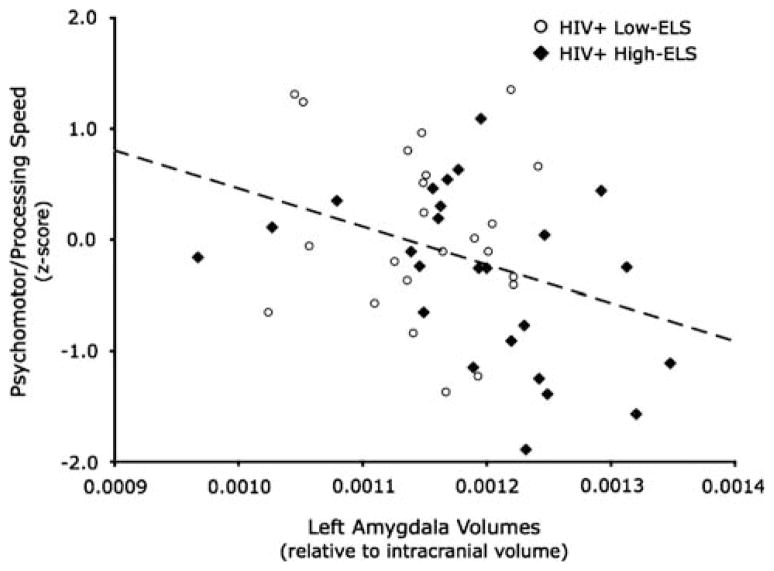
A linear regression analysis was conducted to examine the relation between amygdala volumes and psychomotor/processing speed in HIV+ participants. Left amygdala volumes were significant [β= −.34 (t = −2.24; p = .03)] in the resulting model, revealing that greater left amygdala volumes were associated with reduced psychomotor/processing speed (Figure 3), whereas right amygdala volumes were non-significant [β= .01 (t = .08; p = .94)]. Left amygdala volumes accounted for 10% of the variance in psychomotor/processing speed performance. This finding was maintained even after controlling for education, current stress, depression, cocaine use history, and HCV status [left amygdala: β= −.34 (t = −2.25; p = .03)]. Pearson correlations revealed that this relation was significant in HIV+ High-ELS (r = −.41; p = .04), but not in HIV+ Low-ELS (r = −.12; p = .60), HC High-ELS (r = .25; p = .21), or HC Low-ELS (r = .29; p = .22). However, Fisher’s r-to-z transformation showed that the correlation in HIV+ High-ELS was significantly different from HC Low-ELS (Z = −2.26; p = .01) and HC High-ELS (Z = −2.33; p <.01), but not HIV+ Low-ELS (Z = −1.03; p = .15).
Fig. 3.
Correlations between left amygdala volumes (corrected for intracranial volume) and psychomotor/processing speed composite scores in the HIV+ group.
Lastly, to exclude the possibility that the observed relation between left amygdala volumes and psychomotor/processing speed could result from shared variance between left amygdala and caudate or putamen volumes, which have been shown to be related to psychomotor/processing speed impairments in HIV+ patients (Castelo et al., 2007; Paul et al., 2002), a regression analysis controlling for volumes of these structures was conducted. The results revealed a significant association between left amygdala volumes and psychomotor/processing speed performance [β= −.41 (t = −2.37; p = .02)] even after controlling for caudate and putamen volumes.
DISCUSSION
To our knowledge, this is the first study conducted to examine whether high ELS is associated with increased HIV-related brain abnormalities in HIV+ adults. We report that HIV infection and high ELS exposure have combined effects on amygdala volumes and cognitive function, as HIV+ High-ELS individuals demonstrated larger amygdala volumes and greater impairment on tests of psychomotor/processing speed. Collectively, our results provide strong evidence that high ELS contributes significantly to structural brain and neurocognitive abnormalities in HIV+ patients.
More specifically, we found that HIV+ participants demonstrated greater amygdala volumes compared with HIV-seronegative participants – an effect that was strongly driven by the HIV+ HIGH-ELS group. Our observation of amygdala enlargement in HIV+ patients is novel and parallels prior results indicating HIV-related volumetric increases in other subcortical regions (Castelo et al., 2007). While there is prior evidence of HIV-related abnormalities in temporal limbic regions (Jernigan et al., 1993), there is little to no data regarding HIV-related structural changes in the amygdala specifically. Thus, the results of this study enhance our understating of the effects of HIV on the amygdala. Furthermore, our finding of an ELS effect on the amygdala is in direct agreement with recent findings indicating a positive correlation between ELS exposure and amygdala volumes in children whose HIV status was not examined (Tottenham et al., 2009). Our study extends this literature, as it provides evidence that ELS-related abnormalities in the amygdala remain years after the stressors have been experienced, consistent with results from animal studies revealing enduring stress-related structural changes in the amygdala (Vyas, Pillai, & Chattarji, 2004).
We found that both lower nadir CD4 levels and higher ELS exposure significantly predicted amygdala enlargement in HIV+ participants. These results provide strong evidence that both HIV infection and ELS history influence amygdala morphometry in HIV+ patients. The broader implication of these findings is that high ELS may produce changes in the amygdala that predispose individuals to experiencing greater HIV-related neuropathological effects in this region. Several converging lines of evidence indicate that the amygdala is sensitive to the long-term effects of stress (Baram & Hatalski, 1998; Kikusui & Mori, 2009; Mehta et al., 2009; Ono et al., 2008; Payne, Machado, Bliwise, & Bachevalier, 2010; Salzberg et al., 2007; Tottenham et al., 2009; Tottenham & Sheridan, 2010; Vyas et al., 2004). In addition, the amygdala undergoes rapid changes in early life (Baram & Hatalski, 1998; Payne et al., 2010), which may increase its susceptibility to adverse conditions during this period (Tottenham & Sheridan, 2010). Animal studies have demonstrated that the amygdala can sustain both structural (e.g., accelerated amygdala myelination, increased dendritic arborization) and functional (e.g., hyper-sensitization) changes in response to ELS (Baram & Hatalski, 1998; Brown, 2000; Kikusui & Mori, 2009; Ono et al., 2008; Payne et al., 2010; Salzberg et al., 2007; Tottenham & Sheridan, 2010), which persist even under improved environmental conditions (Vyas et al., 2004). Hence, it is possible that such changes may increase vulnerability to HIV-related neuropathology occurring in temporal limbic regions (Jernigan et al., 1993), leading to the observed amygdala enlargements in HIV+ High-ELS individuals. Our findings, taken together with those of previous studies indicating the susceptibility of the amygdala to ELS, strengthen the suggestion that the amygdala can undergo significant morphologic changes secondary to “legacy” events, such as those associated with HIV infection (i.e., reductions in nadir CD4) or high ELS exposure, which are resistant to recovery even after the causative agent has resolved.
Our study revealed that greater left amygdala enlargement in HIV+ participants was associated with greater impairment on tests of psychomotor and processing speed, illustrating the clinical significance of amygdala enlargements in HIV+ patients, especially those with high ELS. The observation that amygdala volumes may affect cognitive processing speed in HIV+ patients is compelling. While the amygdala is typically considered an affective brain region (Dolan & Vuilleumier, 2003; Liberzon, Phan, Decker, & Taylor, 2003), its activity can also impact cognitive functions (Drevets & Raichle, 1998; Ledoux, Lane, & Nadel, 2000; Yamasaki, LaBar, & McCarthy, 2002; Yun, Krystal, & Mathalon, 2010). These effects are believed to occur through rich connections between the amygdala and frontal-lobe regions responsible for higher-order cognitive functions (Amaral & Price, 1984; Barbas & De Olmos, 1990; Blair et al., 2007; Drevets & Raichle, 1998; Ghashghaei & Barbas, 2002; Hariri, Bookheimer, & Mazziotta, 2000; Pessoa, 2008, 2010; Price, 2003; Quirk, Likhtik, Pelletier, & Pare, 2003; Stefanacci, Suzuki, & Amaral, 1996; Stein et al., 2007; Yamasaki et al., 2002; Yun et al., 2010). Accordingly, our observed relation between left amygdala hypertrophy and reduced processing speed in HIV+ participants could result from abnormalities within amygdala-frontal lobe networks (Drevets & Raichle, 1998; Ledoux et al., 2000; Yamasaki et al., 2002; Yun et al., 2010)—a notion that is supported by evidence indicating a relation between left (but not right) amygdala-frontal lobe connectivity and cognitive performance (Yun et al., 2010). Future studies using functional MRI methods will be best to address this hypothesis.
As a whole, our HIV+ cohort did not display significant cognitive abnormalities compared to the HC group. However, we did observe marked differences in processing speed between the HIV+ High-ELS and HC Low-ELS groups, as HIV+ High ELS participants demonstrated mild impairment in this domain. The lack of an overall HIV effect is not surprising given recent findings in other samples of relatively healthy HIV+ patients on stable cART, indicating that cognitive impairments are commonly mild or even absent in such cohorts (Heaton et al., 2010, 2011; Melrose et al., 2008; Towgood et al., 2012). The fact that cognitive abnormalities were not detected in the HIV+ Low-ELS group underscores the potential importance of high ELS as a contributory factor in the emergence of cognitive decline in HIV+ patients. Notably, stress has been identified as an important precipitating agent in other neurodegenerative disorders (e.g., Alzheimer’s disease) (Tran, Srivareerat, & Alkadhi, 2010; Wilson et al., 2006). Such findings implicate high ELS as a risk factor for developing more rapid decline over time in HIV-infected individuals. While the current study cannot address this question, future longitudinal studies may provide more clarity on this possibility.
Several issues warrant further consideration. First, as with most research relying on retrospective reports, accurate quantification of ELS in the current study may be complicated by participant bias, revisionist recall error, and normal forgetting (Hardt & Rutter, 2004; Maughan & Rutter, 1997). Prospective studies of the effects of ELS on the brain are preferable, but this is less feasible in adult cohorts. That our ELS-related findings in the amygdala are in line with those observed in studies involving children, in which ELS was more discretely quantified (Mehta et al., 2009; Tottenham et al., 2009), provides some assurance against the possibility that our results may be driven by retrospective bias (e.g., that participants with larger amygdalae would have a greater tendency to endorse ELS-related events retrospectively).
Second, our observation of ELS-related effects in the amygdala differs from a previous study, conducted in an adult population, that reported non-significant differences in amygdala volumes between “no-ELS” and “high-ELS” groups (Cohen, Grieve, et al., 2006). The divergent findings are likely attributable to our inclusion of HIV+ patients, given that our ELS effect was driven by the HIV+ High-ELS group and that amygdala volumes in HC High-ELS and HC Low-ELS did not differ. This suggests that in adults [as opposed to children in whom acute effects of high ELS can be observed volumetrically (Mehta et al., 2009; Tottenham et al., 2009)] the effect of high ELS may be expressed as an increase in the susceptibility of the amygdala to later insults (e.g., HIV), while high ELS exposure alone may not be sufficient enough to result in significant amygdala enlargement. This is consistent with the results of Cohen, Grieve, et al. (2006), and in line with studies indicating that high ELS can confer a latent risk for negative health outcomes in later adulthood (Taylor, 2010).
Third, high ELS in the current study was defined as exposure to several different adverse events that may not be easily equated across individuals, and therefore, a simple summation of their effects is admittedly complex. Future studies should examine whether specific types of events [e.g., abuse (Andersen et al., 2008)], experienced within specific developmental periods, may have stronger neurological influences than others. Yet, there is evidence that adverse childhood events commonly co-occur (Dong et al., 2004). Accordingly, an assessment of a wide diversity of adverse events is likely beneficial, as this permits 1) a more comprehensive examination of early-life environmental conditions that have potential to impact the brain and 2) an examination of the possible additive effects of such events. Our results, in combination with prior findings (Cohen, Grieve, et al., 2006; Hedges & Woon, 2011; Paul, Henry, et al., 2008; Seckfort et al., 2008; Tomalski & Johnson, 2010), indicate that ELS history, as a broadly encompassing construct, deserves serious consideration as an important etiological factor with the potential to influence neural development and function over a lifetime. Such findings align well with a rapidly expanding literature documenting the negative effects of high ELS on a variety of health outcomes in adults (e.g., cancer, stroke, heart disease) (Chen, Matthews, & Boyce, 2002; Dube, Felitti, Dong, Giles, & Anda, 2003; Felitti et al., 1998; Gilbert et al., 2009; Repetti, Taylor, & Seeman, 2002; Smith, Hart, Blane, & Hole, 1998; Taylor, 2010).
Lastly, our groups differed with respect to education, current stress, depression, lifetime cocaine use, and HCV status. Although our analyses indicated that group differences in these variables did not account for amygdala volume abnormalities or reduced psychomotor/processing speeds observed in the HIV+ High-ELS group, it remains possible that these demographic differences may have an indirect influence on our findings. Future studies should investigate the brain effects of HIV and ELS in samples in which these demographic variables are more systematically controlled. Similarly, while our sample size is comparable to neuroimaging studies of this type (e.g., Castelo et al., 2007; Towgood et al., 2012), it would be useful to replicate our findings in a larger sample.
In conclusion, our data suggest that high levels of ELS exposure may be a significant risk factor for HAND, as we observed that high ELS is associated with increased neurological (i.e., amygdala) and neurocognitive abnormalities in HIV+ patients. This is the first evidence suggesting that significant exposure to ELS may contribute to cognitive impairment in adults presenting with an underlying neurological vulnerability (i.e., HIV). Notably, these findings suggest that high ELS deserves careful consideration as an important etiological factor in the development of neurodegenerative disease processes observed in adulthood (e.g., HAND, Alzheimer’s disease, etc.). In the case of HIV, additional studies are needed to determine whether high ELS and HIV contribute to increased neuropathology through additive, independent effects on the amygdala, or if high ELS and HIV interact synergistically at a more biological level [e.g., both acting to decrease immune system health and/or increase proinflammatory cytokine activity (Boyce et al., 1995; Glaser, Rice, Speicher, Stout, & Kiecolt-Glaser, 1986; Leserman, 2003, 2008; Miller, Cohen, & Ritchey, 2002; Zachariae, 2009)] to result in increased brain pathology. Such studies will likely lead to improvements in the detection and treatment of patients at risk for developing HAND by identifying possible biomarkers (e.g., cytokines) that may help to better classify patients at risk for developing HAND, as well as identifying those biomarkers that may be targeted pharmacologically in an effort to reduce HAND.
Acknowledgments
This work was supported by the National Institute of Mental Health (Grants R25 MH080663, R01 MH074368-02S1, and R01 MH074368-02) and by the Lifespan/Brown/Tufts Center for AIDS Research Grant (P30 AI042853). The medical team at The Miriam Hospital Immunology Clinic provided valuable aid in our recruitment efforts.
Footnotes
Preliminary data from this study were presented at the mid-year meeting of the International Neuropsychological Society, 2010.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Adimora AA, Schoenbach VJ, Martinson FE, Coyne-Beasley T, Doherty I, Stancil TR, Fullilove RE. Heterosexually transmitted HIV infection among African Americans in North Carolina. Journal of Acquired Immune Deficiency Syndromes. 2006;41(5):616–623. doi: 10.1097/01.qai.0000191382.62070.a5. [DOI] [PubMed] [Google Scholar]
- Alemán-Gómez Y, Melie-García L, Valdés-Hernández P. IBASPM: Toolbox for automatic parcellation of brain structures. Paper presented at the 12th Annual Meeting of the Organization for Human Brain Mapping; Florence, Italy. 2006. Jun 11–15, [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) Journal of Comparative Neurology. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Detre JA. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66(6):862–866. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: Results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: A key triggering mechanism for seizure generation in the developing brain. Trends in Neurosciences. 1998;21(11):471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. Journal of Comparative Neurology. 1990;300(4):549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Becker J, Sanders J, Madsen S, Ragin A, Kingsley L, Maruca V, Thompson P. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging and Behavior. 2011;5(2):77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- Boyce WT, Adams S, Tschann JM, Cohen F, Wara D, Gunnar MR. Adrenocortical and behavioral predictors of immune responses to starting school. Pediatric Research. 1995;38(6):1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict R. Hopkins Verbal Learning Test-Revised. Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Brown R. An introduction to neuroendocrinology. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Cardenas V, Meyerhoff D, Studholme C, Kornak J, Rothlind J, Lampiris H, Weiner M. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. Journal of Neurovirology. 2009;15(4):324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo JM, Courtney MG, Melrose RJ, Stern CE. Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Archives of Neurology. 2007;64(9):1275–1280. doi: 10.1001/archneur.64.9.1275. [DOI] [PubMed] [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- CDC. HIV Transmission among black women – North Carolina, 2004. Morbidity and Mortality Weekly Report. 2005;54(04):89–94. [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128(2):295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, McArthur JC. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52(3):607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- Clark US, Cohen RA. Brain dysfunction in the era of combination antiretroviral therapy: Implications for the treatment of the aging population of HIV-infected individuals. Current Opinion in Investigational Drugs. 2010;11(8):884–900. [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Evans SR, Yang Y, Gulick RM. The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. AIDS. 2005;19(Suppl 3):S64–S71. doi: 10.1097/01.aids.0000192072.80572.43. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Navia B. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. Journal of Neurovirology. 2010;16(6):435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of Neurovirology. 2010;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Paul RH, Stroud L, Gunstad J, Hitsman BL, McCaffery J, Gordon E. Early life stress and adult emotional experience: An international perspective. International Journal of Psychiatry in Medicine. 2006;36(1):35–52. doi: 10.2190/5R62-9PQY-0NEL-TLPA. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, Cohen RA. Neurocognitive Effects of HIV, Hepatitis C, and Substance Use History. Journal of the International Neuropsychological Society. 2012;18(1):68–78. doi: 10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS. Mental health: Culture, race, and ethnicity. A supplement to mental health: A report of the Surgeon General. Washington, DC: US Department of Health and Human Services; 2001. [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect. 2004;28(7):771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12(3):353–385. [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: Evidence from four birth cohorts dating back to 1900. Preventive Medicine. 2003;37(3):268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Muntaner C. Socioeconomic stratification and mental disorder. In: Horwitz AV, Scheid TK, editors. A handbook for the study of mental health: Social contexts, theories, and systems. NewYork: Cambridge University Press; 1999. pp. 259–283. [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: Baseline and 1-year follow-up examinations. Alcoholism, Clinical and Experimental Research. 2009;33(10):1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373(9657):68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK. Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behavioral Neuroscience. 1986;100(5):675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- Hader SL, Smith DK, Moore JS, Holmberg SD. HIV infection in women in the United States: Status at the Millennium. JAMA. 2001;285(9):1186–1192. doi: 10.1001/jama.285.9.1186. [DOI] [PubMed] [Google Scholar]
- Hall M, Whaley R, Robertson K, Hamby S, Wilkins J, Hall C. The correlation between neuropsychological and neuroanatomic changes over time in asymptomatic and symptomatic HIV-1-infected individuals. Neurology. 1996;46(6):1697–1702. doi: 10.1212/wnl.46.6.1697. [DOI] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of child psychology and psychiatry, and allied disciplines. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH The HNRC Group. The HNRC 500-Neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Hedges DW, Woon FL. Early-life stress and cognitive outcome. Psychopharmacology (Berl) 2011;214(1):121–130. doi: 10.1007/s00213-010-2090-6. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Grant I. Clinical factors related to brain structure in HIV: The CHARTER study. Journal of Neurovirology. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin RA, McCutchan JA, Grant I. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Archives of Neurology. 1993;50(3):250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. Journal of Neuroendocrinology. 2009;21(4):427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- Klove H. Grooved pegboard. Lafayette, IN: Lafayette Instruments; 1963. [Google Scholar]
- Krueger LE, Wood RW, Diehr PH, Maxwell CL. Poverty and HIV seropositivity: The poor are more likely to be infected. AIDS. 1990;4(8):811–814. [PubMed] [Google Scholar]
- Ledoux J, Lane RD, Nadel L. Cognitive-emotional interactions: Listen to the brain. In: Lane RD, Nadel L, Ahern GL, editors. Cognitive neuroscience of emotion. New York, NY: Oxford University Press; 2000. pp. 129–155. [Google Scholar]
- Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biological Psychiatry. 2003;54(3):295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic Medicine. 2008;70(5):539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: A PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective & Behavioral Neuroscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: Neurocognitive and neuroimaging features. Neuropsychology Review. 2009;19(2):215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Rutter M. Retrospective reporting of childhood adversity: Issues in assessing long-term recall. Journal of Personality Disorders. 1997;11(1):19–33. doi: 10.1521/pedi.1997.11.1.19. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Sonuga-Barke EJ. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: An fMRI investigation of semantic event sequencing. Behavioural Brain Research. 2008;188(2):337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Grant I. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20(6):879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male Balb/c mice. Neuroscience. 2008;156(4):1103–1110. doi: 10.1016/j.neuroscience.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Paul RH, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience and Biobehavioral Reviews. 2002;26(3):353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, Navia BA. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. Journal of the International Neuropsychological Society. 2008;14(5):725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, Gordon E. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric Disease and Treatment. 2008;4(1):193–201. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2010;20(8):922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L. Emergent processes in cognitive-emotional interactions. Dialogues in Clinical Neuroscience. 2010;12(4):433–448. doi: 10.31887/DCNS.2010.12.4/lpessoa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Roberts B, Cantando JD, Rabinstein A, Duncan R. Hippocampal injury and alterations in neuronal chemokine co-receptor expression in patients with AIDS. Journal of Neuropathology and Experimental Neurology. 2001;60(4):377–385. doi: 10.1093/jnen/60.4.377. [DOI] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reitan RM, Davison LA. Clinical neuropsychology: Current status and applications. Oxford, England: V.H. Winston & Sons; 1974. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: A quantitative Golgi study. Acta Neuropathologica. 2004;107(2):97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Salzberg M, Kumar G, Supit L, Jones NC, Morris MJ, Rees S, O’Brien TJ. Early postnatal stress confers enduring vulnerability to limbic epileptogenesis. Epilepsia. 2007;48(11):2079–2085. doi: 10.1111/j.1528-1167.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: Evidence from a Stroop Match-to-Sample task. Biological Psychiatry. 2005;57(1):67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LM, Gordon E. Early life stress on brain structure and function across the lifespan: A preliminary study. Brain Imaging and Behavior. 2008;2(1):49–58. [Google Scholar]
- Smith GD, Hart C, Blane D, Hole D. Adverse socioeconomic conditions in childhood and cause specific adult mortality: Prospective observational study. BMJ. 1998;316(7145):1631–1635. doi: 10.1136/bmj.316.7145.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- St Louis ME, Conway GA, Hayman CR, Miller C, Petersen LR, Dondero TJ. Human immunodeficiency virus infection in disadvantaged adolescents. Findings from the US Job Corps. JAMA. 1991;266(17):2387–2391. [PubMed] [Google Scholar]
- Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology. 1996;375(4):552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27(4):951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P, Johnson MH. The effects of early adversity on the adult and developing brain. Current Opinion in Psychiatry. 2010;23(3):233–238. doi: 10.1097/YCO.0b013e3283387a8c. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2009;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, Kopelman MD. Mapping the brain in younger and older asymptomatic HIV-1 men: Frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex. 2012;48(2):230–241. doi: 10.1016/j.cortex.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Tran TT, Srivareerat M, Alkadhi KA. Chronic psychosocial stress triggers cognitive impairment in a novel at-risk model of Alzheimer’s disease. Neurobiology of Disease. 2010;37(3):756–763. doi: 10.1016/j.nbd.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Valcour VG, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, Sacktor N. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection–The Hawaii Aging with HIV Cohort. Journal of Neurovirology. 2006;12(5):387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Wenzel SL, Tucker JS. Reemphasizing the context of women’s risk for HIV/AIDS in the United States. Womens Health Issues. 2005;15(4):154–156. doi: 10.1016/j.whi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim CL, Christopherson C, Kidane Y, Kwok S, Masliah E, Soontornniyomkij V. HIV mediates a productive infection of the brain. AIDS. 1999;13(15):2055–2059. doi: 10.1097/00002030-199910220-00007. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology. 2006;27(3):143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun RJ, Krystal JH, Mathalon DH. Working memory overload: Fronto-limbic interactions and effects on subsequent working memory function. Brain Imaging and Behavior. 2010;4(1):96–108. doi: 10.1007/s11682-010-9089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae R. Psychoneuroimmunology: A bio-psychosocial approach to health and disease. Scandinavian Journal of Psychology. 2009;50(6):645–651. doi: 10.1111/j.1467-9450.2009.00779.x. [DOI] [PubMed] [Google Scholar]