Abstract
Tumour-associated macrophages (TAMs) represent a predominant population of inflammatory cells that present in solid tumours. TAMs are mostly characterized as alternatively activated M2-like macrophages and are known to orchestrate nearly all stages of tumour progression. Experimental investigations indicate that TAMs contribute to drug-resistance and radio-protective effects, and clinical evidence shows that an elevated number of TAMs and their M2 profile are correlated with therapy failure and poor prognosis in cancer patients. Recently, many studies on TAM-targeted strategies have made significant progress and some pilot works have achieved encouraging results. Among these, connections between some anti-tumour drugs and their influence on TAMs have been suggested. In this review, we will summarize recent advances in TAM-targeted strategies for tumour therapy. Based on the proposed mechanisms, those strategies are grouped into four categories: (i) inhibiting macrophage recruitment; (ii) suppressing TAM survival; (iii) enhancing M1-like tumoricidal activity of TAMs; (iv) blocking M2-like tumour-promoting activity of TAMs. It is desired that further attention be drawn to this research field and more effort be made to promote TAM-targeted tumour therapy.
Keywords: activation, cancer therapy, recruitment, survival, tumour-associated macrophage
Introduction
To develop new tumour therapies, increasing attention has been paid to the ‘tumour microenvironment’, where tumour cells and non-tumour cells influence each other mutually.1 A highlight in this field is the macrophages that present in tumour tissues, namely tumour-associated macrophages (TAMs).2 TAMs are the main population of inflammatory cells in solid tumours and the cytokines released from them possess diversified significance in tumour development.3–5 TAMs are derived from circulating monocytes and differentiate within the tumour microenvironment.6–8 Although the features of TAMs need to be further characterized, many studies have demonstrated that the majority of TAMs are M2-like macrophages, with properties that differ from the M1 macrophages, which are usually present in tissue areas with acute inflammation.3,8 TAMs generally fail to express pro-inflammatory cytokines for T helper type 1 (Th1) responses but are excellent producers of immunosuppressive cytokines for Th2 responses.4 As TAMs generally exhibit low antigen-presenting and co-stimulating capacity, they ordinarily fail to activate T-cell-mediated adaptive immunity.4,7 Therefore, unlike M1 macrophages, which are highly microbicidal and tumoricidal, the M2-like TAMs are immunosuppressive and facilitate tumour progression.4,7
Experimental and epidemiological studies demonstrated that TAMs play an important role in tumour growth, angiogenesis, metastasis, matrix remodelling and immune evasion in various human and animal tumours.5,7–10 Recently, TAMs are ‘accused’ for their chemo-resistance and radio-protective effects in mouse tumour models, because an increased density of TAMs is associated with poor efficacy in chemotherapy,11,12 and radiotherapy-induced macrophage aggregation is paralleled by decreased radiocurability.13–15
Clinical studies also revealed connections between the state of TAMs and poor outcomes of human tumours. The density, activation and histological location of TAMs can be used to predict patients’ survival time in different types of cancer.16–20 For instance, an increased number of TAMs was correlated with a shortened progress-free survival in classical Hodgkin lymphoma.16 Besides, Kurahara et al.18 observed that a larger number of M2-polarized TAMs correlated with increased density of lymphatic vessels, high incidence of lymph node metastasis and a poor prognosis in patients with pancreatic cancer.
Therefore, TAMs are now considered as a promising target for tumour therapy, and reduction of their tumour-promoting activities has become a hot study area.21 Generally, the approaches to targeting TAMs are by following two routes: decreasing the quantity of TAMs in tumour tissue or shifting TAMs from tumour-promoting to tumoricidal status. Although the clinical application of a TAM-targeted approach is still far from clear, a number of experimental studies have collectively shown the effect of this approach on faster tumour rejection and better therapeutic outcome,22–26 which sheds inspirational light on further clinical studies. In this review, we will discuss current TAM-targeted strategies for anti-tumour therapy.
TAM-targeted anti-tumour strategies
Since the functions of TAMs largely depend on their accumulation and activation in tumour tissues, TAM-targeted anti-tumour approaches are principally based on: (i) inhibiting macrophage recruitment; (ii) suppressing TAM survival; (iii) enhancing M1 tumoricidal activity of TAMs; and (iv) blocking M2 tumour-promoting activity of TAMs. These strategies are summarized in Fig. 1.
Figure 1.
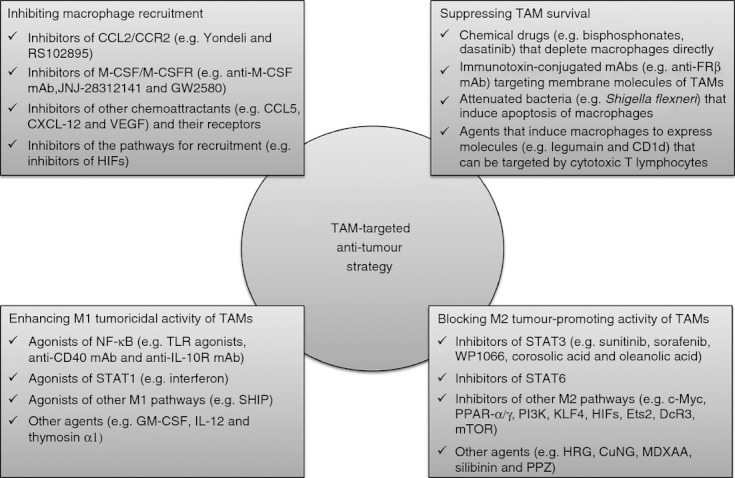
Tumour-associated macrophage (TAM) -targeted anti-tumour strategy. Since the functions of TAMs depend on their accumulation and activation, present TAM-targeted approaches mainly concentrate on four aspects: (i) inhibiting macrophage recruitment; (ii) suppressing TAM survival; (iii) enhancing M1 tumoricidal activity; and (iv) blocking M2 tumour-promoting activity. CCL, C-C motif chemokine ligand; M-CSF, macrophage-colony-stimulating factor; CXCL12, C-X-C motif chemokine ligand 12; VEGF, vascular endothelial growth factor; HIF, hypoxia-inducible factor; FRβ, folate receptor β; NF-κB, nuclear factor κB; TLR, Toll-like receptor; STAT, signal transducers and activators of transcription; SHIP, Src homology 2-containing inositol-5'-phosphatase; GM-CSF, granulocyte–macrophage colony-stimulating factor; PPAR, peroxisome proliferator-activated receptor; PI3K, phosphatidylinositol 3-kinase; KLF4, Krüppel-like factor 4; Ets2, E26 transcription-specific sequence 2; DcR3, Decoy receptor 3; TSC2-mTOR, tuberous sclerosis complex 2-mammalian target of rapamycin; HRG, histidine-rich glycoprotein; MDXAA, 5,6-dimethylxanthenone-4-acetic acid; PPZ, proton pump inhibitor pantoprazole.
Inhibiting macrophage recruitment
Some tumour-released and stroma-released cytokines and chemokines facilitate the recruitment of macrophages to tumour tissues. Therefore, inhibiting macrophage recruitment by modulating the actions of relevant chemoattractants has become a promising approach for tumour therapy. As an example, C-C motif chemokine ligand 2 (CCL2) has been taken into account, because its over-expression was correlated with increased macrophage infiltration and poor prognosis in human cancers,27–29 and macrophage infiltration and the growth of tumours were reduced when CCL2 was inhibited.22,30–33 The tie between CCL2 and M2 macrophages is particularly clear in CCL2+ melanoma. For instance, pharmacological inhibition of CCL2 with bindarit reduced tumour growth, macrophage recruitment and necrotic tumour masses in human melanoma xenograft.30 One of the CCL2-targeting agents, trabectedin, has been efficiently used in clinic to treat human ovarian cancer34 and myxoid liposarcoma.35 According to those reports, trabectedin could suppress the recruitment of monocytes to tumour sites and inhibit their differentiation to mature TAMs, which may contribute to trabectedin-induced tumour rejection. The association of CCL2 with TAM recruitment was further supported by a phase II clinical study, in which anti-interleukin-6 (IL-6) antibody siltuximab reduced macrophage infiltration in tumour tissue via declining the plasma level of some chemoattractants such as CCL2, vascular endothelial growth factor (VEGF) and C-X-C motif chemokine ligand-12 (CXCL-12).36 As an alternative way to suppress the chemoattractive activity of CCL2, neutralizing its receptor, C-C motif chemokine receptor 2 (CCR2), is also challenged. One pharmacological inhibitor of CCR2 (RS102895) has exhibited negative effects on macrophage migration.37 In addition, the efficacy of two humanized monoclonal antibodies (mAbs; CNTO888 and MLN1202) specific for CCL2/CCR2 are under clinical investigation (see ClinicalTrials.gov; study identifier: NCT00537368, NCT00992186, NCT01204996, MLN1202 and NCT01015 560).
Another important chemoattractant for macrophages is macrophage colony-stimulating factor (M-CSF). In human hepatocellular carcinoma, there is a significant association between high M-CSF expression and high macrophage density, each relates to poor overall survival of patients.17 In an M-CSF-deficient mouse model of pancreatic neuroendocrine tumour, macrophage infiltration was decreased by ∼ 50% during all stages of tumour progression.38 In another experiment, treatment with M-CSF antibody suppressed tumour growth by 40% in human MCF-7 breast cancer xenografts.39 More recently, two M-CSF receptor inhibitors (JNJ-28312141 and GW2580) were found to decrease TAM count and suppress tumour growth, angiogenesis and metastasis.40,41 In contrast to standard VEGF inhibition, the continuous M-CSF inhibition did not affect healthy vascular and lymphatic systems outside tumour sites.41 This implies that M-CSF might be a good candidate in the therapies aiming to inhibit macrophage recruitment or angiogenesis. Currently, several phase I clinical trials of M-CSF/M-CSFR inhibitors are in the stage of recruiting patients (see ClinicalTrials.gov; study identifier: NCT01316822, NCT01346358, NCT01440959, NCT01444404, and NCT01004861). These studies should provide more information about whether or not M-CSF/M-CSFR inhibitors are of value in cancer therapy and explore further the role of macrophage depletion.
Other chemoattractants for macrophages, such as VEGF, CXCL-12 and CCL5, also seem to be potential targets for TAM depletion and tumour rejection. For instance, selectively inhibiting VEGFR-2 reduced macrophage density and prevented tumour growth and angiogenesis in orthotropic pancreatic and breast tumours.42,43 In addition, repressing either the CXCL12/C-X-C motif chemokine receptor 4 (CXCR4) or the placental growth factor (PIGF)/VEGFR-1 pathway reduced macrophage count.11,44 As the tumour microenvironment is usually hypoxic and hypoxia-inducible factors (HIFs) are transcriptional activators for VEGF and CXCR4 genes45; HIFs are naturally suggested to play a role in macrophage recruitment. It was reported that HIF-1α deficiency reduced macrophage density, tumour angiogenesis and invasion in murine glioblastoma via blocking the matrix metalloproteinase 9 (MMP9)/VEGF pathway.46 Recent work has shown that HIF-2α mediated macrophage migration to the tumour microenvironment partly through regulating M-CSFR and CXCR4.47 Therefore, HIF inhibitors may be considered as anti-tumour candidates not only for their potential to inhibit angiogenesis, but also for their effects on macrophage recruitment.
Suppressing TAMs survival
To kill TAMs locally is another approach to deplete pro-tumoral TAMs. Two alternative strategies have been tried. One is to directly induce macrophage apoptosis using chemical reagents, immunotoxin-conjugated mAbs or attenuated bacteria; the other is to trigger the immune cells, T lymphocytes for example, to recognize and abrogate TAMs.
Bisphosphonates, generally packed in liposomes, have become prominent drugs for macrophage depletion.48 Two bisphosphonates, clodronate and zoledronic acid, are extensively used in experimental investigations. Several lines of evidence show that clodronate has a selective cytotoxicity to macrophages and this clodronate-induced depletion of macrophages can result in the regression of tumour growth, angiogenesis and metastasis.49–51 Zoledronic acid is a clinical drug for cancer therapy, especially for breast cancers. This compound selectively depletes MMP9-expressing TAMs.23,52 Importantly, current evidence indicates that zoledronic acid not only inhibits macrophage accumulation, but also impairs the differentiation of myeloid cells to TAMs and induces the tumoricidal activity of macrophages.52–55 Given that zoledronic acid can prolong survival in cancer patients,56–58 it is important to clarify whether or not TAM depletion contributes to this efficacy. In addition to clodronate and zoledronic acid, other bisphosphonates (e.g. dichloromethylene bisphosphonate) are also under investigation for their potential in TAM-targeted therapies.59
Dasatinib, a Src kinase inhibitor and a preclinical drug for chronic-phase chronic myeloid leukaemia,60 is also on the study list. As reported, dasatinib could reduce MMP9+ macrophage density and inhibit MMP9 expression in the tumour microenvironment.61 This observation broadened the therapeutic mechanisms of dasatinib.
To deplete TAMs by targeting their surface molecules with immunotoxin-conjugated agents is another approach for tumour therapy. Such studies have been conducted for ovarian cancer treatment by using immunotoxin-conjugated mAbs, where the surface proteins of TAMs, such as scavenger receptor-A and CD52, were targeted.62,63 Folate receptor β (FRβ) is another surface protein worth targeting because it is over-expressed in M2-like TAMs,64,65 and the existence of FRβ+ macrophages positively associates with high vessel density, high incidence of haematogenous metastasis and a poor prognosis in patients with pancreatic cancer.66 Nagai et al.64 reported the inhibitory effects of the folate–immunotoxin conjugate on tumour growth, accomplished with the depletion of TAMs. One benefit of this approach may be that while pro-tumoral M2 TAMs could be depleted, the M1 tumoricidal ones are not affected.
Recent studies demonstrate that several bacteria prefer to take macrophages as targets. For instance, it was reported that Shigella flexneri infection could selectively induce the apoptosis of macrophages,67 and a single injection of an attenuated strain of Shigella flexneri to tumour-bearing mice resulted in the apoptosis of TAMs, followed by a 74% reduction in size of tumours.68 In addition, other bacteria, such as Salmonella typhimurium, Listeria monocytogens, Chlamydia psittaci and Legionella pneumophila, are also considered to be useful for TAM-targeted immunotherapy because they harbour primarily in macrophages.21
Other than directly inducing the apoptosis of TAMs as mentioned above, another available approach for TAM suppression is to evoke acquired immune responses, in which cytotoxic T lymphocytes act as the scavengers of TAMs because they can naturally target the membrane molecules of macrophages. In other words, up-regulating the membrane molecules that could be recognized by T cells in TAMs would be a potential method of TAM depletion. One such molecule is legumain, a lysosomal protease highly expressed in many human tumours; which promotes neoplastic cell invasion and metastasis.69 Luo et al.24 originally found that legumain is over-expressed in M2-like TAMs. In the following studies, they immunized tumour-bearing mice with a novel legumain-based DNA vaccine, and found that this vaccine activated dendritic cells, which then triggered multi-step reactions including the antigen presenting, co-stimulation of cytotoxic CD8+ T cells and the specific abrogation of legumain-expressing TAMs.24,70 They found in those experiments that the macrophage density and the pro-angiogenic factors were reduced in various tumour tissues of vaccine immunized mice, along with attenuated tumour growth, angiogenesis and metastasis.24,70 Given that M1 macrophages do not express legumain, this legumain-based DNA vaccine may be particularly useful for destroying M2-like TAMs. Another membrane protein involved in T-cell-mediated TAM depletion is CD1d, a strict target of Vα24-invariant natural killer T (NKT) cells. NKT cells are an independent factor for favourable outcome in various human cancers.71 The earlier explanation for the tumoricidal role of NKT cells emphasized the expression of CD1d on tumour cells, such as leukaemia and lymphoma cells.71 However, this explanation faced a great challenge because the majority of human tumour cells are actually CD1d-negative. How do NKT cells reject CD1d-negative tumours? Song et al.72,73 provided an alternative answer to this question. They stated that TAMs were the major CD1d-positive cells co-localizing with NKT cells in primary human neuroblastomas and in mouse xenografts of neuroblastoma, and that TAMs were the major targets of NKT cells in CD1d-negative tumours. This discovery is important because it may guide the designs of NKT-mediated immunotherapy, alone or in combination with other standard therapies. According to this notion, the agents that can promote the expression of CD1d in TAMs may improve the tumoricidal function of NKT cells. One such agent is retinoic acid, which can strongly up-regulate the CD1d expression in macrophages74 and is now used as a standard therapeutic drug for high-risk neuroblastoma in clinic.71 However, the contribution of the NKT–TAM axis to the effects of retinoic acid on tumour suppression needs to be further explored.
Enhancing M1 tumoricidal activity of TAMs
Although most TAMs exhibit immunosuppressive M2-like properties, they remain the plasticity for polarization,75 which provides a potential for TAMs to re-polarize from tumour-promoting M2-type to tumoricidal M1-type. It is known that the polarization of macrophages largely depends on the local cytokine profiles. In detail, when high levels of Th1 cytokines, such as tumour necrosis factor (TNF), IL-12 and interferons (IFNs), are present, the pro-inflammatory M1 macrophages will be established; whereas when exposed to Th2 cytokines, such as IL-4, IL-10, IL-13 and transforming growth factor-β (TGF-β), macrophages will polarize to M2 status.4 Until now, several signalling pathways, especially the nuclear factor-κB (NF-κB) and the signalling transducer and activator of transcription (STAT) pathways are known to play pivotal roles in the transcriptional profile of macrophages.6 Among those transcriptional factors, STAT1 and canonical NF-κB (p50p65 heterodimer) are essential for the M1 tumoricidal functions and trigger the expression of pro-inflammatory cytokines.6 In contrast, TAMs harbouring activated STAT3 and STAT6 are not tumoricidal; instead, they exhibit M2 properties and facilitate cancer development.21 Therefore, manipulating these factors and their up-/down-stream regulators will contribute to targeted tumour therapy. In this section, we will primarily discuss the studies aiming to re-polarize TAMs to M1-type. The agents used and the proteins targeted will be outlined. Those studies for hampering the functions of M2-like TAMs will be discussed in the next section.
The NF-κB pathway can positively modulate the transcription of Th1-response cytokines in most circumstances. It is known that the attenuated NF-κB activation in TAMs mediates their immunosuppressive M2 property; whereas NF-κB reactivation can redirect TAMs to a tumoricidal M1-like phenotype.76 By now, several agents with definite roles in activating NF-κB have been reported. They include the agonists of Toll-like receptors (TLRs), anti-CD40 mAb and anti-IL-10R mAb. The TLR agonists are diverse, including PolyI:C (for TLR3), lipopolysaccharide (LPS) and monophosphoryl A (for TLR4), imiquimod and R-848 (for TLR7), and CpG-oligodeoxynucleotide (CpG-ODN, for TLR9). First, PolyI:C is a dsRNA analogy that can reverse tumour-supporting macrophages to tumour-suppressing macrophages via TLR3.77,78 Second, LPS is a well-known activator of the NF-κB pathway and is important in the establishment of the M1 phenotype of macrophages. The detoxified derivative of LPS, monophosphoryl lipid A, has also shown promise as an adjuvant of anti-cancer vaccines.79 Clinical trials of this drug are ongoing. Third, as a ligand for TLR7/8, imiquimod attracts a certain amount of attention, because it could promote the Th1 cytokine production in antigen-presenting cells and enhance the anti-tumour responses of lymphocytes.80,81 In a topical therapy for cutaneous squamous cell carcinoma, imiquimod polarized monocytes/macrophages to an M1 pattern.82 Another agent similar to imiquimod is R-848.83 Importantly, TLR7/8 agonists can enhance the destruction of antibody-coated tumour cells by macrophages.83 Finally, CpG-ODN draws considerable attention because it has been widely used as an adjuvant of tumour-specific antigen vaccines, mechanically standing on the basis that the activation of TLR9 can up-regulate the trans-activity of NF-κB in macrophages.84 Synthetic CpG-ODN is a powerful compound in promoting macrophages to produce IL-12, IFN-α/β and TNF-α.85,86 Moreover, it has been reported that the combined treatment of CpG-ODN with other agents, such as CCL-16 and anti-IL-10R mAb, rapidly switched infiltrating macrophages from M2 type to M1 type, and triggered innate responses debulking large tumours within 16 hr.87 Currently, CpG-ODN-based therapies are in clinical trials for the treatment of various cancers.88–93
The antibodies against the membrane receptors on the up-stream part of the NF-κB pathway are also inspiring for TAM modulation. One such antibody is anti-CD40 mAb. It has been reported that CD40 acts as an inhibitor for cytotoxic function of macrophages, probably because its ligands could inhibit the trans-activity of canonical NF-κB, which is essential for M1 establishment and could up-regulate genes such as CXCL12 and VEGF-C.76 In one study, the ligation of CD40 with anti-CD40 mAb retrieved the activity of NF-κB and induced the destruction of tumour cells.94 In another investigation, the treatment with CD40 mAb resulted in the up-regulation of MHC-II and co-stimulatory molecule CD86 in macrophages, and elevated serum levels of IL-12, TNF-α and IFN-γ, positively correlating with the regression of pancreatic carcinoma in humans and mice.95 The tumour repression effect of anti-CD40 mAb is also attributed to the release of CD40's suppression effect on TLR9 because anti-CD40 mAb promoted TLR9 to respond to CpG-ODN in macrophages.96 In fact, the synergy of CpG-ODN with agonistic anti-CD40 mAb reversed TAMs toward the M1 phenotype, and augmented the apoptogenic effects of macrophages against tumour cells.25,96
However, it should be noted that the activation of the NF-κB pathway does not solely facilitate the M1-phenotype of TAMs.76 For instance, Hagemann et al.97 found that NF-κB participated in pro-tumoral functions of TAMs, and the inhibition of NF-κB activity significantly re-polarized TAMs to M1 tumoricidal phenotype and promoted the regression of mouse ovarian cancers. Moreover, TNF-α and other cytokines involved in NF-κB activation are reported to act positively in the metastasis of certain tumours, such as Lewis lung carcinoma, and these cytokines can protect TAMs and tumour cells from apoptosis.98–100 In addition, NF-κB promotes, in some experiments, the transcription of HIF-1α, which in turn promotes tumour angiogenesis.101 Hence, it is currently still difficult to envisage a broad applicability of NF-κB mediators to re-educate TAMs, further exploration and evaluation are essential.
Like the NF-κB pathway, the STAT1 pathway is generally targeted to reverse TAMs to an M1 transcriptome.6 The natural agonist of STAT1 is IFN. IFN-α and IFN-β have long been known for their anti-tumour potential and have been approved by the US Food and Drug Administration for treatment of several human cancers, including hairy-cell leukaemia and AIDS-related Kaposi sarcoma.102 Experimental studies indicate that the effects of IFN-α/-β on the inhibition of tumour growth is likely to be based on targeting haematopoietic cells rather than tumour cells per se.103 The role of IFN-γ in reversing immunosuppressive and pro-tumoral properties of human TAMs has also been observed.104 It was proposed that IFNs trigger the activation of STAT1 and then the transcription of the genes encoding pro-inflammatory cytokines, such as IL-12, nitric oxide synthase 2 (NOS2) and CXCL-10, in TAMs.105 In this regard, IFNs and IFN-mimics may contribute to TAM-education. However, the STAT1 pathway, similar to NF-κB, also displays pro-tumoral capacity in certain tumours. The presence of STAT1-positive TAMs is associated with adverse survival in human follicular lymphomas,106 and STAT1 deficiency enhanced IL-12-induced tumour regression by a T-cell-dependent mechanism in murine squamous cell carcinoma.107 Therefore, the effects of STAT1 on the modulation of TAM properties should be carefully evaluated before they come to be used in therapy.
In addition, several cytokines, whose signalling pathways are yet to be fully identified, are also involved in TAM re-polarization. One such cytokine is granulocyte–macrophage colony-stimulating factor (GM-CSF), an adjuvant widely used in immunotherapy for human cancers. GM-CSF could induce M1-polarized TAMs with IL-4low, IL-10low, arginase Ilow and NOS2high.108 Clinical immunotherapy with GM-CSF usage has significantly improved the outcome in patients with high-risk neuroblastoma, partly through the increased macrophage density.109 However, further study is needed to explore whether and how TAM-education is responsible for this effect of GM-CSF in human cancers. Another such cytokine is IL-12. IL-12 can rapidly reduce tumour-supportive activity of TAMs, concomitant with IL-12 enhanced pro-inflammatory activity of macrophages.110 The importance of TAMs in IL-12-induced tumour rejection has been highlighted in two studies.111,112 Interestingly, synergy of GM-CSF and IL-12 gene therapy suppressed the growth of orthotropic liver tumours.113 A large number of clinical studies of recombinant IL-12 alone or in combination with other anti-tumour drugs, such as IFN-α, IL-2 and IL-15, have been carried out (see ClinicalTrials.gov). One factor that should be mentioned here is thymosin-α1 (Tα1), a drug used in clinic. An impressive amount of data reported by Shrivastava and his colleagues reveal the benefits of Tα1 to TAM-targeted cancer therapy.114–117 They showed that Tα1 prompted the production of IL-1, TNF, reactive oxygen intermediates and NO in TAMs114,116 and induced M1 TAMs and in turn prolonged the survival time of mice with Dalton lymphoma.116,117
Finally, we would note the effects of re-polarized TAMs on adaptive immunity. In tumour settings, macrophages generally express low levels of MHC-II and so fail to co-stimulate T cells.118,119 However, M1-polarization inducers such as anti-CD40 mAb and IFN-γ are able to up-regulate MHC-II and other co-stimulating factors (e.g. CD86) in macrophages, which enhances the adaptive immune responses that are powerful for tumour rejection. In line with this, the cascade linkages among TAM polarization, MHC-II expression, adaptive immune responses and tumour repression should extend our understanding of the significance of TAM re-polarization and provide novel insight for the connection between innate and adaptive immune responses in anti-tumour immunotherapy.
Blocking M2 tumour-promoting activity of TAMs
In addition to inciting M1 differentiation and tumoricidal activity, inhibiting the signals essential for M2 differentiation and in turn impairing the pro-tumoral and immunosuppressive profile of TAMs is another strategy in development. In this section, we will discuss the pathological role of the STAT3 pathway and STAT6 pathway in M2-like TAM polarization, and the pharmacological effects of the agents that inhibit these pathways. Several other pathways and M2 targeting agents will be outlined at the end of this section.
STAT3 is consistently active in many tumours and acts as a negative regulator for macrophage activation and the host's inflammatory responses.120 When the activation of STAT3 was blocked, either with a dominant negative variant or an antisense oligonucleotide, macrophages could increase the release of IL-12 and RANTES and reverse the systemic immune tolerance.121 Now, some STAT3 inhibitors are under investigation. For instance, a small molecular inhibitor of STAT3 (WP1066) was found to reverse immune tolerance in patients with malignant glioma, correlating with selectively induced expressions of co-stimulatory molecules (CD80 and CD86) on peripheral macrophages and tumour-infiltrating microglias, and immune-stimulatory cytokines (e.g. IL-12).122 Two clinical tyrosine kinase inhibitors (sunitinib and sorafenib) have shown their inhibitory effects on STAT3 in macrophages in vitro.123,124 Sorafenib can restore IL-12 production but suppress IL-10 expression in prostaglandin E2 conditioned macrophages, indicating its effects on reversing the immunosuppressive cytokine profile of TAMs.124 Moreover, two newly identified inhibitors of M2 differentiation are corosolic acid and oleanolic acid. They can also suppress the activation of STAT3.125,126 Actually, other novel STAT3 inhibitors, such as STA-21, IS3 295 and S3I-M2001, have been found to be efficient against tumours,127 although their association with TAM re-polarization needs to be shown.
Another STAT family member important for TAM biology is STAT6. In one study, STAT6–/– mice produced predominantly M1-like tumoricidal TAMs with arginaselow and NOhigh, and > 60% of STAT6–/– mice rejected tumour metastasis.128 Currently, at least three STAT6 inhibitors (AS1517499, leflunomide and TMC-264) have been identified. But their actions as modulators of TAMs remain to be clarified. Instead, several up-/down-stream mediators of STAT6 are more impressive because they could act as modulators of TAM function. These modulators include phosphatidylinositol 3-kinase (PI3K), Src homology 2-containing inositol-5′-phosphatase (SHIP), Krüppel-like factor 4 (KLF4) and c-Myc. PI3K positively regulates STAT6 activation in macrophages, whereas SHIP negatively regulates PI3K. Either PI3K inhibition or SHIP over-expression has been found to decrease the activity of the STAT6 pathway and to reduce M2 skewing of macrophages.129 Therefore, the agents that are able to inhibit PI3K or stabilize SHIP activity may be therapeutic adjuvants for cancer. KLF4 is another interesting modulator protein of STAT6. Liao et al.130 reported that the expression of KLF4 was induced in M2 macrophages and reduced in M1 macrophages. Their mechanistic study indicated that KLF4 cooperated with STAT6 to induce an M2 pattern. Levels of KLF4 can be manipulated by diverse agonists such as statins, resveratrol, bortezomib and dietary compounds, so these factors could be influential for TAM re-education.130 Although still preliminary, the association among c-Myc, STAT6 and M2 polarization has been proposed by recent studies. As reported, c-Myc up-regulated IL-4-mediated STAT6 activation and elevated the expression of 45% of the genes correlated with alternative activation of macrophages.131 In contrast, c-Myc inhibition blocked the expression of some pro-tumoral genes.131
Other proteins and signalling pathways known to promote M2-like properties of macrophages are also the potential targets for tumour therapy. They include peroxisome proliferator-activated receptor (PPARs), HIFs, Ets family member 2 (Ets2), Decoy receptor (DcR3) and mammalian target of rapamycin (mTOR). First, PPAR-γ can promote M2 type differentiation of human macrophages by acting as a transcriptional inhibitor of NF-κB.132 PPAR-α plays a role in macrophages by antagonizing M1 polarization and supporting M2 polarization.133 As synthetic inhibitors of PPAR-α/γ have now been identified, the evaluation of their role in TAM-targeted therapy is essential. Second, HIFs are a hopeful target because of their over-expression in TAMs residing in the hypoxic tumour microenvironment and their ability to induce the production of angiogenic factors, including VEGF, platelet-derived growth factor-β, NOS2, fibroblast growth factor 2, IL-8 and cyclooxygenase-2.134 In fact, macrophage-targeted depletion of HIF-1α reduced tumour growth in mice.135 Therefore, it would be interesting to see whether blocking HIFs could slow or halt tumour recovery. Third, Ets2 is a direct effector of the M-CSF signalling pathway, and so facilitates the formation of M2 macrophage. Zabuawala et al.136 demonstrated that an Ets2-driven transcriptional program in TAMs could promote the angiogenesis and metastasis of murine breast cancer. Interestingly, an Ets2-TAM gene signature consisting of 133 genes retrospectively predicted overall survival of breast cancer patients.136 Investigations of DcR3 and mTOR are also interesting.137,138
Several anti-tumour drugs that are able to suppress M2 macrophages will be introduced as follows. (i) Histidine-rich glycoprotein (HRG): HRG can skew TAMs to M1 type by down-regulation of PIGF, a member of the VEGF family, and can combat tumour malignancy by enhancing immunity and vessel normalization.26 Macrophages are a direct target of HRG; and re-education of TAMs is essential for HRG-mediated anticancer effects.26,139 (ii) Copper chelate (CuNG): A novel CuNG was demonstrated to modulate the cytokine profile of TAMs isolated from chemotherapy-resistant or radiotherapy-resistant cancer patients.140 p38MAPK and ERK1/2 are involved in CuNG-induced IFN-γ and IL-12 up-regulation, as well as TGF-β down-regulation in TAMs.141 (iii) 5,6-Dimethylxanthenone-4-acetic acid (MDXAA): MDXAA can significantly induce the release of various immune-stimulatory cytokines and chemokines from TAMs, followed by CD8+ T-cell infiltration and tumour rejection.142 (iv) Cisplatin: Cisplatin promotes macrophages to produce large amounts of NO, a reactive oxygen intermediate and pro-inflammatory cytokines, leading to enhanced tumoricidal activity.143 (v) Silibinin: Silibinin is now under clinical trials. Experimental studies have shown that silibinin inhibited the production of angiogenic cytokines and interleukins in macrophages, leading to angiogenesis regression.144 (vi) Proton pump inhibitor pantoprazole (PPZ): In addition to the ability of inducing tumour cell apoptosis, PPZ also affects the state of TAMs. It enhances TAM recruitment but augments TAMs to an M1-like tumoricidal state.145 Although the drugs listed above show their encouraging potential for TAM-targeted therapy, the specificity is yet to be certain. What's more, our understanding of TAM modulation is till limited, which means that more extensive biological and pharmacological studies are required.
Conclusion and perspective
TAMs serve as pivotal inflammatory orchestrators in the development of various solid tumours. These immunosuppressive cells are closely associated with poor prognosis in cancer patients. Therefore, targeting TAMs potentially offers a new approach for cancer therapy. The recent ongoing experimental and pre-clinical TAM-targeted studies have indeed made some encouraging progress. Since the pro-tumoral activity of TAMs largely depends on their recruitment and activation, the present TAM-targeted therapeutic attempts are mainly concentrated on four aspects: (i) inhibiting macrophage recruitment; (ii) suppressing TAM survival; (iii) enhancing M1 tumoricidal activity of TAMs; and (iv) blocking M2 tumour-promoting activity of TAMs.
Although a number of strategies previously mentioned in this review are not clinically available, they are feasible at least in experimental and preclinical studies. Up to now, many agents have been identified as candidate drugs, either as inhibitors of macrophage accumulation or as modulators of TAM properties. In fact, achievements in experimental investigations revealed that TAM-targeting is essential for some already approved drugs, which are listed in Table 1. Anyhow, using immune system to combat cancer is a promising approach that perhaps possesses the greatest potential to provide a cure for cancer.146 Interestingly, melanoma and renal cell carcinoma show the highest response rate to immunotherapies among malignant solid tumours, which has been partly explained by the involvement of macrophages and local immune environment.30,123 As TAMs contribute to chemo-resistance and radio-protective effects,11–14 TAM-targeted strategies may also improve the efficacy of conventional therapies in some cases. Moreover, technical advances in TAM-targeted image and drug delivery are encouraging for both investigators and clinicians.147–151 We would like to believe that in the near future TAM-targeted strategy will be clinically accepted as a valuable adjuvant therapy for cancer patients.
Table 1.
Clinical anti-tumour drugs with potential to target tumour-associated macrophages (TAMs)
| Name | Development phase | Clinical use | Experimental tumour type | How to target TAMs | References |
|---|---|---|---|---|---|
| Zoledronic acid | Approved | Various cancers, especially breast cancer | Kinds of murine tumour models | Depleting MMP9-expressing macrophages; inhibiting MMP9 expression and impairing maturation of TAMs | 23,52–54 |
| Trabectedin | Approved in Europe | Soft tissue sarcomas | Human ovarian cancer and myxoid liposarcoma (clinical trial) | Inhibiting CCL2 expression and reducing macrophage recruitment in tumours | 34,35 |
| Siltuximab (anti-IL-6 mAb) | Phase II | Ovarian cancer | Human ovarian cancer (clinical trial) | Declining levels of IL-6-regulated CCL2, CXCL12, and VEGF; and reducing macrophage recruitment | 36 |
| Dasatinib | Approved | Chronic myeloid leukaemia | Human Colo205 tumour mouse model | Inhibiting MMP9-expressing macrophage recruitment and suppressing MMP9 expression | 61 |
| CpG-ODN | Approved | Various tumours | Various tumours | Activating macrophages to produce IL-12, IFN-α/β and TNF-α, so enhancing Th1 responses; switching TAMs from M2 to M1 phenotype | 85–87 |
| Interferon-α,-β,-γ | Approved | Hairy-cell leukaemia and AIDS-related Kaposi sarcoma | Various tumours | Triggering the activity of STAT1 for some genes encoding pro-inflammatory cytokines, resulting in M1 pattern of TAMs with high levels of IL-12, NOS2 and CXCL-10 | 105 |
| GM-CSF | Approved | Various cancers | Murine breast cancer | Inducing M1-polarized TAMs with IL-4low, IL-10low, arginase Ilow and NOS2high, and blocking macrophage VEGF activity | 108 |
| IL-12 | Approved | Various tumours | Murine Lewis lung carcinoma | Reducing tumour supportive cytokines (IL-10, CCL2, MIF, and TGF) and increasing pro-inflammatory and pro-immunogenic cytokines (TNF-α, IL-15, and IL-18) | 110–112 |
| Thymosin α 1 | Phase II | Malignant melanoma and hepatic cancer | Murine Dalton's lymphoma | Activating TAMs to produce high levels of IL-1, TNF, reactive oxygen intermediate and NO | 114–117 |
| Sunitinib | Approved | Renal cell carcinoma and gastrointestinal stromal tumour | Murine renal cell carcinoma | Regressing the activation of STAT3 in TAMs | 123 |
| 5,6-Dimethylxanthenone-4-acetic acid | Phase I | Refractory solid tumours | Murine lung cancer and mesothelioma | Activating TAMs to release a variety of immune-stimulatory cytokines and chemokines, leading to CD8+ T-cell-dependent anti-tumour immune responses | 142 |
| Silibinin | Phase III | Hepatocellular carcinoma | Murine lung adenocarcinoma | Decreasing TAMs and their release of angiogenesis-related cytokines (e.g. IL-13) | 144 |
MMP9, matrix metalloproteinase 9; IL, interleukin; CCL2, C-C motif chemokine ligand 2; CXCL, C-X-C motif chemokine ligand; VEGF, vascular endothelial growth factor; COX-2, cyclooxygenase 2; STAT, signal transducers and activators of transcription; GM-CSF, granulocyte–macrophage colony-stimulating factor; NOS2, nitric oxide synthase 2; MIF, migration inhibitory factor; TGF, transforming growth factor; TNF, tumour necrosis factor; NO, nitric oxide.
However, we have come to appreciate the fact that cancer is a systemic disease and TAMs are involved in tumour progression through rather complex mechanisms. TAM-targeted therapy, therefore, requires an overall understanding about TAM functions in tumour development. One major gap in our knowledge is why TAM infiltration is associated with poor prognosis in many types of cancers but with favourable survival in others. Although a few pieces of evidence indicate the micro-anatomical location and macrophage phenotype might be responsible for this dichotomy,152–154 clinical evidence is substantially lacking. Second, it would be interesting to identify TAM-specific molecules that could serve as targets for tumour therapy, because previous identified factors (e.g. VEGF, MMPs, TGF-β and CXCL-12) important for TAM-mediated tumour progression,3,4,7–9,75 are also produced by cancer cells themselves. Hopefully, recent clinical and experimental investigations have identified several tumour-promoting molecules (e.g. CCL-18 and IRAK-M) predominantly produced by M2 TAMs.155,156 Third, what should not be neglected is the close interaction between macrophages and other stromal cells within the tumour microenvironment. A better understanding of those connections will contribute to TAM-targeted adjuvant therapies. The fourth inherent issue is how to keep the balance between ‘cancer-inhibiting inflammatory responses’ and ‘cancer-promoting inflammatory responses’.157,158 More biological understandings and pharmacological approaches are needed to fill this gap of our knowledge. Furthermore, a practical issue for developing TAM-targeted therapy is that, clinically, how should a drug be administered at the right time and to the right place so that the tumour-promoting TAMs could be depleted or re-educated whereas the tumoricidal macrophages in tumours or healthy tissues remain unaffected. In summary, more comprehensive understanding of the properties of TAMs and their interactions with the tumour microenvironment, together with advances in diagnostic/therapeutic techniques, will be required to facilitate the development and clinical application of TAM-targeted adjuvant cancer therapies.
Acknowledgments
Our deepest gratitude goes first and foremost to Dr Meiyi Pu for her critical reading of the manuscript and her great contribution to the English improvement. Without her help, this article could not have reached its present form. We also thank Dr Changhua Zou for her wonderful suggestion. This work was supported by a grant from the West China Hospital of Sichuan University (Huaxi Grant 13708002).
Disclosures
The authors declare having no conflicts of interest.
References
- 1.Tarin D. Clinical and biological implications of the tumor microenvironment. Cancer Microenviron. 2012;5:95–112. doi: 10.1007/s12307-012-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–5. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 3.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol. 2010;32:153–8. [PubMed] [Google Scholar]
- 6.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 8.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–7. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 9.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–75. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Zhu XD, Sun HC, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–30. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 13.Chen FH, Chiang CS, Wang CC, Tsai CS, Jung SM, Lee CC, McBride WH, Hong JH. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res. 2009;15:1721–9. doi: 10.1158/1078-0432.CCR-08-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Y, Beckett MA, Liang H, Mauceri HJ, Rooijen Nv, Cohen KS, Weichselbaum RR. Blockade of tumor necrosis factor α signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70:1534–43. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn GO, Tseng D, Liao C-H, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA. 2010;107:8363–8. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X-D, Zhang J-B, Zhuang P-Y, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–16. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 18.Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2009;167:e211–9. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol. 2000;7:263–9. doi: 10.1046/j.1442-2042.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 20.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–74. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 21.Weigert A, Sekar D, Brüne B. Tumor-associated macrophages as targets for tumor immunotherapy. Immunotherapy. 2009;1:83–95. doi: 10.2217/1750743X.1.1.83. [DOI] [PubMed] [Google Scholar]
- 22.Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884–92. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 23.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, Rakhmilevich AL. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132:226–39. doi: 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–54. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–85. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–8. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gazzaniga S, Bravo AI, Guglielmotti A, et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–41. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani K, Sud S, McGregor NA, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–42. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian B-Z, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Fujita M, Snyder L, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2010;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent Yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–71. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 35.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010;70:2235–44. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 36.Coward J, Kulbe H, Chakravarty P, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–96. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin G, Kawsar HI, Hirsch SA, et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS One. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, Joyce JA. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene. 2011;31:1459–67. doi: 10.1038/onc.2011.337. [DOI] [PubMed] [Google Scholar]
- 39.Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349–56. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- 40.Manthey CL, Johnson DL, Illig CR, et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther. 2009;8:3151–61. doi: 10.1158/1535-7163.MCT-09-0255. [DOI] [PubMed] [Google Scholar]
- 41.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dineen SP, Lynn KD, Holloway SE, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–6. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 43.Roland CL, Dineen SP, Lynn KD, et al. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther. 2009;8:1761–71. doi: 10.1158/1535-7163.MCT-09-0280. [DOI] [PubMed] [Google Scholar]
- 44.Welford AF, Biziato D, Coffelt SB, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–73. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang H-Y, Hughes R, Murdoch C, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–59. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du R, Lu KV, Petritsch C, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imtiyaz HZ, Williams EP, Hickey MM, et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers T, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AHM, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiraoka K, Zenmyo M, Watari K, et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. 2008;99:1595–602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miselis NR, Wu ZJ, Van Rooijen N, Kane AB. Targeting tumor-associated macrophages in an orthotopic murine model of diffuse malignant mesothelioma. Mol Cancer Ther. 2008;7:788–99. doi: 10.1158/1535-7163.MCT-07-0579. [DOI] [PubMed] [Google Scholar]
- 52.Tsagozis P, Eriksson F, Pisa P. Zoledronic acid modulates antitumoral responses of prostate cancer-tumor associated macrophages. Cancer Immunol Immunother. 2008;57:1451–9. doi: 10.1007/s00262-008-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veltman JD, Lambers MEH, van Nimwegen M, Hendriks RW, Hoogsteden HC, Hegmans JPJJ, Aerts JGJV. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer. 2010;103:629–41. doi: 10.1038/sj.bjc.6605814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coscia M, Quaglino E, Iezzi M, et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803–15. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green JR, Guenther A. The backbone of progress–preclinical studies and innovations with zoledronic acid. Crit Rev Oncol Hematol. 2011;77(Suppl 1):S3–12. doi: 10.1016/S1040-8428(11)70003-8. [DOI] [PubMed] [Google Scholar]
- 56.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 57.Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188–94. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 58.Coleman R, Cook R, Hirsh V, Major P, Lipton A. Zoledronic acid use in cancer patients. Cancer. 2011;117:11–23. doi: 10.1002/cncr.25529. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi T, Ibata M, Yu Z, et al. Rejection of intradermally injected syngeneic tumor cells from mice by specific elimination of tumor-associated macrophages with liposome-encapsulated dichloromethylene diphosphonate, followed by induction of CD11b+/CCR3–/Gr-1– cells cytotoxic against the tumor cells. Cancer Immunol Immunother. 2009;58:2011–23. doi: 10.1007/s00262-009-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 61.Liang W, Kujawski M, Wu J, et al. Antitumor activity of targeting Src kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin Cancer Res. 2010;16:924–35. doi: 10.1158/1078-0432.CCR-09-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pulaski H, Spahlinger G, Silva I, et al. Identifying alemtuzumab as an anti-myeloid cell antiangiogenic therapy for the treatment of ovarian cancer. J Transl Med. 2009;7:49. doi: 10.1186/1479-5876-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bak SP, Walters JJ, Takeya M, Conejo-Garcia JR, Berwin BL. Scavenger receptor-A-targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Res. 2007;67:4783–9. doi: 10.1158/0008-5472.CAN-06-4410. [DOI] [PubMed] [Google Scholar]
- 64.Nagai T, Tanaka M, Tsuneyoshi Y, et al. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor β. Cancer Immunol Immunother. 2009;58:1577–86. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puig-Kröger A, Sierra-Filardi E, Domínguez-Soto A, et al. Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 66.Kurahara H, Takao S, Kuwahata T, et al. Clinical significance of folate receptor β-expressing tumor-associated macrophages in pancreatic cancer. Ann Surg Oncol. 2012;19:2264–71. doi: 10.1245/s10434-012-2263-0. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki T, Franchi L, Toma C, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galmbacher K, Heisig M, Hotz C, et al. Shigella mediated depletion of macrophages in a murine breast cancer model is associated with tumor regression. PLoS One. 2010;5:e9572. doi: 10.1371/journal.pone.0009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C, Sun C, Huang H, Janda K, Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003;63:2957–64. [PubMed] [Google Scholar]
- 70.Lewēn S, Zhou H, Hu H-d, Cheng T, Markowitz D, Reisfeld R, Xiang R, Luo Y. A legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–15. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140:119–29. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song L, Ara T, Wu H-W, et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117:2702–12. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song L, Asgharzadeh S, Salo J, et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Q, Ross AC. Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp Biol Med. 2007;232:488–94. [PMC free article] [PubMed] [Google Scholar]
- 75.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 76.Biswas SK, Lewis CE. NF-κB as a central regulator of macrophage function in tumors. J Leukoc Biol. 2010;88:877–84. doi: 10.1189/jlb.0310153. [DOI] [PubMed] [Google Scholar]
- 77.Seya T, Shime H, Matsumoto M. TAMable tumor-associated macrophages in response to innate RNA sensing. OncoImmunology. 2012;1:1–2. doi: 10.4161/onci.19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shime H, Matsumoto M, Oshiumi H, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci USA. 2012;109:2066–71. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cluff CW. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: Clinical results. In: Jeannin J-F, editor. Lipid A in Cancer Therapy. Vol. 667. New York: Springer; 2010. pp. 111–23. [DOI] [PubMed] [Google Scholar]
- 80.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–34. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schön M, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–9. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 82.Smith KJ, Hamza S, Skelton H. Topical imidazoquinoline therapy of cutaneous squamous cell carcinoma polarizes lymphoid and monocyte/macrophage populations to a Th1 and M1 cytokine pattern. Clin Exp Dermatol. 2004;29:505–12. doi: 10.1111/j.1365-2230.2004.01593.x. [DOI] [PubMed] [Google Scholar]
- 83.Butchar JP, Mehta P, Justiniano SE, et al. Reciprocal regulation of activating and inhibitory Fcγ receptors by TLR7/8 activation: implications for tumor immunotherapy. Clin Cancer Res. 2010;16:2065–75. doi: 10.1158/1078-0432.CCR-09-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 85.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discovery. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 86.Wilson HL, Dar A, Napper SK, Marianela Lopez A, Babiuk LA, Mutwiri GK. Immune mechanisms and therapeutic potential of CpG oligodeoxynucleotides. Int Rev Immunol. 2006;25:183–213. doi: 10.1080/08830180600785868. [DOI] [PubMed] [Google Scholar]
- 87.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–46. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 88.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–7. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- 89.Ursu R, Carpentier A, Metellus P, et al. Phase II trial of intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma. J Clin Oncol. 2009;27:2043. [Google Scholar]
- 90.Carpentier A, Metellus P, Ursu R, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro-Oncology. 2010;12:401–8. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weber JS, Zarour H, Redman B, Trefzer U, O'Day S, van den Eertwegh AJM, Marshall E, Wagner S. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer. 2009;115:3944–54. doi: 10.1002/cncr.24473. [DOI] [PubMed] [Google Scholar]
- 92.Zent CS, Smith BJ, Ballas ZK, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:211–7. doi: 10.3109/10428194.2011.608451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hennessy EJ, Parker AE, O'Neill LAJ. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 94.Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-γ-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174:6013–22. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 95.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–18. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 97.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-κB. J Exp Med. 2008;205:1261–8. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lo SZY, Steer JH, Joyce DA. TNF-α renders macrophages resistant to a range of cancer chemotherapeutic agents through NF-κB-mediated antagonism of apoptosis signalling. Cancer Lett. 2011;307:80–92. doi: 10.1016/j.canlet.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Kim S, Takahashi H, Lin W-W, Descargues P, Grivennikov S, Kim Y, Luo J-L, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer. 2012;12:121–32. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 101.Rius J, Guma M, Schachtrup C, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 103.Dunn GP, Bruce AT, Sheehan KCF, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 104.Duluc D, Corvaisier M, Blanchard S, et al. Interferon-γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer. 2009;125:367–73. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 105.Mancino A, Lawrence T. Nuclear factor-κB and tumor-associated macrophages. Clin Cancer Res. 2010;16:784–9. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Álvaro T, Lejeune M, Camacho FI, et al. The presence of STAT1-positive tumor-associated macrophages and their relation to outcome in patients with follicular lymphoma. Haematologica. 2006;91:1605–12. [PubMed] [Google Scholar]
- 107.Torrero MN, Xia X, Henk W, Yu S, Li S. STAT1 deficiency in the host enhances interleukin-12-mediated tumor regression. Cancer Res. 2006;66:4461–7. doi: 10.1158/0008-5472.CAN-05-3554. [DOI] [PubMed] [Google Scholar]
- 108.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, Marsh CB. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 2009;69:2133–40. doi: 10.1158/0008-5472.CAN-08-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–62. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 111.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 112.Watkins SK, Li B, Richardson KS, Head K, Egilmez NK, Zeng Q, Suttles J, Stout RD. Rapid release of cytoplasmic IL-15 from tumor-associated macrophages is an initial and critical event in IL-12-initiated tumor regression. Eur J Immunol. 2009;39:2126–35. doi: 10.1002/eji.200839010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang C-J, Chen Y-H, Huang K-W, Cheng H-W, Chan S-F, Tai K-F, Hwang L-H. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology. 2007;45:746–54. doi: 10.1002/hep.21560. [DOI] [PubMed] [Google Scholar]
- 114.Shrivastava P, Singh SM, Singh N. Effect of thymosin-α1 on the production of nitric oxide by tumor-associated macrophages. Neoplasma. 2003;50:47–53. [PubMed] [Google Scholar]
- 115.Shrivastava P, Singh SM, Singh N. Effect of thymosin α1 on the antitumor activity of tumor-associated macrophage-derived dendritic cells. J Biomed Sci. 2004;11:623–30. doi: 10.1007/BF02256128. [DOI] [PubMed] [Google Scholar]
- 116.Shrivastava P, Singh SM, Singh N. Activation of tumor-associated macrophages by thymosin α1. Int J Immunopathol Pharmacol. 2004;17:39–47. doi: 10.1177/039463200401700106. [DOI] [PubMed] [Google Scholar]
- 117.Shrivastava P, Singh SM, Singh N. Antitumor activation of peritoneal macrophages by thymosin α-1. Cancer Invest. 2005;23:316–22. doi: 10.1081/cnv-58813. [DOI] [PubMed] [Google Scholar]
- 118.Chang Y-C, Chen T-C, Lee C-T, Yang C-Y, Wang H-W, Wang C-C, Hsieh S-L. Epigenetic control of MHC class II expression in tumor-associated macrophages by decoy receptor 3. Blood. 2008;111:5054–63. doi: 10.1182/blood-2007-12-130609. [DOI] [PubMed] [Google Scholar]
- 119.Chan LLY, Cheung BKW, Li JCB, Lau ASY. A role for STAT3 and cathepsin S in IL-10 down-regulation of IFN-γ-induced MHC class II molecule on primary human blood macrophages. J Leukoc Biol. 2010;88:303–11. doi: 10.1189/jlb.1009659. [DOI] [PubMed] [Google Scholar]
- 120.Lin T, Bost KL. STAT3 activation in macrophages following infection with Salmonella. Biochem Biophys Res Commun. 2004;321:828–34. doi: 10.1016/j.bbrc.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 121.Cheng F, Wang H-W, Cuenca A, et al. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425–36. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 122.Hussain SF, Kong L-Y, Jordan J, Conrad C, Madden T, Fokt I, Priebe W, Heimberger AB. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 123.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of STAT3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Edwards JP, Emens LA. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE2 in murine macrophages. Int Immunopharmacol. 2010;10:1220–8. doi: 10.1016/j.intimp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujiwara Y, Komohara Y, Kudo R, Tsurushima K, Ohnishi K, Ikeda T, Takeya M. Oleanolic acid inhibits macrophage differentiation into the M2 phenotype and glioblastoma cell proliferation by suppressing the activation of STAT3. Oncol Rep. 2011;226:1533–7. doi: 10.3892/or.2011.1454. [DOI] [PubMed] [Google Scholar]
- 126.Fujiwara Y, Komohara Y, Ikeda T, Takeya M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011;102:206–11. doi: 10.1111/j.1349-7006.2010.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: rationality and rationale design of inhibitors. Expert Opin Investig Drugs. 2011;20:1263–75. doi: 10.1517/13543784.2011.601739. [DOI] [PubMed] [Google Scholar]
- 128.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 129.Weisser SB, McLarren KW, Voglmaier N, van Netten-Thomas CJ, Antov A, Flavell RA, Sly LM. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol. 2011;41:1742–53. doi: 10.1002/eji.201041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liao X, Sharma N, Kapadia F, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–49. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pello OM, De Pizzol M, Mirolo M, et al. Role of c-Myc in alternative activation of human macrophages and tumor-associated macrophage biology. Blood. 2012;119:411–21. doi: 10.1182/blood-2011-02-339911. [DOI] [PubMed] [Google Scholar]
- 132.Bouhlel MA, Derudas B, Rigamonti E, et al. PPAR-g activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 133.Van Ginderachter JA, Movahedi K, Van den Bossche J, De Baetselier P. Macrophages, PPARs, and Cancer. PPAR Res. 2008;2008:169414. doi: 10.1155/2008/169414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–35. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zabuawala T, Taffany DA, Sharma SM, et al. An Ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–33. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tai S-K, Chang H-C, Lan K-L, et al. Decoy receptor 3 enhances tumor progression via induction of tumor-associated macrophages. J Immunol. 2012;188:2464–71. doi: 10.4049/jimmunol.1101101. [DOI] [PubMed] [Google Scholar]
- 138.Chen W, Ma T, Shen X-n, Xia X-f, Xu G-d, Bai X-l, Liang T-b. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–72. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 139.Tugues S, Honjo S, König C, et al. Genetic deficiency in plasma protein HRG enhances tumor growth and metastasis by exacerbating immune escape and vessel abnormalization. Cancer Res. 2012;72:1953–63. doi: 10.1158/0008-5472.CAN-11-2194. [DOI] [PubMed] [Google Scholar]
- 140.Chatterjee S, Mookerjee A, Mookerjee BasuJ, et al. A novel copper chelate modulates tumor associated macrophages to promote anti-tumor response of T cells. PLoS One. 2009;4:e7048. doi: 10.1371/journal.pone.0007048. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Chakraborty P, Chatterjee S, Ganguly A, Saha P, Adhikary A, Das T, Chatterjee M, Choudhuri SK. Reprogramming of TAM toward proimmunogenic type through regulation of MAP kinases using a redox-active copper chelate. J Leukoc Biol. 2012;91:609–19. doi: 10.1189/jlb.0611287. [DOI] [PubMed] [Google Scholar]
- 142.Jassar AS, Suzuki E, Kapoor V, et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–61. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 143.Chauhan P, Sodhi A, Shrivastava A. Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology. 2009;214:197–209. doi: 10.1016/j.imbio.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 144.Tyagi A, Singh RP, Ramasamy K, et al. Growth inhibition and regression of lung tumors by Silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-κB and signal transducers and activators of transcription 3. Cancer Prev Res. 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vishvakarma NK, Singh SM. Immunopotentiating effect of proton pump inhibitor pantoprazole in a lymphoma-bearing murine host: implication in antitumor activation of tumor-associated macrophages. Immunol Lett. 2010;134:83–92. doi: 10.1016/j.imlet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 146.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang Z, Zhang Z, Jiang Y, Zhang D, Chen J, Dong L, Zhang J. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J Controlled Release. 2011;158:286–92. doi: 10.1016/j.jconrel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 148.Daldrup-Link HE, Golovko D, Ruffell B, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17:5695–704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keliher EJ, Yoo J, Nahrendorf M, Lewis JS, Marinelli B, Newton A, Pittet MJ, Weissleder R. 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging. Bioconjug Chem. 2011;22:2383–9. doi: 10.1021/bc200405d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leimgruber A, Berger C, Cortez-Retamozo V, et al. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia. 2009;11:459–68. doi: 10.1593/neo.09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Melancon MP, Lu W, Huang Q, Thapa P, Zhou D, Ng C, Li C. Targeted imaging of tumor-associated M2 macrophages using a macromolecular contrast agent PG-Gd-NIR813. Biomaterials. 2010;31:6567–73. doi: 10.1016/j.biomaterials.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dai F, Liu L, Che G, et al. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Welsh T, Green R, Richardson D, Waller D, O'Byrne K, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–67. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 155.Chen J, Yao Y, Gong C, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Standiford TJ, Kuick R, Bhan U, Chen J, Newstead M, Keshamouni VG. TGF-β-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene. 2011;30:2475–84. doi: 10.1038/onc.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 158.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]


