Abstract
Chymase has been extensively studied with respect to its role in the pathophysiology of cardiovascular disease, and is notable for its role in the generation of angiotensin II, a mediator crucial in vascular remodelling. However, in more recent years, an association between chymase and several inflammatory diseases, including gastrointestinal (GI) disorders such as inflammatory bowel diseases (IBD) have been described. Such studies, to date, with respect to IBD at least, are descriptive in the clinical context; nonetheless, preclinical studies implicate chymase in the pathogenesis of gut inflammation. However, studies to elucidate the role of chymase in functional bowel disease are in their infancy, but suggest a plausible role for chymase in contributing to some of the phenotypic changes observed in such disorders, namely increased epithelial permeability. In this short review, we have summarized the current knowledge on the pathophysiological role of chymase and its inhibition with reference to inflammation and tissue injury outside of the GI tract and discussed its potential role in GI disorders. We speculate that chymase may be a novel therapeutic target in the GI tract, and as such, inhibitors of chymase warrant preclinical investigation in GI diseases.
Keywords: chymase, gastrointestinal, permeability, inflammation, mast cell
Introduction
Derived from bone marrow, mast cells (MC) are classically recognized as key cells involved in a number of non-allergic diseases and hypersensitivity (He, 2004). They contain secretory granules, which, upon stimulation, release various cytokines, biogenic amines, proteoglycans and proteases such as, tryptase, carboxypeptidase A and notably, in the context of this review, chymase (Pejler et al., 2010; Scandiuzzi et al., 2010).
In humans MC classification is less complex than that in rodents and is based on their protease content, the latter being predominantly tryptase or chymase, thereby leading to their classification as MCT, those expressing MC tryptase only, and MCTC, a subgroup expressing both tryptase and chymase (Scandiuzzi et al., 2010).
With respect to MC chymase (EC 3.4.21.39), it is a serine protease that possesses chymotrypsin-like cleavage specificity. Pereira et al. (1999) determined the crystal structure of human chymase complexed with the peptide inhibitor, succinyl-Ala-Ala-Pro-Phe-chloromethylketone. Human chymase is synthesized as an inactive prepro-enzyme, which is then converted to the pro-enzyme. The thiol proteinase, dipeptidyl peptidase I is necessary for activation of chymase within the secretory granules (Murakami et al., 1995). When stored in secretory granules of MC, chymase remains enzymatically inactive at pH 5.5; its optimum enzymatic activity being achieved at a pH of 7 to 9 following release into the interstitial tissues (where a pH 7.4 is observed) (Takai et al., 2010) (Figure 1). MC containing chymases reside primarily in the connective tissue and the submucosa (Irani et al., 1986). This chymotrypsin-like serine protease is common to several other species, namely primates, dogs, sheep and hamsters. Humans possess a single α-chymase gene, whereas rats and mice possess not only a single α-chymase gene, mouse mast cell protease-5 (mMCP-5) and rat mast cell protease-5 (rMCP-5), but also up to 14 β-chymase genes (Gallwitz and Hellman, 2006). Moreover, significant species variability has contributed to the controversy over the possible physiological roles for chymase (Miyazaki et al., 2006). Despite such complexity, however, rodent models have been generated with targeted deletions in the phylogenetic homologues to the human α-chymase allowing for extrapolation of preclinical data to human conditions. Identifying the functional homologue to human chymase has been the focus of several studies (Kunori et al., 2002; Hochegger et al., 2005), not all yielding the expected outcome. For example, Kunori et al. (2002), using a molecular phylogenetics approach, demonstrated rMCP-5 and mMCP-5, although homologous to human chymase, exhibited marked differences in substrate specificity conferring elastase-like, rather than chymase activity, and the closest homologues to human chymase in terms of functional properties and tissue localization appear to be murine β-chymase, mMCP-4 and rMCP-1 (Tchougounova et al., 2003; Andersson et al., 2008).
Figure 1.
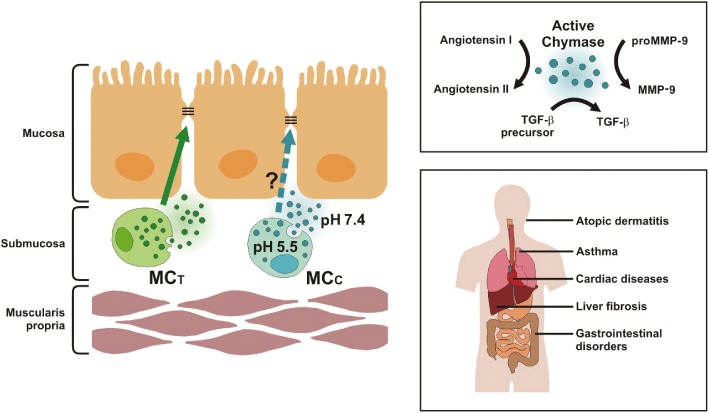
Role of chymase in disease pathogenesis. Angiotensin converting enzyme inhibitor-independent angiotensin II synthesis by chymase has been implicated in the structural remodelling associated with cardiovascular disease and can induce activation of TGF-β, a major regulator of tissue fibrosis, and MMP-9 activity (top right panel), thereby collectively contributing to the pathological role of chymase in cardiovascular, fibrotic and inflammatory diseases, including IBD (bottom right panel). Both mast cell tryptase (MCT) and mast cell chymase (MCC) have been implicated in regulating epithelial barrier function in the gastrointestinal tract. Thus, in addition to inhibiting downstream TGF-β and MMP-9 activity, chymase inhibition may enhance epithelial barrier function, which is known to be compromised in inflammatory and functional bowel diseases. (left-hand pictogram).
Chymase inhibition: a validated target in cardiovascular and inflammatory diseases
Chymase-dependent angiotensin (Ang) II formation in cardiovascular disease
Chymase has largely been documented for its involvement in the synthesis of Ang II from Ang I in addition to endothelin-1. Studies to decipher the pathophysiological role of chymase in cardiac disease have gained momentum in recent times, and ACE-independent Ang II synthesis by chymase has been implicated in the structural remodelling associated with cardiovascular disease (Doggrell and Wanstall, 2004; 2005). Evidence suggests Ang II formation may depend to a greater extent on chymase rather than ACE, with one recent study suggesting that up to 75% of Ang II formation is dependent on chymase in human cardiac tissue (Takai et al., 2011). Studies with coronary artery homogenates from human hearts demonstrated that chymostatin had the capacity to reduce Ang II formation in contrast to the ACE inhibitor, captopril (Doggrell and Wanstall, 2004). Therefore, studies have focussed on reducing the availability of the enzyme through the use of the MC stabilizer, tranilast, rather than chymase inhibition per se, in animal models of cardiovascular disease. However, although tranilast proved effective pre-clinically, unfortunately, clinical trials proved unsuccessful (Doggrell and Wanstall, 2004).
Chymase-induced activation of TGF-β and MMP-9: implications in cardiac disease
In addition to its direct enzymatic effects, chymase can also induce activation of TGF-β, a major regulator of tissue fibrosis, as well as MMP-9 (Takai et al., 2010), the latter being involved in extracellular matrix (ECM) turnover with potential to induce organ damage (Takai et al., 2010). Thus, chymase has a crucial role to play in tissue fibrosis and remodelling thereby implicating a role for this protease in regulating homeostatic function (Pejler et al., 2010) and it is in this context that chymase may contribute to abdominal aortic aneurysm (AAA) lesions (Takai et al., 2010). This was substantiated in vitro in vascular tissue from human AAA lesions, in which a significant increase in MMP-9 activity was observed when the lesions were incubated with purified human chymase (Takai et al., 2010). Not surprisingly therefore, mice lacking mMCP-4 had markedly reduced AAA formation in an experimental model of AAAs. The underlying mechanisms by which mMCP-4 contribute to AAA lesions include vascular cell apoptosis, elastase degradation and angiogenesis (Sun et al., 2007).
Atherosclerosis is associated with aortic aneurysm development, and a recent study by Bot et al. (2011) examined the contribution of chymase to atherosclerotic plaque stability in the apolipoprotein mouse model (apoE−/− mice) of human atherosclerosis. Bot et al. had previously demonstrated that perivascular MC play a crucial role in plaque destabilization and progression, and their subsequent findings were the first to demonstrate that chymase inhibition, using the selective chymase inhibitor, RO5066852 (Tables 1 and 2) reduced plaque progression and enhanced plaque stability (Bot et al., 2011). Specifically, oral administration of RO5066852 to apoE−/− mice reduced spontaneous atherosclerosis and plaque progression, changes which corresponded to enhanced plaque stability and diminished frequency and area of intraplaque haemorrhages (Bot et al., 2011). Of note is the reported presence of mMCP-4-containing MC in aortic atherosclerotic plaques, thereby further implicating chymase in plaque formation (Sun et al., 2007). In a related study, using hamsters with elevated cholesterol, chymase secretion contributed to plaque vulnerability and treatment with the MC stabilizer, tranilast enhanced plaque stability presumably through inhibition of chymase release (Guo et al., 2009).
Table 1.
Therapeutic application of chymase inhibitors
| Chymase inhibitors | Therapeutic potential | Reference |
|---|---|---|
| SUN-C8257 | Atherosclerosis, pulmonary fibrosis, skin disease | Doggrell (2008) |
| SUN13834a | Atopic dermatitis | Ogata et al. (2011) |
| BCEAB | Cardiac disease | Doggrell (2008) |
| Compound 17 | Cardiac or circulatory disease, asthma | Doggrell (2008) |
| NK3201 | Cardiac disease | Doggrell (2008) |
| TEI-E548 | Cardiac disease | Hoshino et al. (2003) |
| RO5066852 | Atherosclerosis | Bot et al. (2011) |
| JNJ-10311795 | Anti-inflammatory | De Garavilla et al. (2005) |
| Suc-Val-Pro-PheP (OPh)2 | Cardiac adhesions | Soga et al. (2004) |
| Y-40613 | Atopic dermatitis | Akahoshi et al. (2001) |
| Imada et al. (2002) | ||
| Y-40079b | Akahoshi et al. (2001) | |
| TY-51469 | Cardiac disease, liver fibrosis, gastrointestinal disease, diabetes | Oyamada et al. (2011) |
| Komeda et al. (2010) | ||
| Kakimoto et al. (2010) | ||
| Takai et al. (2009) | ||
| Chymostatin | Glaucoma, chorioretinal, gastrointestinal diseases | Doggrell (2008) |
| Groschwitz et al. (2009) |
Currently in phase II clinical trial.
Yet to be tested in experimental disease models.
Table 2.
Species in which chymase inhibitors have been validated in preclinical disease models
| Chymase inhibitor | Substrate specificity | Species | Reference |
|---|---|---|---|
| SUN-C8257 | Chymase, cathepsin G | Human, hamster, dog | Matsumoto et al. (2003) |
| Doggrell (2008) | |||
| SUN13834a | Chymase, cathepsin G | Mouse, human | Ogata et al. (2011) |
| BCEAB | Chymase | Hamster | Doggrell (2008) |
| Compound 17 | Chymase | Sheep | Doggrell (2008) |
| NK3201 | Chymase | Hamster | Doggrell (2008) |
| TEI-E548 | Chymase | Hamster | Hoshino et al. (2003) |
| RO5066852 | Chymase | Mice | Bot et al. (2011) |
| Suc-Val-Pro-PheP (OPh)2 | Chymase | Hamster | Soga et al. (2004) |
| JNJ-10311795: RWJ-355871 | Cathepsin G, chymase | Rats, mice, sheep | De Garavilla et al. (2005) |
| Maryanoff et al. (2010) | |||
| Y-40613 | Chymase | Mice | Akahoshi et al. (2001) |
| Imada et al. (2002) | |||
| TY-51469 | Chymase | Rats, hamster, pigs | Komeda et al. (2010) |
| Kakimoto et al. (2010) | |||
| Takai et al. (2009) | |||
| Oyamada et al. (2011) | |||
| Chymostatin | Chymase | Rabbit | Doggrell (2008) |
In a more acute cardiac process, Oyamada et al. (2011) recently assessed the effects of the chymase inhibitor, TY-51469 on myocardial protection and fibrosis after acute myocardial ischaemia/reperfusion in which chymase inhibition played a crucial role in myocardial protection and was associated with decreased MMP-9 activity. Additionally, myocardial infarct size and neutrophil infiltration were decreased, as was inflammatory gene expression (Oyamada et al., 2011). However, MMP-9 is not the only significant downstream effector molecule activated by chymase, and as already alluded to, chymase can activate TGF-β, which in turn promotes tissue fibrosis. With this in mind, Soga et al. (2004) investigated the role of chymase in cardiac adhesion formation using the chymase specific inhibitor, Suc-Val-Pro-PheP(OPh)2 and assessed cardiac adhesions in a post operative hamster model. Suc-Val-Pro-PheP(OPh)2 was administered into the thoracic cavity surrounding the surgical lesions and was effective in reducing cardiac chymase activity, the density of MC, in addition to suppressing the levels of TGF-β1, resulting in a reduction in postoperative cardiac adhesions (Soga et al., 2004).
Chymase inhibitors: anti-inflammatory properties as a pharmacological mechanism of action
Chymase promotes inflammation, tissue remodelling and matrix destruction by several mechanisms, including the enzymatic activation of TGF-β and MMP-9 (Figure 1). Therefore, chymase inhibition is likely to attenuate both TGF-β and MMP-9 activation, thereby directly influencing tissue inflammation and fibrosis beyond cardiac diseases. Indeed, chymase has been associated with the pathogenesis of inflammatory disorders such as atopic dermatitis (AD) (Ong, 2009), one of the most common chronic inflammatory or allergic skin diseases, and autoimmune arthritis (Magnusson et al., 2009). Moreover, the association of chymase with AD has been strengthened by several genetic studies in which a correlation between an allelic polymorphism in the chymase gene and the incidence of AD has been reported (Mao et al., 1996; Tanaka et al., 1999; Watanabe et al., 2007). Moreover, the results obtained after targeting chymase in pre-clinical animal models of AD have been promising, and the chymase inhibitor, SUN13834 is currently in phase II clinical trials for the treatment of AD (Asubio Pharmaceuticals Inc, 2011; Ogata et al., 2011).
It is widely established that MC are present in increased numbers and have been implicated in airway remodelling associated with asthma (Lazaar et al., 2002). Despite the evidence discussed thus far on the benefits of chymase inhibition, it is noteworthy that a possible protective effect of chymase has been suggested from studies in humans with severe asthma (Balzar et al., 2005), and have been corroborated by studies in mMCP-4-deficient mice, which suggest the absence of chymase may lead to increased tissue inflammation and smooth muscle layer thickening (Waern et al., 2009). In contrast to in vivo studies, in vitro studies on human airway smooth muscle suggest a detrimental effect of chymase and its contribution to airway remodelling (Lazaar et al., 2002), perhaps suggesting that the microenvironment in vivo determines the final outcome in terms of smooth muscle remodelling. Moreover, a single nucleotide polymorphism in CMA1, the MC chymase gene, was found to be associated with childhood asthma (Hossny et al., 2008). The evidence from renal studies similarly suggests a potential dual role for chymase, or MC at least, in glomerulonephritis (Hochegger et al., 2005; Scandiuzzi et al., 2010). Hochegger et al. reported a dramatic increase in glomerular damage in mice deficient in MC, providing evidence of the protective properties of MC (Hochegger et al., 2005). In contrast, Scandiuzzi et al. (2010) investigated the role of MC chymase in experimental antiglomerular basement membrane-induced glomerulonephritis. Here they demonstrated that mMCP-4-deficient mice developed less severe renal damage. Contrary to the previous study these results highlight the possible detrimental role of chymase in kidney disease.
Chymase inhibition: a novel therapeutic target in gastrointestinal (GI) disorders?
The role of chymase in inflammatory bowel disease (IBD)
With regard to inflammation in the GI tract, an association between IBD and increased MC number has long been reported (Dvorak et al., 1980; Nolte et al., 1990; Gelbmann et al., 1999). However, until relatively recently, little was known about the role of chymase in IBD patients. Andoh et al. (2006) were one of the first to report a marked change in the distribution and number of chymase immunopositive MC in IBD biopsies. IBD is characterized by chronic inflammation and encompasses both ulcerative colitis (UC) and Crohn's disease (CD); in UC, however, the inflammation is limited to the mucosa of the colon, while in CD any region of the intestine may be affected and this inflammation is often transmural (Abraham and Cho, 2009). Given the ability of chymase to convert TGF-β and MMP-9 into their active forms (Takai et al., 2010), and the association of both these chymase-induced mediators in the pathophysiology of IBD, one may speculate that targeting chymase in IBD may be of therapeutic relevance. TGF-β, for example, is known to play a central role in severe fibrotic diseases, such as CD, through an increased production of ECM components (Pohlers et al., 2009). Of note, however, with respect to chymase in IBD are the divergent changes which occur in chymase-containing MC number between UC and CD, as well as between active and inactive phases of inflammation (Andoh et al., 2006). In particular, Andoh et al. (2006) noted a marked increase in chymase immunopositive MC in the colonic mucosa of active CD patients, not only in the laminia propria but also in the submucosa, propria muscularis and in the surrounding fatty tissue. Given that CD is characterized by tissue fibrosis, and MC have been implicated in this process (Gelbmann et al., 1999), this finding is perhaps expected. Indeed, such an observation would be in-keeping with the pro-fibrotic characteristics of chymase observed in patients with chronic hepatitis and liver fibrosis (Satomura et al., 2003), which in preclinical models display sensitivity to chymase inhibition with TY-51469 (Komeda et al., 2010). Conversely, decreased chymase has been associated with active UC in patient biopsies (Andoh et al., 2006). Nonetheless, mechanistic insight into the contribution of chymase in UC emerges from studies that have focussed on determining the role of chymase-dependent MMP-9 activation. MMP-9 is known to play an important role in the degradation of ECM proteins and has been shown to be consistently up-regulated during active inflammation, both in patients and in animal models of colitis (Takai et al., 2010). Tchougounova et al. (2005) further confirmed, by examining a functionally relevant murine protease to human chymase, that mMCP-4 is responsible for the conversion of proMMP-9 to its active form, MMP-9 in vivo. Significantly, this chymase-MMP-9 pathway may well be of significance in IBD, as Ishida et al. (2008) found that both chymase and MMP-9 were significantly increased in the dextran sodium sulphate animal model of colitis. Furthermore, they demonstrated that this increased activity could be suppressed by the chymase inhibitor, NK3201 suggesting an important role for chymase in the development of colitis via MMP-9 activation, and indicative of an indirect role for chymase in the degradation of the ECM. Indeed, this chymase-dependent MMP-9 activation has also been shown to play a significant role in other animal models of GI inflammation, for example, in rats with indomethacin-induced small intestinal damage. Here again, inhibition of chymase by use of TY-51469 significantly attenuated MMP-9 activation and ameliorated small intestinal damage (Kakimoto et al., 2010). Therefore, the preclinical evidence suggests that inhibition of chymase in IBD may be of benefit, but via indirect down-regulation of TGF-β and MMP-9 activation; whether this is of clinical relevance in IBD patients awaits investigation. Of note, however, is the increased presence of chymase-positive MC, not only in active CD, but also in inactive CD, relative to healthy patients (Andoh et al., 2006); again, the clinical significance of this finding is unknown. However, we speculate, as in irritable bowel syndrome (IBS), and given the ability of chymase to increase colonic epithelial permeability (Groschwitz et al., 2009), that these persisting chymase-positive MC may contribute to the increased permeability characteristically observed in quiescent CD biopsies, and therefore may contribute to the rate of relapse in these patients (Wyatt et al., 1993; Hilsden et al., 1999).
The potential role of chymase in the pathophysiology of IBS
Unlike IBD, IBS is a common sensory and motility disorder of the GI tract, characterized by chronic, episodic abdominal pain and discomfort as well as altered bowel habits, the pathophysiology of which is not fully understood (Quigley et al., 2006). Evidence for MC involvement in the pathogenesis of IBS has been mounting with studies in human (Park et al., 2003; Barbara et al., 2004) and in preclinical rodent models (Gue et al., 1997; Barreau et al., 2008; Hyland et al., 2009) in agreement. MC hyperplasia in biopsy tissue obtained from IBS patients occurs in the colon (Barbara et al., 2004), ceacum and rectum (Park et al., 2003), as well as in the small intestine (Guilarte et al., 2007) and, more specifically, their products have been associated with symptom severity in IBS (Park et al., 2003; Barbara et al., 2004). Whether MC, or indeed their products, transpire to be robust and reliable biomarkers of IBS remains unclear. However, a sensitive and specific assay for chymase activity in serum has been developed. Raymond et al. (2009) exploited the α2-macroglobulin binding properties of chymase to assess for chymase activity in the serum of subjects with mastocytosis. Indeed, recent evidence has proposed the use of secretory leucocyte protease inhibitor (SLPI) cleavage products, as a novel biomarker of chymase activity (Belkoski et al., 2008). These authors showed that chymase has the ability to cleave SLPI in a site-specific and unique nature from other proteases. This cleavage was easily detectable in human saliva and correlated with the severity of allergic symptoms observed (Belkoski et al., 2008).
Emerging evidence suggests that patients with IBS have increased intestinal permeability (Dunlop et al., 2006). However, to date, studies have focussed on the involvement of tryptase (Guilarte et al., 2007; Piche et al., 2009; Lee et al., 2010). Guilarte et al. (2007) reported jejunal MC hyperplasia and tryptase release, particularly in diarrhoea-predominant IBS (D-IBS) patients. However, despite these findings, studies examining the integrity of the gut mucosa in colonic biopsies of IBS patients have demonstrated an increase in intestinal epithelial barrier permeability irrespective of IBS subtype and that soluble mediators released from the biopsies of IBS patients significantly increased paracellular permeability of colonic epithelial cells in vitro (Piche et al., 2009). While histamine has been precluded from mediating this effect (Piche et al., 2009), other investigators, using the tryptase inhibitor nafamostat, demonstrated that increased permeability was tryptase-dependant, in D-IBS patients at least (Lee et al., 2010). However, to date, little attention has been given to chymase, or chymase-containing MC, in contributing to the deficits in intestinal permeability in IBS, despite evidence that functionally, and relevant to man, the mouse homologue of human chymase, mMCP-4, regulates intestinal barrier function (Groschwitz et al., 2009). In preclinical studies, largely conducted in rat, the β-chymase, rMCP-2 has been most studied. However, little information exists on rMCP-1, which is functionally more akin to human chymase (Andersson et al., 2008). Increased numbers of mucosal MC expressing rMCP-2 have been identified in both mucosal and connective tissue in an early life stress-induced model of IBS (Barreau et al., 2008; Hyland et al., 2009). While most studies measure circulating or mucosal rMCP-2 release do so as a marker of increased protease activity (Moriez et al., 2007), few have appreciated its effects on epithelial permeability and the implications this may have for IBS. However, of those who have considered the direct effects of rMCP-2 on epithelial barrier function, the data support a direct and detrimental effect on the epithelium. Vergnolle et al. (1998), by exogenously adding rMCP-2, demonstrated increased epithelial paracellular permeability in vitro, while Scudamore et al. (1995) demonstrated the relationship between intestinal mucosal permeability and the release of rMCP-2 in an anaphylaxis response. Likewise, studies in endotoxaemic rats, administered lippopolysaccharide, showed a rMCP-2-dependent increase in colonic permeability, the functional significance of which is yet to be determined (Moriez et al., 2007) but may be very relevant to the pathogenesis of IBS. Moreover, similar chymase-induced effects have been observed outside of the GI tract; human chymase was shown to decrease barrier function and migration of corneal epithelial cells and this activity was inhibited by chymostatin (Ebihara et al., 2005).
Even if we do recognize that chymase may have a role to play in the barrier breakdown associated with IBS, is it doing so independently of tryptase? Evidence suggests that tryptase release is under the regulatory control of chymase (He and Xie, 2004), therefore, it is possible that both chymase and tryptase, in concert, contribute to the symptoms associated with IBS. Despite the identification of a chymostatin-sensitive tryptase-release pathway in MC isolated from the GI tract (He and Xie, 2004), to date, this pathway has not been investigated in IBS.
Conclusions and perspective
Mice and rats have been the experimental models of choice in characterizing the role of chymase in the pathogenesis of several diseases primarily due to availability (Table 2). However, extrapolating data from such animal models to humans is complicated by multiple β-chymases in contrast to the fewer α-chymase(s) present in humans, primates, dogs, hamsters and sheep. Nonetheless, human chymase and rodent, rMCP-1 and mMCP-4 have well-characterized chymotrytic substrate recognition profiles (Andersson et al., 2008; 2009) and have been shown to display similar substrate specificities (Andersson et al., 2008). While mMCP-4 knockout mice have been used to examine the contribution of this human chymase homologue in several disease models, the total impact of all the murine chymases cannot be excluded (Pejler et al., 2010). The rationale behind the extensive duplication of the chymase gene in rodents remains unclear. However, these gene duplications may be important in the process of speciation where, a particular function, such as immunity, a sense of taste and smell, may provide selective advantages in a novel environment (Gallwitz and Hellman, 2006). Indeed, such diversity must be taken into account when characterizing chymase inhibitors, as demonstrated by Kervinen et al. (2010) who noted a strikingly wide variation of inhibitory potency across species. Despite such limitations, numerous orally-active chymase inhibitors have been developed and patented, for example, BCEAB, Compound 17, NK3201 and SUN-C8257 (Doggrell, 2008), although few have been tested in preclinical models of GI disease. Such studies may well be compounded by species variability, but have, in the setting of non-GI disorders, been useful tools in characterizing the role of chymase in the pathogenesis of cardiovascular and inflammatory diseases among others. Unfortunately, there remains a paucity of studies investigating the pathophysiological role of chymase in GI disorders, and particularly so with respect to IBS, with the mechanisms involved still to be fully elucidated. However, the current preclinical data are tantalizing with respect to a role for chymase in both inflammatory and functional bowel diseases.
Acknowledgments
The authors acknowledge financial support from the Health Research Board (Ireland) and a centre grant to the Alimentary Pharmabiotic Centre from Science Foundation Ireland. We also acknowledge Dr. Marcela Julio-Pieper for producing Figure 1.
Glossary
- AAA
abdominal aortic aneurysm
- AD
atopic dermatitis
- Ang
angiotensin
- CD
Crohn's disease
- CMA1
mast cell chymase gene
- D-IBS
diarrhoea-predominant irritable bowel syndrome
- ECM
extracellular matrix
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- MC
mast cells
- mMCP
mouse mast cell protease
- rMCP
rat mast cell protease
- SLPI
secretory leucocyte protease inhibitor
- UC
ulcerative colitis
Conflicts of interest
None.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Eng J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahoshi F, Ashimori A, Sakashita H, Yoshimura T, Imada T, Nakajima M, et al. Synthesis, structure-activity relationships, and pharmacokinetic profiles of nonpeptidic α-keto heterocycles as novel inhibitors of human chymase. J Med Chem. 2001;44:1286–1296. doi: 10.1021/jm000496v. [DOI] [PubMed] [Google Scholar]
- Andersson MK, Karlson U, Hellman L. The extended cleavage specificity of the rodent [beta]-chymases rMCP-1 and mMCP-4 reveal major functional similarities to the human mast cell chymase. Mol Immunol. 2008;45:766–775. doi: 10.1016/j.molimm.2007.06.360. [DOI] [PubMed] [Google Scholar]
- Andersson MK, Enoksson M, Gallwitz M, Hellman L. The extended substrate specificity of the human mast cell chymase reveals a serine protease with well-defined substrate recognition profile. Int Immunol. 2009;21:95–104. doi: 10.1093/intimm/dxn128. [DOI] [PubMed] [Google Scholar]
- Andoh A, Deguchi Y, Inatomi O, Yagi Y, Bamba S, Tsujikawa T, et al. Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol Rep. 2006;16:103–107. [PubMed] [Google Scholar]
- Asubio Pharmaceuticals Inc. 2011. A multicenter, randomized, placebo-controlled, double-blind study of SUN13834 in adult subjects with atopic dermatitis. NCT00717769.
- Balzar S, Chu HW, Strand M, Wenzel S. Relationship of small airway chymase-positive mast cells and lung function in severe asthma. Am J Respir Crit Care Med. 2005;171:431–439. doi: 10.1164/rccm.200407-949OC. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve–mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–590. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- Belkowski SM, Masucci J, Mahan A, Kervinen J, Olson M, de Garavilla L, et al. Cleaved SLPI, a novel biomarker of chymase activity. Biol Chem. 2008;389:1219–1224. doi: 10.1515/BC.2008.138. [DOI] [PubMed] [Google Scholar]
- Bot I, Bot M, Van Heiningen SH, Van Santbrink PJ, Lankhuizen IM, Hartman P, et al. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE−/− mice. Cardiovasc Res. 2011;89:244. doi: 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- De Garavilla L, Greco MN, Sukumar N, Chen ZW, Pineda AO, Mathews F, et al. A novel, potent dual inhibitor of the leukocyte proteases cathepsin G and chymase. J Biolog Chem. 2005;280:18001–18007. doi: 10.1074/jbc.M501302200. [DOI] [PubMed] [Google Scholar]
- Doggrell SA. Therapeutic potential of non-peptide chymase inhibitors. Exp Opin Therc Patents. 2008;18:485–499. [Google Scholar]
- Doggrell SA, Wanstall JC. Vascular chymase: pathophysiological role and therapeutic potential of inhibition. Cardiovasc Res. 2004;61:653–662. doi: 10.1016/j.cardiores.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Doggrell SA, Wanstall JC. Cardiac chymase: pathophysiological role and therapeutic potential of chymase inhibitors. Can J Physiol Pharmacol. 2005;83:123–130. doi: 10.1139/y04-136. [DOI] [PubMed] [Google Scholar]
- Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Monahan RA, Osage JE, Dickersin GR. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980;11:606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- Ebihara N, Funaki T, Murakami A, Takai S, Miyazaki M. Mast cell chymase decreases the barrier function and inhibits the migration of corneal epithelial cells. Curr Eye Res. 2005;30:1061–1069. doi: 10.1080/02713680500346625. [DOI] [PubMed] [Google Scholar]
- Gallwitz M, Hellman L. Rapid lineage-specific diversification of the mast cell chymase locus during mammalian evolution. Immunogenetics. 2006;58:641–654. doi: 10.1007/s00251-006-0123-4. [DOI] [PubMed] [Google Scholar]
- Gelbmann C, Mestermann S, Gross V, Köllinger M, Schölmerich J, Falk W. Strictures in Crohn's disease are characterised by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut. 1999;45:210–217. doi: 10.1136/gut.45.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Åbrink M, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gue M, Del Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- Guilarte M, Santos J, De Torres I, Alonso C, Vicario M, Ramos L, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Chen WQ, Zhang C, Zhao YX, Zhang Y. Chymase activity is closely related with plaque vulnerability in a hamster model of atherosclerosis. Atherosclerosis. 2009;207:59–67. doi: 10.1016/j.atherosclerosis.2009.04.014. [DOI] [PubMed] [Google Scholar]
- He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309–318. doi: 10.3748/wjg.v10.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SH, Xie H. Inhibition of tryptase release from human colon mast cells by protease inhibitors. World J Gastroenterol. 2004;10:332–336. doi: 10.3748/wjg.v10.i3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsden RJ, Meddings JB, Hardin JGD, Sutherland G, Lloyd R. Intestinal permeability and postheparin plasma diamine oxidase activity in the prediction of Crohn's disease relapse. Inflamm Bowel Dis. 1999;5:85–91. doi: 10.1097/00054725-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Hochegger K, Siebenhaar F, Vielhauer V, Heininger D, Mayadas TN, Mayer G, et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol. 2005;35:3074–3082. doi: 10.1002/eji.200526250. [DOI] [PubMed] [Google Scholar]
- Hoshino F, Urata H, Inoue Y, Saito Y, Yahiro E, Ideishi M, et al. Chymase inhibitor improves survival in hamsters with myocardial infarction. J Cardiovasc Pharmacol. 2003;41:S11–S18. [PubMed] [Google Scholar]
- Hossny E, Amr N, Elsayed S, Nasr R, Ibraheim E. Association of polymorphisms in the mast cell chymase gene promoter region (-1903 G/A) and (TG) n (GA) m repeat downstream of the gene with bronchial asthma in children. J Investig Allergol Clin Immunol. 2008;18:376–381. [PubMed] [Google Scholar]
- Hyland N, Julio-Pieper M, O'Mahony S, Bulmer D, Lee K, Quigley E, et al. A distinct subset of submucosal mast cells undergoes hyperplasia following neonatal maternal separation: a role in visceral hypersensitivity? Gut. 2009;58:1029–1030. doi: 10.1136/gut.2008.167882. [DOI] [PubMed] [Google Scholar]
- Imada T, Komorita N, Kobayashi F, Naito K, Yoshikawa T, Miyazaki M, et al. Development and application of chymase inhibitors: therapeutic potential of a specific chymase inhibitor in atopic dermatitis. Jpn J Pharmacol. 2002;90:214–217. doi: 10.1254/jjp.90.214. [DOI] [PubMed] [Google Scholar]
- Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Takai S, Murano M, Nishikawa T, Inoue T, Murano N, et al. Role of chymase-dependent matrix metalloproteinase-9 activation in mice with dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2008;324:422–426. doi: 10.1124/jpet.107.131946. [DOI] [PubMed] [Google Scholar]
- Kakimoto K, Takai S, Murano M, Ishida K, Yoda Y, Inoue T, et al. Significance of chymase-dependent matrix metalloproteinase-9 activation on indomethacin-induced small intestinal damages in rats. J Pharmacol Exp Ther. 2010;332:684–689. doi: 10.1124/jpet.109.162933. [DOI] [PubMed] [Google Scholar]
- Kervinen J, Crysler C, Bayoumy S, Abad MC, Spurlino J, Deckman I, et al. Potency variation of small-molecule chymase inhibitors across species. Biochem Pharmacol. 2010;80:1033–1041. doi: 10.1016/j.bcp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Komeda K, Takai S, Jin D, Tashiro K, Hayashi M, Tanigawa N, et al. Chymase inhibition attenuates tetrachloride induced liver fibrosis in hamsters. Hepatol Res. 2010;40:832–840. doi: 10.1111/j.1872-034X.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- Kunori Y, Koizumi M, Masegi T, Kasai H, Kawabata H, Yamazaki Y, et al. Rodent chymases are elastase like proteases. Eur J Biochem. 2002;269:5921–5930. doi: 10.1046/j.1432-1033.2002.03316.x. [DOI] [PubMed] [Google Scholar]
- Lazaar AL, Plotnick MI, Kucich U, Crichton I, Lotfi S, Das SKP, et al. Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J Immunol. 2002;169:1014–1020. doi: 10.4049/jimmunol.169.2.1014. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park JH, Park DIL, Kim HJ, Cho YK, Sohn CIL, et al. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci. 2010;55:2922–2928. doi: 10.1007/s10620-009-1094-8. [DOI] [PubMed] [Google Scholar]
- Magnusson SE, Pejler G, Kleinau S, Åbrink M. Mast cell chymase contributes to the antibody response and the severity of autoimmune arthritis. FASEB J. 2009;23:875–882. doi: 10.1096/fj.08-120394. [DOI] [PubMed] [Google Scholar]
- Mao X, Shirakawa T, Yoshikawa T, Yoshikawa K, Kawai M, Sasaki S, et al. Association between genetic variants of mast-cell chymase and eczema. Lancet. 1996;348:581–583. doi: 10.1016/s0140-6736(95)10244-2. [DOI] [PubMed] [Google Scholar]
- Maryanoff BE, De Garavilla L, Greco MN, Haertlein BJ, Wells GI, Andrade-Gordon P, et al. Dual inhibition of cathepsin G and chymase is effective in animal models of pulmonary inflammation. Am J Respir Crit Care Med. 2010;181:247–253. doi: 10.1164/rccm.200904-0627OC. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Wada A, Tsutamoto T, Ohnishi M, Isono T, Kinoshita M. Chymase inhibition prevents cardiac fibrosis and improves diastolic dysfunction in the progression of heart failure. Circulation. 2003;107:2555–2558. doi: 10.1161/01.CIR.0000074041.81728.79. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Takai S, Jin D, Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112:668–676. doi: 10.1016/j.pharmthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Moriez R, Leveque M, Salvador-Cartier C, Barreau F, Theodorou V, Fioramonti J, et al. Mucosal mast cell proteases are involved in colonic permeability alterations and subsequent bacterial translocation in endotoxemic rats. Shock. 2007;28:118–124. doi: 10.1097/SHK.0b013e3180315ba9. [DOI] [PubMed] [Google Scholar]
- Murakami M, Karnik SS, Husain A. Human prochymase activation. J Biol Chem. 1995;270:2218–2223. [PubMed] [Google Scholar]
- Nolte H, Spjeldnaes N, Kruse A, Windelborg B. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut. 1990;31:791–794. doi: 10.1136/gut.31.7.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata A, Fujieda Y, Terakawa M, Muto T, Tanaka T, Maruoka H, et al. Pharmacokinetic/pharmacodynamic analyses of chymase inhibitor SUN13834 in NC/Nga mice and prediction of effective dosage for atopic dermatitis patients. Int Immunopharmacol. 2011;11:1628–1632. doi: 10.1016/j.intimp.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Ong PY. Emerging drugs for atopic dermatitis. Expert Opin Emerg Drugs. 2009;1:165–179. doi: 10.1517/14728210902721248. [DOI] [PubMed] [Google Scholar]
- Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther. 2011;339:143–151. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci. 2003;18:204–210. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- Pereira PJB, Wang ZM, Rubin H, Huber R, Bode W, Schechter NM, et al. The 2.2 Å crystal structure of human chymase in complex with succinyl-ala-ala-pro-phe-chloromethylketone: structural explanation for its dipeptidyl carboxypeptidase specificity 1. J Mol Biol. 1999;286:163–173. doi: 10.1006/jmbi.1998.2462. [DOI] [PubMed] [Google Scholar]
- Piche T, Barbara G, Aubert P, Bruley Des Varannes S, Dainese R, Nano JL, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, et al. TGF-β and fibrosis in different organs – molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Quigley E, Bytzer P, Jones R, Mearin F. Irritable bowel syndrome: the burden and unmet needs in Europe. Dig Liver Dis. 2006;38:717–723. doi: 10.1016/j.dld.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Raymond WW, Su S, Makarova A, Wilson TM, Carter MC, Metcalfe DD, et al. α2-Macroglobulin capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity. J Immunol. 2009;182:5770–5777. doi: 10.4049/jimmunol.0900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomura K, Yin M, Shimizu S, Kato Y, Nagano T, Komeichi H, et al. Increased chymase in livers with autoimmune disease: colocalization with fibrosis. J Nihon Med Sch. 2003;70:490–495. doi: 10.1272/jnms.70.490. [DOI] [PubMed] [Google Scholar]
- Scandiuzzi L, Beghdadi W, Daugas E, Åbrink M, Tiwari N, Brochetta C, et al. Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol. 2010;185:624–633. doi: 10.4049/jimmunol.0902129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudamore CL, Thornton EM, McMillan L, Newlands GF, Miller HR. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med. 1995;182:1871–1881. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga Y, Takai S, Koyama T, Okamoto Y, Ikeda T, Nishimura K, et al. Attenuation of adhesion formation after cardiac surgery with a chymase inhibitor in a hamster model. J Thorac Cardiovasc Surg. 2004;127:72–78. doi: 10.1016/s0022-5223(03)00697-4. [DOI] [PubMed] [Google Scholar]
- Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, Miyazaki M. New approaches to blockade of the renin–angiotensin–aldosterone system: chymase as an important target to prevent organ damage. J Pharmacol Sci. 2010;113:301–309. doi: 10.1254/jphs.10r05fm. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, Miyazaki M. Targets of chymase inhibitors. Expert Opin Ther Targets. 2011;15:519–527. doi: 10.1517/14728222.2011.555401. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, Ohzu M, Tanaka K, Miyazaki M. Chymase inhibition provides pancreatic islet protection in hamsters with streptozotocin-induced diabetes. J Pharmacol Sci. 2009;110:459–465. doi: 10.1254/jphs.09115fp. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Sugiura H, Uehara M, Sato H, Hashimoto-Tamaoki T, Furuyama J. Association between mast cell chymase genotype and atopic eczema: comparison between patients with atopic eczema alone and those with atopic eczema and atopic respiratory disease. Clin Exp Allergy. 1999;29:800–803. doi: 10.1046/j.1365-2222.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- Tchougounova E, Pejler G, Åbrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Åbrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Renaux B, Hollenberg MD, Befus AD, Macnaughton WK, Wallace JL. Role of rat mast cell protease-2 (RMCP-2) in intestinal transport and barrier function. Gastroenterology. 1998;114(Suppl. 1):A429. [Google Scholar]
- Waern I, Jonasson S, Hjoberg J, Bucht A, Åbrink M, Pejler G, et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Tomimori Y, Terakawa M, Ishiwata K, Wada A, Muto T, et al. Oral administration of chymase inhibitor improves dermatitis in NC/Nga mice. J Invest Dermatol. 2007;127:971–973. doi: 10.1038/sj.jid.5700708. [DOI] [PubMed] [Google Scholar]
- Wyatt J, Vogelsang H, Hübl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. The Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]


