Abstract
Sulfate is ubiquitous in groundwater, with both natural and anthropogenic sources. Sulfate reduction reactions play a significant role in mediating redox conditions and biogeochemical processes for subsurface systems. They also serve as the basis for innovative in-situ methods for groundwater remediation. An overview of sulfate reduction in subsurface environments is provided, along with a brief discussion of characterization methods and applications for addressing acid mine drainage. We then focus on two innovative, in-situ methods for remediating sulfate-contaminated groundwater, the use of zero-valent iron (ZVI) and the addition of electron-donor substrates. The advantages and limitations associated with the methods are discussed, with examples of prior applications.
Keywords: groundwater pollution, stable isotope analysis, enhanced bioremediation, natural attenuation
1. Introduction
Sulfate occurs extensively in both natural and anthropogenic water systems. Primary natural sources of sulfate include atmospheric deposition, sulfate mineral dissolution, and sulfide mineral oxidation (e.g., Krouse and Mayer, 1999). Anthropogenic sources include coal mines, power plants, phosphate refineries, and metallurgical refineries (e.g., Seller and Canter, 1980). Higher levels of sulfate are common in the western part of the United States (MDH, 2008).
High concentrations of sulfate in ingested water can cause diarrhea in humans, especially infants (EPA, 1999). However, adults generally become accustomed to high sulfate concentrations after a few days (EPA, 1999). Sulfate in drinking water has a secondary maximum contaminant level (SMCL) of 250 mg/L, based on aesthetic effects (i.e., taste and odor) (EPA, 2009). It is estimated that about 3% of the public drinking water systems in the United States may have sulfate concentrations of 250 mg/L or greater (EPAwebsite).
In addition to its classification as a secondary contaminant, there is great interest in the transport and fate of sulfate in subsurface environments in relation to biogeochemical processes affecting natural and contaminated systems. For example, sulfate serves as a primary electron acceptor for anaerobic microbially-mediated transformation processes. Sulfate is reduced during such processes, and thus sulfate reduction is of great significance for many subsurface systems. In addition, sulfate reduction is of particular interest for systems with acidic metal-rich waters, such as mining-impacted sites, because the production of sulfide from sulfate reduction can effectively precipitate some heavy metals in the form of highly insoluble metal sulfides (e.g., Langmuir, 1997).
In light of growing interest in sulfate as a contaminant and, concomitantly, the feasibility of remediating groundwater contaminated by sulfate, we present an overview of sulfate reduction in subsurface systems. Methods available for characterizing sulfate reduction are discussed. The role of sulfate reduction in addressing acid mine drainage is reviewed. Finally, two innovative, in-situ methods for remediating sulfate-contaminated groundwater, the use of zero-valent iron (ZVI) and the addition of electron-donor substrates, are discussed.
2. Sulfate Reduction in Natural Groundwater Systems
Sulfate is the completely oxidized form of sulfur, and it is the most stable aqueous form of sulfur under aerobic conditions. Sulfur occurs in several oxidation states ranging from S2- to S6+, and its geochemical behavior, including transport and fate, is coupled to the aqueous redox conditions (e.g., Hem 1985; Langmuir, 1997). Sulfate is typically the first or second most abundant anion in natural waters (e.g., Hem 1985). Similar to how aqueous carbonate geochemistry is generally considered to exert a primary control over acid-base reactions for natural systems, sulfur geochemistry may be considered to represent a primary control over redox reactions for many environmental systems.
Sulfate reduction occurs extensively in natural groundwater systems. Sulfate is a redox-defining species in the Terminal Electron Accepting Process (TEAP) sequence, with sulfate reduction occurring after iron reduction and before methanogenic conditions. As such, it is the second most reducing condition in natural groundwater systems. Sulfate reduction involves the consumption of a substantial amount of hydrogen ions and the production of HS- at circumneutral pH. The general form of the reaction is given as (e.g., Christensen et al., 2000):
| (1) |
Furthermore, dissimilatory sulfate reduction under environmental conditions is conducted by a specialized group of prokaryotic bacteria through chemical reactions in which organic carbon (2) or H2 (3) is oxidized while sulfate is reduced (e.g., Canfield 2001a,b):
| (2) |
| (3) |
Thus, hydrogen ions are consumed and bicarbonate is produced, which leads to increases in pH. These aqueous geochemical signatures along with changes in redox conditions and redox sensitive species may be used as evidence for the occurrence and characterization of sulfate reduction.
Sulfate reduction in the subsurface is almost exclusively mediated by sulfate reducing bacteria (SRB) (e.g., Chambers and Trudinger, 1979; Gibson, 1990; Hao et al., 1996; Canfield, 2001a,b; Berner et al., 2002; and Aravena and Mayer, 2009). These bacteria are anaerobes that gain energy for growth from oxidation of organic matter using sulfate as the terminal electron acceptor (Hao et al., 1996; Barton and Tomei, 1995). While a large number of organic compounds have been shown to support SRB growth (Barton and Tomei, 1995), preferred carbon sources include organic acids (e.g., lactate, pyruvate, formate, and malate), volatile acids (e.g., acetate), and alcohols (e.g., ethanol, propanol, methanol, and butanol) (Hao et al., 1996). SRB are ubiquitous in the subsurface, and are present (but not likely metabolically active) under aerobic conditions as well as anaerobic conditions (e.g., Gibson, 1990; Bottrell et al., 1991; Fortin et al., 2002; Wargin et al., 2007; Praharaj and Fortin, 2008; Winch et al., 2009). A relatively wide range of genera of SRB has been identified (Hao et al., 1996).
The rate of sulfate reduction in groundwater has been examined in a number of studies. Both zeroth-order and first-order equations have been used to represent field data, depending on prevalent sulfate concentrations. Tabulated zeroth-order reaction rate coefficients for sulfate reduction can be found in several publications (Detmers et al., 2001; Jakobsen and Postma, 1994; McGuire et al., 2002). For example, McGuire et al. (2002) compiled zeroth-order sulfate reduction rate coefficients published in the literature. These values vary from about 2 × 10-6 mg/L/day to 3000 mg/L/day). Half-lives associated with first-order sulfate reduction rate coefficients reported for several field studies are compiled in Table 1. Considerable variation is observed among the studies, which may be caused by several factors, including differences in site conditions (e.g., microbial community, redox conditions, aqueous geochemistry, presence of organic contaminants) and measurement methods. The amount of substrate (organic carbon) and nutrients present are often limiting factors for sulfate reduction. For example, larger values are observed for systems with petroleum-hydrocarbon contaminants present compared to uncontaminated aquifers. Finally, it's worth noting that sulfate reduction rate coefficients have been measured for marine and lake sediments, and are typically orders of magnitude higher than those obtained for aquifers (Jakobsen and Postma, 1994).
Table 1.
Summary of first-order sulfate reduction half-lives from field studies.
| Location | Method | Sulfate Conc. (mg/L) | Half-lives | Contamination | Citation | |
|---|---|---|---|---|---|---|
| Uncontaminated | Sturgeon Falls, Canada | Depth profile (GW age vs conc.) | 6-27 | 1 year | None | (Robertson and Schiff, 1994) |
| Fuhrberger, Germany | Depth profile (GW age vs conc.) | 105-175 | 30-40 years | None | (Strebel et al., 1990) | |
| Contaminated | Hanahan, South Carolina | Flowpath (Conc. Vs Distance ) | 0-14 | 9-35 days | BTEX | (Chapelle et al., 1996) |
| Seal Beach, California | Tracer Test (BTC) | 80 | 7 days | Fuel hydrocarbons | (Cunningham et al., 2000) | |
| Studen, Switzerland | Single-well push pull (BTC) | 96 | 5-16 days | Petroleum hydrocarbons | (Schroth et al., 2001) | |
| Wurtsmith AFB, MI | Single-well push pull (BTC) | 10 | 0.1-0.2 days | Petroleum hydrocarbons and chlorinated solvents | (McGuire et al., 2002) | |
3. Characterizing Sulfate Reduction
Several methods or lines of evidence can be used to evaluate the occurrence of sulfate reduction. The baseline approach involves monitoring temporal and spatial changes in sulfate concentrations. However, decreases in sulfate concentrations can be caused by factors other than sulfate reduction, such as hydraulic processes (e.g., dilution) and precipitation of sulfate-bearing minerals (e.g., gypsum). Concurrently, the dissolution of sulfate minerals and the oxidation of sulfide-bearing minerals contribute to increases in sulfate concentrations, which may obscure the occurrence of sulfate reduction. Thus, observation of changes in sulfate concentration is not deterministic evidence for sulfate reduction. Consequently, the use of sulfate concentration data to characterize magnitudes and rate of reduction is influenced by great uncertainty.
As mentioned above, sulfate reduction can significantly impact aqueous geochemical conditions. Thus, changes in concentration of various aqueous-phase constituents are often monitored to evaluate the occurrence of sulfate reduction. For example, reduction of sulfate is often accompanied by the production of H2S, which has a rotten egg odor. This is strong, qualitative evidence for sulfate reduction. However, it is often difficult to quantify the concentration of H2S present. This is especially true when there are substantial amounts of iron or other metals present, as they can form metal sulfides that may obscure the actual amount of H2S produced. Sulfate reduction also affects the speciation and concentrations of metals present, as for example, through formation of metal sulfides and consumption of H+ (pH change). In addition, concurrent reduction of Fe(III) and sulfate may occur, which complicates assessment of changes in redox-sensitive species as an indicator of sulfate reduction.
Monitoring concentrations of sulfate and other redox-sensitive species can provide evidence of sulfate reduction. However, as noted, there is often significant uncertainty associated with such evaluations, particularly for quantitative assessments. Stable isotope analysis provides a means by which to improve the assessment of microbially-mediated transformation processes. Specifically, sulfur and oxygen isotopes of dissolved sulfate can be used to confirm the occurrence of sulfate reduction in groundwater.
Stable isotope analysis is based on the isotopic fractionation that occurs during biotransformation of compounds due to differential reaction rates among isotopes of a given element. Specifically, given the dynamics of isotopic fractionation, the parent compound becomes enriched in the heavier isotope and depleted in the lighter isotope as it undergoes biotransformation. The use of isotope fractionation for sulfur has been of interest for some time (e.g., Kaplan and Rittenberg, 1964), and a seminal review of isotopic fractionation for both oxidation and reduction of sulfur was presented by Chambers and Trudinger (1979). A number of studies have investigated sulfur isotope fractionation during sulfate reduction in the subsurface under natural conditions (Strebel et al., 1990; Robertson and Schiff, 1994; Aravena and Robertson, 1998; Krouse and Mayer, 1999; Spence et al., 2001; Berner et al., 2002; Einsiedl and Mayer, 2005; Li et al., 2008). For sulfate reduction, the residual aqueous sulfate would be enriched in the heavier sulfur and oxygen isotopes (i.e., 34S and 18O) versus the lighter isotopes (i.e. 32S and 16O) as the reaction proceeds and the concentration of aqueous sulfate decreases.
Sulfur and oxygen isotopes are expressed in delta (δ) notation as δ34SSO4 and δ18OSO4, respectively, which is defined for sulfur as:
| (4) |
where Rsample is the isotopic ratio (34S/32S) of the number of 34S and 32S atoms measured in a sample and Rstandard is the ratio for the reference material, which is a standard recommended by the International Atomic Energy Agency. Values of δ34SSO4 for sulfate that has undergone microbially-mediated reduction range from 20‰-56‰ based on field studies (Berner et al., 2002; Dogramaci et al., 2001; Robertson et al., 1989). Depending on site conditions, the overall sulfur isotope fractionation during bacterial dissimilatory reduction can vary from less than 10‰ to more than 45‰ (Aravena and Mayer, 2009).
Stable isotope analysis has proven to be a useful tool for characterizing sulfate reduction in groundwater for both natural and anthropogenically-affected systems. For example, isotopic analysis has been used to characterize sulfate sources and transformation processes at mining sites (Taylor, 1984; Sracek et al., 2004). However, there are several factors that can complicate and limit the application of stable isotope analysis, and which, in some cases, can result in false positives. For example, different sources of sulfate (e.g., sulfuric acid, atmospheric deposition, mineral dissolution) have different isotopic signatures. Thus, mixing of sulfate from multiple sources could result in temporal and spatial changes in the composite isotopic signature, which could lead to an incorrect conclusion that sulfate reduction was occurring (a false-positive result). Another constraint to isotope analysis is obtaining robust values for the source signature, which serves as the datum for isotope data analysis. In some cases, the original source may no longer be present at the site. In these cases, the sample with the least enrichment of the heavier isotope (i.e., that subjected to the least amount of transformation) is used as a surrogate. This approach introduces greater uncertainty to the analysis. While stable isotope analysis is a powerful tool, it should be used in combination with other approaches such as geochemical analysis, microbial methods, and mathematical modeling to enhance the robustness of the characterization efforts (e.g., Nijenhuis et al., 2007; Carreón-Diazconti et al., 2009; Carroll et al., 2009).
4. The Role of Sulfate Reduction in Remediation of Acid Mine Drainage
The US Forest Service reported between 27,000 and 39,000 abandoned mines on National Forest System federal lands nationwide (USFS, 2005). Most of these abandoned sites involved metals extraction. A major environmental impact of such mining is the generation of acid mine drainage (or acid rock drainage). Waste rock piles or tailings impoundments are exposed to air and rain that contain oxygen. Sulfide-rich minerals (e.g., pyrite) in the tailings are subject to a series of oxidation reactions that produce sulfate and hydrogen and can lead to the release of heavy metals such as As, Cd, Cu, Ni, Pb, and Zn. The general forms of the acid and sulfate generating reactions are:
| (5) |
| (6) |
Acid mine drainage has a low pH and typically has elevated concentrations of sulfate and heavy metals. It is estimated that about 20,000 to 50,000 mines are generating acidic drainage on US forest service lands (USDA, 1993). Acid mine drainage may be discharged into surface water and cause significant and persistent contamination. In the western US, between 5,000 and 10,000 miles of streams are impacted by metals mining (USDA, 1993). Infiltration of acid mine drainage can cause significant groundwater contamination.
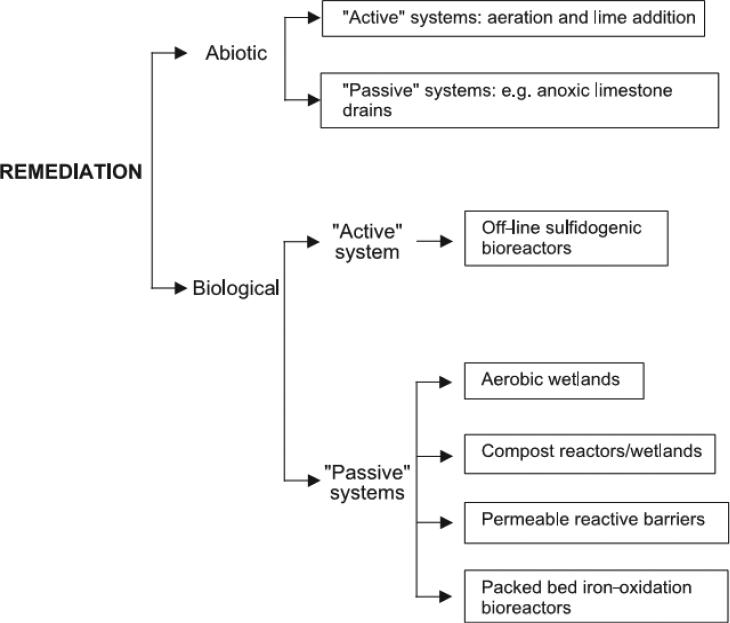
Johnson and Hallberg (2005) reviewed remediation options for acid mine drainage. Ideally, preventive actions should be taken to control the source to prevent contamination of groundwater. Source control measures include flooding/sealing of underground mines, underwater storage of mine tailings, and land-based storage in sealed waste heaps (Johnson and Hallberg, 2005). These approaches are aimed to prevent the contact of mine tailings with oxygen to minimize the production of acid mine drainage. Common remedial methods for sites with contaminated groundwater can be categorized into abiotic and biological methods, which include both active and passive systems as shown in Figure 1.
Figure 1.
Remediation options for acid mine drainage; figure reproduced from Johnson and Hallberg (2005).
Sulfate reduction plays a very important role in the treatment of acid mine drainage because the production of sulfide from sulfate reduction can substantially reduce heavy metal concentrations due to formation of low-solubility metal-sulfide precipitates, and because the coupled oxidation of organic matter in sulfate reduction produces bicarbonate that serves as a buffer for low pH. It has been shown that SRB can survive in very acid conditions (Church et al., 2007; Koschorreck, 2008). It is worth noting that not only can SRB reduce sulfate, but they can also reduce heavy metals directly. For example, Chang et al. (2001) described SRB that could reduce and immobilize uranium from U(VI) to U(IV) at a uranium mill tailings site. One species, Desulfotomaculum, was found to have remarkable tolerance and adaptation to high levels of uranium (Chang et al., 2001).
The use of wetlands is a common approach for treating surface water and groundwater contaminated by acid mine drainage. Anaerobic conditions are maintained due to the abundant natural organic matter, which also serves as substrate for bacteria such as SRB. Sulfate is reduced, forming insoluble metal sulfides and raising pH (Johnson and Hallberg, 2005).
Permeable reactive barriers (PRB) have been increasingly used for passive treatment of groundwater contaminated by acid mine drainage. Benner et al. (1997) described a full-scale PRB installed at the Nickel Rim mine site in Canada for treatment of acid mine drainage. The PRB is composed of 50% organic material, including municipal compost, leaf compost and wood chips, and 50% gravel to maintain high hydraulic conductivity. After passing through the PRB, sulfate concentrations decreased from 2400-4600 mg/L to 200-3600 mg/L; iron concentrations decreased from 250-1300 mg/L to 1.0-40 mg/L; and pH increased from 5.8 to 7.0 (Benner et al., 1997).
In-situ bioprecipitation (ISBP) is an emerging technology that has promise for treating subsurface acid mine drainage contamination. In this technology, electron donors (substrates for bacteria) are injected into wells to stimulate in-situ sulfate reduction and form a reactive zone for metal precipitation (e.g., Janssen and Temminghoff, 2004; Moon et al., 2009). Substrates that can be injected include stock reagents such as methanol, ethanol, lactate, and hydrogen (Bilek and Wagner, 2009) and less expensive waste products such as manure and sludge (Diels et al., 2005). A successful pilot field application in an aquifer with very unfavorable conditions (high Eh, low pH, low organic matter content, and low sulfate concentrations) illustrates the potential effectiveness of this approach (Janssen and Temminghoff, 2004).
The effectiveness of ISBP depends on many factors, including the type and concentration of amended electron donors, the type and concentration of contaminants, background sulfate concentration, pH, redox potential, and soil type (Diels et al., 2005). The selection of appropriate electron donors is generally the most important factor, as it influences the rates of reaction and the type and stability of the metal precipitates (Diels et al., 2005). Potential drawbacks to ISBP include non-persistence of precipitates after termination of substrate supply, potential clogging of pore spaces, and long lag time (Bilek, 2006; Vanbroekhoven et al., 2008). The long-term stability of the metal-sulfide precipitates generated is generally the primary issue for this method. While the results of some studies suggest that some metal precipitates do not undergo significant leaching (Bilek, 2006; Schols et al., 2008; Vanbroekhoven et al., 2009), the results of others have shown they are not always stable under various conditions (Beyenal and Lewandowski, 2004; Prommer et al., 2007).
5. Remediation of Sulfate-Contaminated Groundwater
Pump and treat is the standard method used for remediation of groundwater for which sulfate is the primary contaminant. This approach is effective at controlling the contaminant plume, but is generally cost and time intensive. Electrokinetic methods are another possible in-situ alternative for remediation (e.g., Runnells and Wahli, 1993). However, their use would typically be restricted to very small, shallow sites with relatively high concentrations. Interest is growing in the use of methods that are based on microbially-mediated processes as an alternative or adjunct to pump and treat. Two innovative, in-situ methods under current investigation involve the use of zero-valent iron (ZVI) and the addition of electron-donor substrates.
Zero valent iron has been used successfully for in-situ treatment of organic compounds such as chlorinated solvents, as well as for a range of inorganics such as arsenic (Kanel et al., 2005; Lackovic et al., 2000), nitrate (Della Rocca et al., 2007; Yang et al., 2008), heavy metals (e.g., nickel, mercury, silver, and cadmium), and radionuclides (e.g., UO22+) (Cantrell et al., 1995; Li et al., 2006; Ponder et al., 2000; Zhang, 2003). The results of several laboratory studies have indicated that significant decreases in sulfate concentrations can be achieved with treatment by ZVI (Bilek, 2006; Bilek and Wagner, 2009; Burghardt et al., 2007; Diels et al., 2005; Janssen and Temminghoff, 2004). Zero-valent iron can react directly with sulfate via abiotic reaction (Equation 7). More significantly, ZVI reacts with water to produce H2 and ferrous iron (Equation 8), after which sulfate is reduced by H2 to sulfide via microbially-mediated reactions and forms iron sulfide precipitates (Equation 9):
| (7) |
| (8) |
| (9) |
The results of laboratory studies have shown that minimal decreases in sulfate typically occur in the presence of sterile ZVI, indicating very low rates of abiotic sulfate reduction (Burghardt et al., 2007; Karri et al., 2005). Conversely, much higher rates of reduction are observed for systems with SRB present (Karri et al., 2005), which indicates the prime importance of microbially-mediated reduction for sulfate.
There are two primary methods by which ZVI may be employed. The common approach involves a permeable reactive barrier system, wherein the ZVI is placed within a trench to control for example the leading edge of a contaminant plume. To date, PRB applications for inorganics have been designed to target various metals, and have not been used for cases wherein sulfate is the sole contaminant. However, in some cases, sulfate was present as a co-contaminant, and significant concentration decreases were observed along with induced precipitation of metal sulfides (e.g. Johnson et al., 2008; Ludwig et al., 2009; Wilkin et al., 2005). Successful use of ZVI for sulfate treatment would possibly entail supplementation with an amendment to enhance activity of SRB populations.
The second approach for use of ZVI involves injection of colloidal forms of ZVI to target specific zones of contamination. Several field studies have been conducted illustrating the injection of colloidal ZVI for remediation of chlorinated-solvent contamination (Bennett et al., 2010; Elliott and Zhang, 2001; Elsner et al., 2010; Henn and Waddill, 2006; Quinn et al., 2005; Wei et al., 2010; Zhang, 2003). To the best of our knowledge, field studies of direct injection of colloidal ZVI for sulfate contamination have not been reported.
The advantages of colloidal ZVI injection compared to PRB systems are greater flexibility in targeting the source and high reactivity due to extremely high specific surface area of the colloidal ZVI (typically up to 30 times greater than granular iron used for PRBs) (Zhang, 2003). However, the drawbacks include relatively short longevity of the colloidal ZVI (weeks compared to years with PRB) and potential limited transport due to aggregation and strong attachment (He and Zhao, 2007; He et al., 2010; Saleh et al., 2005; Schrick et al., 2004; Zhang, 2003). Highly dispersed nanoscale ZVI particles stabilized with carboxymethyl cellulose (CMC) have been used to improve delivery (He and Zhao, 2005; He and Zhao, 2007; He et al., 2007). However, the results of field studies showed that transport distances were still limited for injected particles, with estimated maximum travel distances in the range of 1-2 meters (Bennett et al., 2010; He et al., 2010). Given these disadvantages, it appears that ZVI injection may be best suited for small, localized contaminant sources.
There is great interest in the use of electron-donor substrates to promote microbially-mediated reduction for the remediation of groundwater contaminated by a variety of inorganic elements. For example, this approach has been applied to systems with uranium-contaminated groundwater (Istok et al., 2004; Wu et al., 2006; Wu et al., 2007; Kelly et al., 2008; Englert et al., 2009; Hwang et al., 2009; Wilkins et al., 2009; Williams et al., 2009; Xu et al., 2010). Sulfate was a co-contaminant in several of these studies, and significant decreases in sulfate concentrations were typically observed after substrate addition. Based on these results, it is likely that addition of electron-donor substrates would serve as a potential method for sulfate remediation for systems wherein it is the principal contaminant.
Several laboratory studies have been conducted to evaluate the impact of electron-donor addition on sulfate reduction for various applications, including acid mine drainage (e.g., El Bayoumy et al., 1999; Glombitza, 2001; Johnson and Hallberg, 2002; Gibert et al., 2002), recovery of industrial wastewater (e.g., García-Saucedo et al., 2008; Eljamal et al., 2009; Meulepas et al., 2010), remediation of contaminated lakes and sediments (e.g. Smith and Klug, 1981; Lovley et al., 1995), and to investigate isotopic fractionation (e.g. Bolliger et al., 2001; Kleikemper et al., 2004). Results from these studies indicate that electron donors are essential for the treatment of sulfate-containing water and sediments by biologically mediated sulfate reduction. It is also clear that different electron donors can result in different magnitudes and rates of reaction. Liamleam and Annachhatre (2007) summarized sulfate reduction data including reduction rates, microbial biomass yield, and free energies of reactions by electron donor.
A primary concern for the use of the electron-donor approach for sulfate is the fate of sulfide produced from sulfate reduction. For the wetlands and ISBP technologies noted above for treatment of acid mine drainage, the sulfide is primarily sequestered through formation of metal-sulfide precipitates because of the abundance of metals generally associated with these systems. For the situation wherein sulfate is the primary contaminant, the fate of the sulfides will depend upon site conditions. If one or more metals (e.g., Fe, Mn) are abundant, the sulfide may form precipitates and be sequestered. The long-term stability of these precipitates would be of concern, as discussed above for the ISBP method. For sites with low abundance of metals, sulfate will to a great extent be converted to hydrogen sulfide. For circumneutral pH conditions, HS- will predominate, and it will be subject to aqueous-phase advective-dispersive transport. For lower-pH conditions, hydrogen sulfide gas may form, which can transport via gas-phase processes. Hydrogen sulfide is quite labile, and subject to re-oxidation to sulfate when it encounters groundwater with higher redox potential.
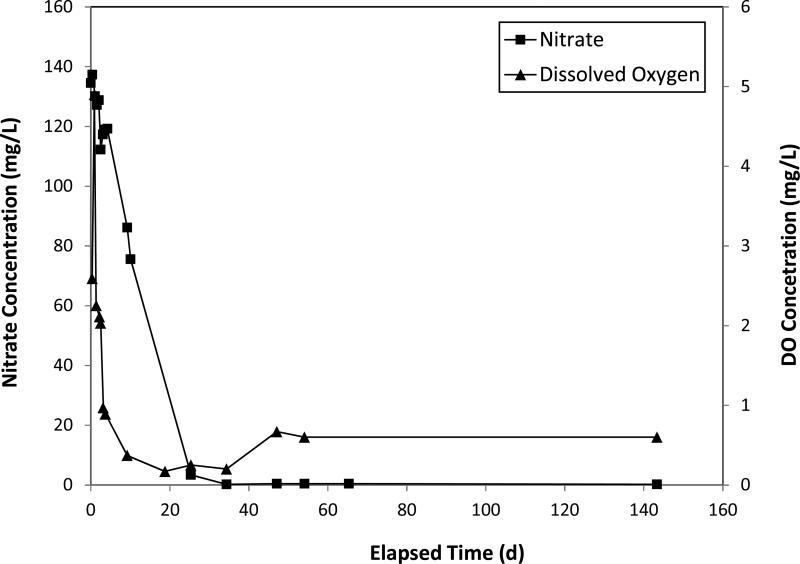
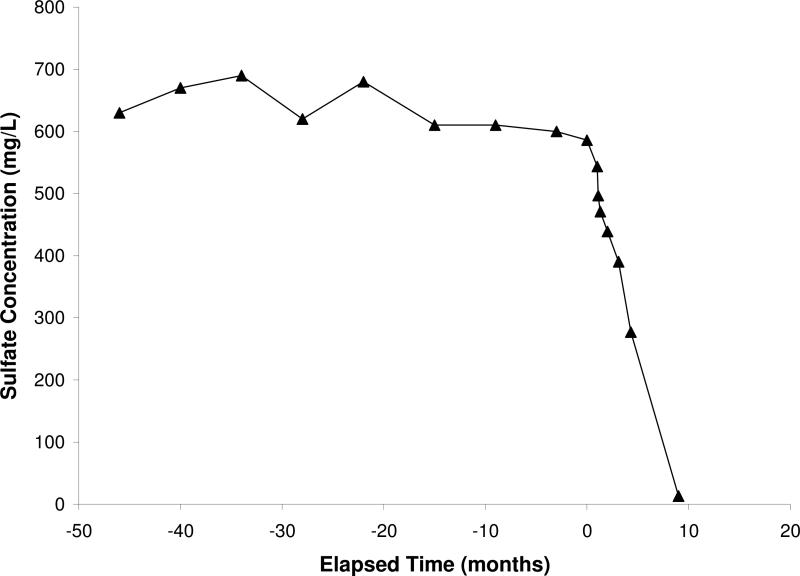
A pilot test using electron-donor addition for remediation of nitrate and sulfate contaminated groundwater was recently conducted at a former uranium mining site in Arizona. Ethanol was used as the substrate, and the test was conducted using the single-well injection method (Borden, 2010). The results showed that the concentration of nitrate and dissolved oxygen decreased (Figure 2), while the concentration of nitrous oxide (a product of denitrification) increased. Continued monitoring after completion of the pilot test has shown that nitrate concentrations surrounding the injection well have remained at levels three orders of magnitude lower than the initial value (~120 mg/L as N), indicating that the impact of the pilot test has been sustained for several months. Concentrations of sulfate were observed to decrease significantly after the ethanol injection test (see Figure 3), with concentrations of approximately 600 mg/L observed before the test decreasing to approximately 10 mg/L within one year after the test. Concomitantly, concentrations of hydrogen sulfide were observed to increase. These results suggest that introduction of the electron donor resulted in significant decreases in concentrations of both nitrate and sulfate via microbially-mediated reduction. The formation and fate of reaction products such as hydrogen sulfide, which will impact the long-term viability of this approach, need to be evaluated.
Figure 2.
Concentration of nitrate and dissolved oxygen for a pilot test of ethanol amendment injection conducted at Monument Valley, AZ.
Figure 3.
Sulfate concentrations measured for the pilot test conducted at Monument Valley, AZ. The zero point for elapsed time represents the start of the pilot test.
7. Summary
Sulfate is ubiquitous in groundwater, with both natural and anthropogenic sources. It is often used to help characterize hydrologic and biogeochemical interactions in subsurface environments. The standard approach for remediating groundwater contaminated by sulfate, pump and treat, is relatively expensive and time intensive. Innovative remediation methods include the use of zero valent iron or electron-donor substrates to promote in-situ sulfate reduction, primarily via microbially-mediated processes.
Pilot tests employing ZVI for other contaminants have been conducted successfully, and sulfate, present as a co-contaminant, has been observed to undergo reduction. These results indicate that the use of ZVI may be a viable approach for remediation of sulfate-contaminated groundwater. The results of a pilot-scale electron-donor test illustrate that the injection of a substrate to enhance biotransformation can effectively reduce sulfate concentrations. Enhanced reduction thus appears to also be a viable method for in-situ remediation of sulfate-contaminated groundwater. However, there is a need to evaluate potential constraints associated with these approaches, and to identify site conditions that would support successful implementation.
Acknowledgements
This research was supported by the University of Arizona TRIF Water Sustainability Program through the Center for Environmentally Sustainable Mining, Schlumberger Inc., and the NIEHS Superfund Research Program (P42 ES04940).
References
- Aravena R, Mayer B. Isotopes and processes in the nitrogen and sulfur cycles. In: Aelion CM, Höhener P, Hunkeler D, Aravena R, editors. Environmental Isotopes in Biodegradation and Bioremediation. CRC Press; 2009. pp. 203–246. [Google Scholar]
- Aravena R, Robertson WD. Use of multiple isotope tracers to evaluate denitrification in ground water: Study of nitrate from a large-flux septic system plume. Ground Water. 1998;36(6):975–982. [Google Scholar]
- Barton LL, Tomei FA. Characteristics and activities of sulfate-reducing bacteria. In: Barton LL, editor. Sulfate Reducing Bacteria. Springer-Verlag; New York: 1995. pp. 1–32. [Google Scholar]
- Benner SG, Blowes DW, Ptacek CJ. A full-scale porous reactive wall for prevention of acid mine drainage. Ground Water Monitoring and Remediation. 1997;17(4):99–107. [Google Scholar]
- Bennett P, He F, Zhao DY, Aiken B, Feldman L. In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. Journal of Contaminant Hydrology. 2010;116(1-4):35–46. doi: 10.1016/j.jconhyd.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Berner ZA, Stuben D, Leosson MA, Klinge H. S- and O-isotopic character of dissolved sulphate in the cover rock aquifers of a Zechstein salt dome. Applied Geochemistry. 2002;17(12):1515–1528. [Google Scholar]
- Beyenal H, Lewandowski Z. Dynamics of lead immobilization in sulfate reducing biofilms. Water Research. 2004;38(11):2726–2736. doi: 10.1016/j.watres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Bilek F. Column tests to enhance sulphide precipitation with liquid organic electron donators to remediate AMD-influenced groundwater. Environmental Geology. 2006;49(5):674–683. [Google Scholar]
- Bilek F, Wagner S. Testing in situ sulfate reduction by H2 injection in a bench-scale column experiment. Water Air and Soil Pollution. 2009;203(1-4):109–122. [Google Scholar]
- Bolliger C, Schroth MH, Bernasconi SM, Kleikemper J, Zeyer J. Sulfur isotope fractionation during microbial sulfate reduction by toluene-degrading bacteria. Geochimica Et Cosmochimica Acta. 2001;65(19):3289–3298. [Google Scholar]
- Borden A. M.S. Thesis. University of Arizona; Tucson, AZ: 2010. Pilot Test of Enhanced Nitrate Attenuation at the Uranium Mill Tailing Site in Monument Valley, Arizona. [Google Scholar]
- Bottrell SH, Smart PL, Whitaker F, Raiswell R. Geochemistry and isotope systematics of sulfur in the mixing zone of Bahamian blue holes. Applied Geochemistry. 1991;6(1):97–103. [Google Scholar]
- Burghardt D, Simon E, Knoller K, Kassahun A. Immobilization of uranium and arsenic by injectible iron and hydrogen stimulated autotrophic sulphate reduction. Journal of Contaminant Hydrology. 2007;94(3-4):305–314. doi: 10.1016/j.jconhyd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Canfield DE. Stable Isotope Geochemistry. Vol. 43. Mineralogical Soc America; Washington: 2001a. Biogeochemistry of sulfur isotopes. pp. 607–636. Reviews in Mineralogy & Geochemistry. [Google Scholar]
- Canfield DE. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochimica Et Cosmochimica Acta. 2001b;65(7):1117–1124. [Google Scholar]
- Cantrell KJ, Kaplan DI, Wietsma TW. Zero-valent iron for the in-situ remediation of selected metals in groundwater. Journal of Hazardous Materials. 1995;42(2):201–212. [Google Scholar]
- Carreon-Diazconti C, Santamaria J, Berkompas J, Field JA, Brusseau ML. Assessment of in situ reductive dechlorination using compound-specific stable isotopes, functional gene PCR, and geochemical data. Environmental Science & Technology. 2009;43(12):4301–4307. doi: 10.1021/es803308q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KC, Jordan FL, Glenn EP, Waugh WJ, Brusseau ML. Comparison of nitrate attenuation characterization methods at the Uranium mill tailing site in Monument Valley, Arizona. Journal of Hydrology. 2009;378(1-2):72–81. [Google Scholar]
- Chambers LA, Trudinger PA. Microbiological fractionation of stable sulfur isotopes - review and critique. Geomicrobiology Journal. 1979;1(3):249–293. [Google Scholar]
- Chang YJ, Peacock AD, Long PE, Stephen JR, McKinley JP, Macnaughton SJ, et al. Diversity and characterization of sulfate-reducing bacteria in groundwater at a uranium mill tailings site. Applied and Environmental Microbiology. 2001;67(7):3149–3160. doi: 10.1128/AEM.67.7.3149-3160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapelle FH, Bradley PM, Lovley DR, Vroblesky DA. Measuring rates of biodegradation in a contaminated aquifer using field and laboratory methods. Ground Water. 1996;34(4):691–698. [Google Scholar]
- Christensen TH, Bjerg PL, Banwart SA, Jakobsen R, Heron G, Albrechtsen HJ. Characterization of redox conditions in groundwater contaminant plumes. Journal of Contaminant Hydrology. 2000;45(3-4):165–241. [Google Scholar]
- Church CD, Wilkin RT, Alpers CN, Rye RO, McCleskey RB. Microbial sulfate reduction and metal attenuation in pH 4 acid mine water. Geochemical Transactions. 2007;8(10) doi: 10.1186/1467-4866-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JA, Hopkins GD, Lebron CA, Reinhard M. Enhanced anaerobic bioremediation of groundwater contaminated by fuel hydrocarbons at Seal Beach, California. Biodegradation. 2000;11(2-3):159–170. doi: 10.1023/a:1011167709913. [DOI] [PubMed] [Google Scholar]
- Della Rocca C, Belgiorno V, Meric S. Overview of in-situ applicable nitrate removal processes. Desalination. 2007;204(1-3):46–62. [Google Scholar]
- Detmers J, Schulte U, Strauss H, Kuever J. Sulfate reduction at a lignite seam: Microbial abundance and activity. Microbial Ecology. 2001;42(3):238–247. doi: 10.1007/s00248-001-1014-8. [DOI] [PubMed] [Google Scholar]
- Diels L, Geets J, Dejonghe W, Van Roy S, Vanbroekhoven K, Szewczyk A, et al. Heavy metal immobilization in groundwater by in situ bioprecipitation: comments and questions about carbon source use, efficiency and sustainability of the process. 9th International Mine Water Congress in Oviedo. 2005:355–360. [Google Scholar]
- Dogramaci SS, Herczeg AL, Schiff SL, Bone Y. Controls on delta S-34 and delta O-18 of dissolved sulfate in aquifers of the Murray Basin, Australia and their use as indicators of flow processes. Applied Geochemistry. 2001;16(4):475–488. [Google Scholar]
- Einsiedl F, Mayer B. Sources and processes affecting sulfate in a karstic groundwater system of the Franconian Alb, southern Germany. Environmental Science & Technology. 2005;39(18):7118–7125. doi: 10.1021/es050426j. [DOI] [PubMed] [Google Scholar]
- El Bayoumy M, Bewtra JK, Ali HI, Biswas T. Removal of heavy metals and cod by SRB in UAFF reactor. Journal of Environmental Engineering-Asce. 1999;125(6):532–539. [Google Scholar]
- Eljamal O, Jinno K, Hosokawa T. Modeling of solute transport and biological sulfate reduction using low cost electron donor. Environmental Geology. 2009;56(8):1605–1613. [Google Scholar]
- Elliott DW, Zhang WX. Field assessment of nanoscale biometallic particles for groundwater treatment. Environmental Science & Technology. 2001;35(24):4922–4926. doi: 10.1021/es0108584. [DOI] [PubMed] [Google Scholar]
- Elsner M, Couloume GL, Mancini S, Burns L, Lollar BS. Carbon isotope analysis to evaluate nanoscale Fe(O) treatment at a chlorohydrocarbon contaminated site. Ground Water Monitoring and Remediation. 2010;30(3):79–95. [Google Scholar]
- Englert A, Hubbard SS, Williams KH, Li L, Steefel CI. Feedbacks between hydrological heterogeneity and bioremediation induced biogeochemical transformations. Environmental Science & Technology. 2009;43(14):5197–5204. doi: 10.1021/es803367n. [DOI] [PubMed] [Google Scholar]
- EPA . Health effects from exposure to high levels of sulfate in drinking water study. U.S. Environmental Protection Agency; 1999. [Google Scholar]
- EPA . National secondary drinking water regulation. U.S. Environmental Protection Agency; 2009. [Google Scholar]
- EPA website . Sulfate in drinking water. U.S. Environmental Protection Agency; http://www.epa.gov/safewater/contaminants/unregulated/sulfate.html. [Google Scholar]
- Fortin D, Rioux JP, Roy M. Geochemistry of iron and sulfur in the zone of microbial sulfate reduction in mine tailings. Water, Air, & Soil Pollution: Focus. 2002;2(3):37–56. [Google Scholar]
- Garcia-Saucedo C, Fernandez FJ, Buitron G, Cuervo-Lopez FM, Gomez J. Effect of loading rate on TOC consumption efficiency in a sulfate reducing process: sulfide effect in batch culture. Journal of Chemical Technology and Biotechnology. 2008;83(12):1648–1657. [Google Scholar]
- Gibert O, de Pablo J, Cortina JL, Ayora C. Treatment of acid mine drainage by sulphate-reducing bacteria using permeable reactive barriers: A review from laboratory to full-scale experiments. Reviews in Environmental Science and Biotechnology. 2002;1(4):327–333. [Google Scholar]
- Gibson GR. Physiology and ecology of the sulphate-reducing bacteria. Journal of Applied Microbiology. 1990;69:769–797. doi: 10.1111/j.1365-2672.1990.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Glombitza F. Treatment of acid lignite mine flooding water by means of microbial sulfate reduction. Waste Management. 2001;21(2):197–203. doi: 10.1016/s0956-053x(00)00061-1. [DOI] [PubMed] [Google Scholar]
- Hao OJ, Chen JM, Huang L, Buglass RL. Sulfate-reducing bacteria. Critical Reviews in Environmental Science and Technology. 1996;26(2):155–187. [Google Scholar]
- He F, Zhao DY. Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environmental Science & Technology. 2005;39(9):3314–3320. doi: 10.1021/es048743y. [DOI] [PubMed] [Google Scholar]
- He F, Zhao DY. Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environmental Science & Technology. 2007;41(17):6216–6221. doi: 10.1021/es0705543. [DOI] [PubMed] [Google Scholar]
- He F, Zhao DY, Liu JC, Roberts CB. Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Industrial & Engineering Chemistry Research. 2007;46(1):29–34. [Google Scholar]
- He F, Zhao DY, Paul C. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Research. 2010;44(7):2360–2370. doi: 10.1016/j.watres.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Hem JD. Study and interpretation of the chemical characteristics of natural water. U.S. Geological Survey Water-Supply; 1985. Paper 2254. [Google Scholar]
- Henn KW, Waddill DW. Utilization of nanoscale zero-valent iron for source remediation—A case study. Remediation Journal. 2006;16(2):57–77. [Google Scholar]
- Hwang CC, Wu WM, Gentry TJ, Carley J, Corbin GA, Carroll SL, et al. Bacterial community succession during in situ uranium bioremediation: spatial similarities along controlled flow paths. Isme Journal. 2009;3(1):47–64. doi: 10.1038/ismej.2008.77. [DOI] [PubMed] [Google Scholar]
- Istok JD, Senko JM, Krumholz LR, Watson D, Bogle MA, Peacock A, et al. In situ bioreduction of technetium and uranium in a nitrate-contaminated aquifer. Environmental Science & Technology. 2004;38(2):468–475. doi: 10.1021/es034639p. [DOI] [PubMed] [Google Scholar]
- Jakobsen R, Postma D. In-situ rates of sulfate reduction in an aquifer (Romo, Denmark) and implications for the reactivity of organic-matter. Geology. 1994;22(12):1103–1106. [Google Scholar]
- Janssen G, Temminghoff EJM. In situ metal precipitation in a zinc-contaminated, aerobic sandy aquifer by means of biological sulfate reduction. Environmental Science & Technology. 2004;38(14):4002–4011. doi: 10.1021/es030131a. [DOI] [PubMed] [Google Scholar]
- Johnson DB, Hallberg KB. Pitfalls of passive mine water treatment. Reviews in Environmental Science and Biotechnology. 2002;1(4):335–343. [Google Scholar]
- Johnson DB, Hallberg KB. Acid mine drainage remediation options: a review. Science of the Total Environment. 2005;338(1-2):3–14. doi: 10.1016/j.scitotenv.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Thoms RB, Johnson RO, Nurmi JT, Tratnyek PG. Mineral precipitation upgradient from a zero-valent iron permeable reactive barrier. Ground Water Monitoring and Remediation. 2008;28(3):56–64. [Google Scholar]
- Kanel SR, Manning B, Charlet L, Choi H. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environmental Science & Technology. 2005;39(5):1291–1298. doi: 10.1021/es048991u. [DOI] [PubMed] [Google Scholar]
- Kaplan IR, Rittenberg SC. Microbiological fractionation of sulphur isotopes. Journal of General Microbiology. 1964;34(2):195–212. doi: 10.1099/00221287-34-2-195. [DOI] [PubMed] [Google Scholar]
- Karri S, Sierra-Alvarez R, Field JA. Zero valent iron as an electron-donor for methanogenesis and sulfate reduction in anaerobic sludge. Biotechnology and Bioengineering. 2005;92(7):810–819. doi: 10.1002/bit.20623. [DOI] [PubMed] [Google Scholar]
- Kelly SD, Kemner KM, Carley J, Criddle C, Jardine PM, Marsh TL, et al. Speciation of uranium in sediments before and after in situ biostimulation. Environmental Science & Technology. 2008;42(5):1558–1564. doi: 10.1021/es071764i. [DOI] [PubMed] [Google Scholar]
- Kleikemper J, Schroth MH, Bernasconi SM, Brunner B, Zeyer J. Sulfur isotope fractionation during growth of sulfate-reducing bacteria on various carbon sources. Geochimica Et Cosmochimica Acta. 2004;68(23):4891–4904. [Google Scholar]
- Koschorreck M. Microbial sulphate reduction at a low pH. Fems Microbiology Ecology. 2008;64(3):329–342. doi: 10.1111/j.1574-6941.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- Krouse HR, Mayer B. Sulfur and oxygen isotopes in sulphate. In: Cook PG, Herczeg AL, editors. Environmental Tracers in Subsurface Hydrology. Kluwer; Boston: 1999. pp. 195–231. [Google Scholar]
- Lackovic JA, Nikolaidis NP, Dobbs GM. Inorganic arsenic removal by zero-valent iron. Environmental Engineering Science. 2000;17(1):29–39. [Google Scholar]
- Langmuir D. Aqueous Environmental Geochemistry. Prentice-Hall; 1997. [Google Scholar]
- Li L, Fan MH, Brown RC, Van Leeuwen JH, Wang JJ, Wang WH, et al. Synthesis, properties, and environmental applications of nanoscale iron-based materials: A review. Critical Reviews in Environmental Science and Technology. 2006;36(5):405–431. [Google Scholar]
- Li XQ, Zhou AG, Liu CF, Cai HS, Wu JB, Gan YQ, et al. 34S and 18O isotopic evolution of residual sulfate in groundwater of the Hebei plain. Diqiu Xuebao/Acta Geoscientica Sinica. 2008;29(6):745–751. [Google Scholar]
- Liamleam W, Annachhatre AP. Electron donors for biological sulfate reduction. Biotechnology Advances. 2007;25(5):452–463. doi: 10.1016/j.biotechadv.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lovley DR, Coates JD, Woodward JC, Phillips EJP. Benzene oxidation coupled to sulfate reduction. Applied and Environmental Microbiology. 1995;61(3):953–958. doi: 10.1128/aem.61.3.953-958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig RD, Smyth DJA, Blowes DW, Spink LE, Wilkin RT, Jewett DG, et al. Treatment of arsenic, heavy metals, and acidity using a mixed ZVI-compost PRB. Environmental Science & Technology. 2009;43(6):1970–1976. doi: 10.1021/es802394p. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Long DT, Klug MJ, Haack SK, Hyndman DW. Evaluating behavior of oxygen, nitrate, and sulfate during recharge and quantifying reduction rates in a contaminated aquifer. Environmental Science & Technology. 2002;36(12):2693–2700. doi: 10.1021/es015615q. [DOI] [PubMed] [Google Scholar]
- MDH . Sulfate in well water. Minnesota Department of Health, Well Management Section, Environmental Health Division; 2008. [Google Scholar]
- Meulepas R, Stams A, Lens P. Biotechnological aspects of sulfate reduction with methane as electron donor. Reviews in Environmental Science and Biotechnology. 2010;9(1):59–78. [Google Scholar]
- Moon HS, Komlos J, Jaffe PR. Biogenic U(IV) oxidation by dissolved oxygen and nitrate in sediment after prolonged U(VI)/Fe(III)/SO42- reduction. Journal of Contaminant Hydrology. 2009;105(1-2):18–27. doi: 10.1016/j.jconhyd.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Nijenhuis I, Nikolausz M, Koth A, Felfoldi T, Weiss H, Drangmeister J, et al. Assessment of the natural attenuation of chlorinated ethenes in an anaerobic contaminated aquifer in the Bitterfeld/Wolfen area using stable isotope techniques, microcosm studies and molecular biomarkers. Chemosphere. 2007;67(2):300–311. doi: 10.1016/j.chemosphere.2006.09.084. [DOI] [PubMed] [Google Scholar]
- Ponder SM, Darab JG, Mallouk TE. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environmental Science & Technology. 2000;34(12):2564–2569. [Google Scholar]
- Praharaj T, Fortin D. Seasonal variations of microbial sulfate and iron reduction in alkaline Pb-Zn mine tailings (Ontario, Canada). Applied Geochemistry. 2008;23(12):3728–3740. [Google Scholar]
- Prommer H, Grassi ME, Davis AC, Patterson BM. Modeling of microbial dynamics and geochemical changes in a metal bioprecipitation experiment. Environmental Science & Technology. 2007;41(24):8433–8438. doi: 10.1021/es071123n. [DOI] [PubMed] [Google Scholar]
- Quinn J, Geiger C, Clausen C, Brooks K, Coon C, O'Hara S, et al. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environmental Science & Technology. 2005;39(5):1309–1318. doi: 10.1021/es0490018. [DOI] [PubMed] [Google Scholar]
- Robertson WD, Cherry JA, Schiff SL. Atmospheric sulfur deposition 1950-1985 inferred from sulfate in groundwater. Water Resources Research. 1989;25(6):1111–1123. [Google Scholar]
- Robertson WD, Schiff SL. Fractionation of sulfur isotopes during biogenic sulfate reduction below a sandy forested recharged area in south-central Canada. Journal of Hydrology. 1994;158(1-2):123–134. [Google Scholar]
- Runnells DD, Wahli C. Insitu electromigration as a method for removing sulfate, metals, and other contaminants from ground-water. Ground Water Monitoring and Remediation. 1993;13(1):121–129. [Google Scholar]
- Saleh N, Phenrat T, Sirk K, Dufour B, Ok J, Sarbu T, et al. Adsorbed triblock copolymers deliver reactive iron nanoparticles to the oil/water interface. Nano Letters. 2005;5(12):2489–2494. doi: 10.1021/nl0518268. [DOI] [PubMed] [Google Scholar]
- Schols E, Swennen R, Smolders E, Diels L, B. L, Vanbroekhoven K. Stability of metal precipitation formed during immobilization of metals from polluted groundwater by means of in situ precipitation.. UFZ/TNO International Conference on Soil–Water Systems; Milano, Italy. 3–6 June 2008. [Google Scholar]
- Schrick B, Hydutsky BW, Blough JL, Mallouk TE. Delivery vehicles for zerovalent metal nanoparticles in soil and groundwater. Chemistry of Materials. 2004;16(11):2187–2193. [Google Scholar]
- Schroth MH, Kleikemper J, Bolliger C, Bernasconi SM, Zeyer J. In situ assessment of microbial sulfate reduction in a petroleum-contaminated aquifer using push-pull tests and stable sulfur isotope analyses. Journal of Contaminant Hydrology. 2001;51(3-4):179–195. doi: 10.1016/s0169-7722(01)00128-0. [DOI] [PubMed] [Google Scholar]
- Seller LE, Canter LW. Sulfates in surface and ground water. National Center for Ground Water Research; Norman, Oklahoma: 1980. [Google Scholar]
- Smith RL, Klug MJ. Electron-donors utilized by sulfate-reducing bacteria in eutrophic lake-sediments. Applied and Environmental Microbiology. 1981;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence MJ, Bottrell SH, Thornton SF, Lerner DN. Isotopic modelling of the significance of bacterial sulphate reduction for phenol attenuation in a contaminated aquifer. Journal of Contaminant Hydrology. 2001;53(3-4):285–304. doi: 10.1016/s0169-7722(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Sracek O, Choquette M, Gelinas P, Lefebvre R, Nicholson RV. Geochemical characterization of acid mine drainage from a waste rock pile, Mine Doyon, Quebec, Canada. Journal of Contaminant Hydrology. 2004;69(1-2):45–71. doi: 10.1016/S0169-7722(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Strebel O, Bottcher J, Fritz P. Use of isotope fractionation of sulfate-sulfur and sulfate-oxygen to assess bacterial desulfurication in a sandy aquifer. Journal of Hydrology. 1990;121(1-4):155–172. [Google Scholar]
- Taylor BE, Wheeler MC, Nordstrom DK. Isotope composition of sulfate in acid-mine drainage as measure of bacterial oxidation. Nature. 1984;308(5959):538–541. [Google Scholar]
- USDA . Acid drainage from mines on the National Forest: A management challenge. U.S. Forest Service Publication(1505); 1993. pp. 1–12. [Google Scholar]
- USFS Distribution of abandoned and inactive mines on National Forest System Lands. RMRS Publications: RM General Technical Reports (GTR) 2005 [Google Scholar]
- Vanbroekhoven K, Satyawali Y, Roy SV, Vangeel S, Gemoets J, Muguet S, et al. Tenth International In Situ and On-Site Bioremediation Symposium, Baltimore, MD, May 5–8 2009. Battelle Memorial Institute; Columbus, OH: Stability of metal precipitates formed after in situ bioprecipitation induced by sulfidogenesis. (Vol. Paper A-04) [Google Scholar]
- Vanbroekhoven K, Van Roy S, Diels L, Gemoets J, Verkaeren P, Zeuwts L, et al. Sustainable approach for the immobilization of metals in the saturated zone: In situ bioprecipitation. Hydrometallurgy. 2008;94(1-4):110–115. [Google Scholar]
- Wargin A, Olanczuk-Neyman K, Skucha M. Sulphate-reducing bacteria, their properties and methods of elimination from groundwater. Polish Journal of Environmental Studies. 2007;16(4):639–644. [Google Scholar]
- Wei YT, Wu SC, Chou CM, Che CH, Tsai SM, Lien HL. Influence of nanoscale zero-valent iron on geochemical properties of groundwater and vinyl chloride degradation: A field case study. Water Research. 2010;44(1):131–140. doi: 10.1016/j.watres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wilkin RT, Su CM, Ford RG, Paul CJ. Chromium-removal processes during groundwater remediation by a zerovalent iron permeable reactive barrier. Environmental Science & Technology. 2005;39(12):4599–4605. doi: 10.1021/es050157x. [DOI] [PubMed] [Google Scholar]
- Wilkins MJ, VerBerkmoes NC, Williams KH, Callister SJ, Mouser PJ, Elifantz H, et al. Proteogenomic monitoring of geobacter physiology during stimulated uranium bioremediation. Applied and Environmental Microbiology. 2009;75(20):6591–6599. doi: 10.1128/AEM.01064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KH, Kemna A, Wilkins MJ, Druhan J, Arntzen E, N'Guessan AL, et al. Geophysical monitoring of coupled microbial and geochemical processes during stimulated subsurface bioremediation. Environmental Science & Technology. 2009;43(17):6717–6723. doi: 10.1021/es900855j. [DOI] [PubMed] [Google Scholar]
- Winch S, Mills HJ, Kostka JE, Fortin D, Lean DRS. Identification of sulfate-reducing bacteria in methylmercury-contaminated mine tailings by analysis of SSU rRNA genes. Fems Microbiology Ecology. 2009;68(1):94–107. doi: 10.1111/j.1574-6941.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- Wu WM, Carley J, Gentry T, Ginder-Vogel MA, Fienen M, Mehlhorn T, et al. Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. Reduction of U(VI) and geochemical control of U(VI) bioavailability. Environmental Science & Technology. 2006;40(12):3986–3995. doi: 10.1021/es051960u. [DOI] [PubMed] [Google Scholar]
- Wu WM, Carley J, Luo J, Ginder-Vogel MA, Cardenas E, Leigh MB, et al. In situ bioreduction of uranium (VI) to submicromolar levels and reoxidation by dissolved oxygen. Environmental Science & Technology. 2007;41(16):5716–5723. doi: 10.1021/es062657b. [DOI] [PubMed] [Google Scholar]
- Xu MY, Wu WM, Wu LY, He ZL, Van Nostrand JD, Deng Y, et al. Responses of microbial community functional structures to pilot-scale uranium in situ bioremediation. Isme Journal. 2010;4(8):1060–1070. doi: 10.1038/ismej.2010.31. [DOI] [PubMed] [Google Scholar]
- Yang GCC, Hung CH, Tu HC. Electrokinetically enhanced removal and degradation of nitrate in the subsurface using nanosized Pd/Fe slurry. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering. 2008;43(8):945–951. doi: 10.1080/10934520801974517. [DOI] [PubMed] [Google Scholar]
- Zhang WX. Nanoscale iron particles for environmental remediation: An overview. Journal of Nanoparticle Research. 2003;5(3-4):323–332. [Google Scholar]