Abstract
BACKGROUND AND PURPOSE
Betahistine, the main histamine drug prescribed to treat vestibular disorders, is a histamine H3 receptor antagonist.
Here, we explored the potential for modulation of the most recently cloned histamine receptor (H4 receptor) to influence vestibular system function, using a selective H4 receptor antagonist JNJ 7777120 and the derivate compound JNJ 10191584.
EXPERIMENTAL APPROACH
RT-PCR was used to assess the presence of H4 receptors in rat primary vestibular neurons. In vitro electrophysiological recordings and in vivo behavioural approaches using specific antagonists were employed to examine the effect of H4 receptor modulation in the rat vestibular system.
KEY RESULTS
The transcripts of H4 and H3 receptors were present in rat vestibular ganglia. Application of betahistine inhibited the evoked action potential firing starting at micromolar range, accompanied by subsequent strong neuronal depolarization at higher concentrations. Conversely, reversible inhibitory effects elicited by JNJ 10191584 and JNJ 7777120 began in the nanomolar range, without inducing neuronal depolarization. This effect was reversed by application of the selective H4 receptor agonist 4-methylhistamine. Thioperamide, a H3/H4 receptor antagonist, exerted effects similar to those of H3 and H4 receptor antagonists, namely inhibition of firing at nanomolar range and membrane depolarization above 100 µM. H4 receptor antagonists significantly alleviated the vestibular deficits induced in rats, while neither betahistine nor thioperamide had significant effects.
CONCLUSIONS AND IMPLICATIONS
H4 receptor antagonists have a pronounced inhibitory effect on vestibular neuron activity. This result highlights the potential role of H4 receptors as pharmacological targets for the treatment of vestibular disorders.
Keywords: vestibular primary neurons, histamine H4 receptor, antagonists, in vitro electrophysiology, vestibular disorders
Introduction
Inner ear-related dizziness represents about 80% of all vertigo. Pharmacological treatments of these vestibular disorders employ several families of compounds, including histamine drugs (for review, see Soto and Vega, 2010). Among them, the histamine H3 receptor antagonist betahistine (Arrang et al., 1985) is the most commonly used compound in Europe, Canada and Latin America for the treatment of Ménière's disease and vestibular drop attacks (Lacour and Sterkers, 2001; Smith et al., 2005; Lacour et al., 2007; Huppert et al., 2011).
The clinical effect of betahistine was originally attributed to increased blood flow in the vestibular system (Martinez, 1972). However, more recent studies have established that the clinical action of betahistine is more likely to be due to its inhibitory influence exerted both at the level of the vestibular nuclei (Tighilet et al., 2005) and peripheral end organs (Soto et al., 2001; Chávez et al. 2005). This inhibitory action modulates the afferent sensory input and the release of other neurotransmitters, facilitating the restoration of balanced activity between contralateral afferent neurons.
While expression of both H3 receptor mRNA and protein was recently confirmed in mammal vestibular primary neurons (Tritto et al., 2009), pharmacological effects of betahistine has only been demonstrated in lower vertebrates (Botta et al., 1998; Soto et al., 2001; Chávez et al. 2005). The present study was designed to assess the functional effects of betahistine in vestibular primary neurons isolated from the rat Scarpa's ganglion. The pronounced inhibitory effect of thioperamide, a H3/H4 receptor antagonist, on the excitability of the rat vestibular neurons led us to further investigate the expression of the histamine H4 receptor, as well as the pharmacological effects of selective H4 receptor antagonists.
The H4 receptor was first described in bone marrow, eosinophils and other types of immune system cells (Oda et al., 2000; Liu et al., 2001; Zhu et al., 2001). More recently, its expression has also been reported in the nervous system (Strakhova et al., 2009), with functional expression in neurons of the CNS (Connelly et al., 2009) and enteric nervous system (Breunig et al., 2007). As H3 and H4 receptors share significant homology (Liu et al., 2001), various compounds bind to both receptors (Gbahou et al., 2006). As an example, the standard H3 receptor inverse agonist thioperamide (Arrang et al., 1987) displays comparable potencies for the human H4 receptor (Oda et al., 2002; Hofstra et al., 2003; Gbahou et al., 2006). Cloning of H4 receptor has led to the identification of several specific ligands (Engelhardt et al., 2009). 4-Methylhistamine, originally developed as a relatively selective H2 receptor agonist, displays 100-fold higher selectivity for the H4 receptor than for other histamine receptors (Lim et al., 2005). The first selective non-imidazole H4 receptor neutral antagonist discovered, JNJ 7777120, displays comparable activity on human, mouse and rat receptors (Jablonowski et al., 2003; Thurmond et al., 2004). A derivate compound, JNJ 10191584, also has high selectivity for H4 receptors (Terzioglu et al., 2004) with improved liver microsome stability (Venable et al., 2005).
In the present study, we examined the effects of H4 receptor ligands, relative to well-known H3 receptor antagonists, on the excitability of mammalian primary vestibular neurons by means of in vitro electrophysiological recordings. We further investigated the effects of H4 receptor antagonists on vestibular function of adult rats in vivo following induced vestibular deficits. Two different approaches based on previously described paradigms (Llorens et al., 1993; Brugeaud et al., 2007) were employed for induction of vestibular deficits. In the first model, systemic 3,3′-iminodipropionitrile (IDPN) injection caused progressive bilateral hair cell degeneration in the sensory vestibular epithelia without neuronal injury, as previously described (Llorens et al., 1993; Llorens and Demêmes, 1996). At the point of histamine receptor antagonist testing (4 to 7 days after IDPN injection), degeneration occurred differentially in the vestibular epithelia (cristae > maculae; central > peripheral regions; type I hair cells > type II hair cells) resulting in a perturbation of vestibular information processing and strong vestibular deficits (Llorens and Demêmes, 1996). In the second model, a unilateral vestibular deficit was induced by transient excitotoxicity from repeated transtympanic injections of kainate in one ear leading to swelling of primary vestibular neuronal terminals and synaptic uncoupling (Dyhrfjeld-Johnsen et al., 2010).
Altogether, our results demonstrate the efficacy of H4 receptor antagonists as vestibulomodulators suggesting a potential direct application in improving current anti-vertigo medication.
Methods
Animals
Experiments used female Wistar or Long-Evans rats (CERJ, Le Genest, France) in accordance with the French Ministry of Agriculture regulations and European Community Council Directive no. 86/609/EEC, OJL 358. A total of 350 postnatal (2–8 days-old) and 60 adult rats were used. The animals were kept in an animal facility on a 12 h light/dark cycle, temperature 20–24°C and food (from Harlan Laboratories) available ad libitum. The results of all studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
Cell culture
Postnatal rats (2–8 days-old) were killed by decapitation and the brainstem was hemi-sectioned aseptically and the eighth nerve cut immediately rostral to the otocyst. The vestibular ganglia were excised, placed in Leibovitz medium (Invitrogen, Cergy Pontoise, France) and enzymatically dissociated (collagenase 0.75 mg·mL−1, dispase 1 mg·mL−1and DNAse 0.75 mg·mL−1, 15 min at 25°C). After washing, a pipette gently dissociated the ganglion neurons in culture medium (Dulbecco's modified Eagle medium and Ham's F-12 medium 1:1, 2% N2 nutrient, 28 mM Glc, 1.5 mM Gln, 15 mM HEPES, 2 µM AraC, 10 ng·mL−1 brain-derived neurotrophic factor and 1% penicillin/streptomycin). The neurons were plated onto culture dishes previously coated with 5 µg·mL−1 poly-L-ornithine, 10 µg·mL−1laminin. These low density cultures were maintained at 37°C in 5% CO2 for 7 to 8 days before the patch-clamp experiments.
Patch-clamp recordings
Whole-cell responses (current-clamp configuration) were recorded using an Axopatch 200B amplifier (Axon Instruments; Molecular Devices Corp., Sunnyvale, CA, USA). The recording solution contained (in mM): NaCl 135, HEPES 10, glucose 10, MgCl2 1, KCl 5, and CaCl2 4, pH 7.35. Recording pipettes (2–3 MΩ) were filled with (in mM): KCl 135, HEPES 10, glucose 10, NaCl 5, EGTA 5, Mg-ATP 3, GTP-Na 1, pH 7.35 (with KOH). The osmolarity of all solutions was adjusted to 300 mOsm·L−1. Voltage transients were recorded from neurons with resting membrane potentials between −45 and −65 mV. Depolarizing pulses (1 s duration, 200 pA) applied every 6.5 s elicited trains of action potentials. A multiple capillary perfusion system (flow rate 500 µL·min−1) allowed application of control and test solutions. To verify the response reversibility, neurons were washed with control recording medium after each tested drug treatment.
RT-PCR
Adult rats were killed by an overdose of pentobarbital (i.p.) before removal of various organs. Spleen (tissue control for H4 receptor) and vestibular ganglia were flash frozen in liquid nitrogen immediately after dissection from adult rats. For a single experiment, at least 12 vestibular ganglia and one spleen were used to extract the total RNA using a standard protocol with TRIzol (RiboPure, Ambion, Austin, TX, USA) and chloroform. First-strand cDNA synthesis (reverse transcription, RT) was performed with 0.5–1 µg of total RNA DNAse treated (Qiagen, Courtaboeuf, France) and Oligo(dT) primers (Kit Quantitect reverse transcription, Qiagen). Reactions were performed at 42°C for 1 h followed by 95°C for 2 min. PCR was performed with 1–2 µL of RT reaction material. Amplification was for 35 cycles for spleen and vestibular ganglia with 30 s at 95°C, 30 s at the primer-specific annealing temperature (58–60°C) and 1 min at 72°C. A final extension was done at 72°C for 7 min. For H4 receptor, RT was preferentially done using sequence specific primer and PCR products (1 µL) resolved on 2% agarose gels containing ethidium bromide (10%). Results were obtained in three different experiments (two independent) and PCR products were identified at least once by direct sequencing. To avoid misinterpretation of our results through genomic DNA contaminations, primer sets were designed across intron-exon boundaries as determined from rat sequences using the free NCBI/Primer-Blast software (Table 1, Figure 5A). For H4 receptors, primers were systematically tested for quality on spleen tissue.
Table 1.
Primer sequences for RT-PCR of histamine H3 and H4 receptor subunits and GAPDH.
| Code | Primer sequence (5′→ 3′) | Gen bank accession | Fragment size |
|---|---|---|---|
| H3R F1 | ACAGTACTGGGCAACGCGCTG | ||
| H3R R1 | GTGGCCCTCGGGGATGGAAC | NM_053506 | 420 bp |
| H4R R | GCTAGCTTCCTGCCTCTGAG | NM_131909 | |
| H4R F1 | TGTGATGTCGGAGTCTAACG | NM_131909 | 536 bp |
| H4R R1 | CGAGGATGTACCACTCAGTA | ||
| H4R F2 | GCCACTGACTGCTCAAGTTCCCT | NM_131909 | 477 bp |
| H4R R2 | CCAGGCTCGCACTCCTCTGTGT | ||
| GAPDH F1: | GGTGAAGGTCGGTGTGAACGGATTT | NM_017008 | 502 bp |
| GAPDH R1: | GATGCCAAAGTTGTCATGGATGACC |
Figure 5.
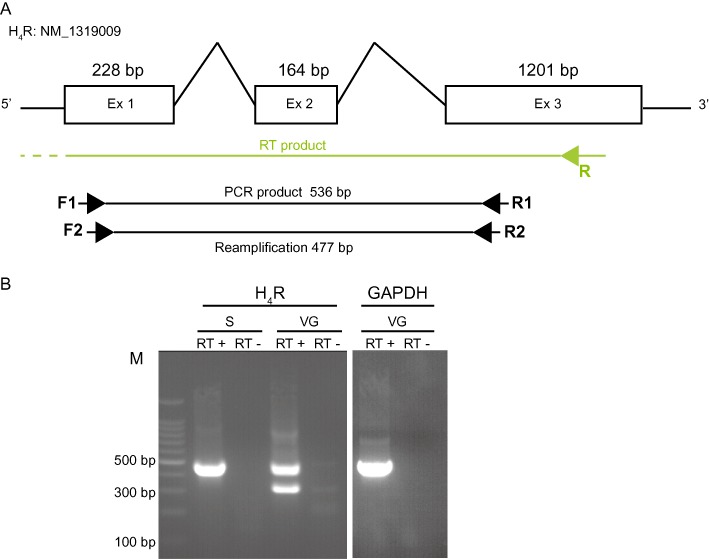
Vestibular ganglion of adult rats expresses H4 receptor mRNA. (A) Structure of H4 receptor gene with lengths of exons (rectangles) and introns shown in base pairs. Arrows represent orientation and approximate locations of primers used in our RT-PCR experiments. A sequence specific primer was used to reverse transcribe RNA transcript, F1/R1 and F2/R2 were used for PCR and reamplification, respectively. (B) The figure shows a representative example of PCR products obtained for H4 receptors (477 bp) in the spleen (S) and vestibular ganglia (VG). GAPDH (502 bp): positive control to attest the success of the PCR reaction. RT (–): negative RT control where water has been substituted for reverse transcriptase. Size of the PCR products if genomic DNA was present in our samples as a template for amplification should be 2500 bp (GAPDH) and larger than 15 kb (H4 receptor), respectively. No band with this predicted size was observed.
Behavioural experiments
For surgical procedures the rats were anaesthetized with 4% isoflurane and maintained with 2% for 10 min. Vestibular deficits were induced in female Wistar rats (200–225 g) by a single injection of IDPN (3,3′-iminodipropionitrile) i.p. (1000 mg·kg−1) or in female Long-Evans rats (185–200 g) by three transtympanic applications of 100 µL 25 mM kainate in the middle ear (in saline completed with 10 mg·mL−1 benzyl alcohol) at 1 h intervals. The vestibular symptoms of the IDPN model were evaluated using a previously described panel of six behavioural tests (Llorens et al., 1993; Llorens and Rodríguez-Farré, 1997; Brugeaud et al., 2007), each scored on a discrete scale indicating normal behaviour (0) to maximal impairment (4): head bobbing, circling, retropulsion, tail-hanging, contact-inhibition and air-righting reflex. The effect of a single administration of the histamine receptor antagonists studied on vestibular deficit scores was tested 3 to 7 days post-IDPN lesion, followed by vestibular behaviour testing 30 min to 96 h post-injection. In the kainate model, four of the six previously described tests (head bobbing, circling, tail-hanging and air righting reflex) appropriate for a unilateral model were scored on the scale of 0–4, along with the degree of lateral head tilt towards the injured side (Dyhrfjeld-Johnsen et al., 2010). Tests were carried out 1.5 to 72 h post-injection.
Drugs/chemicals
Standard solutions of JNJ 10191584 (Tocris Bioscience, Bristol, UK) and JNJ 7777120 (Sigma-Aldrich, St-Quentin Fallavier, France) were prepared at 50 mM and 20 mM in 100% dimethyl sulfoxide (DMSO), respectively, as recommended by the suppliers. Stock solutions of betahistine, thioperamide, (Sigma-Aldrich) and 4-methylhistamine (Tocris) were prepared in recording medium. Drugs were further diluted in the recording solution before use. IDPN was purchased from Fisher Scientific (Illkirch, France) and kainic acid from Ascent Scientific (Bristol, UK).
Data analysis and statistical methods
For electrophysiological data, the results are expressed as means ± SEM. A single Hill equation of the form 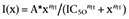 was fitted to dose–response relationships. Differences between groups were tested using Student's t-test or anova with P < 0.05 as the threshold for significance. Behavioural scoring was analysed using SigmaPlot (v11.0, Systat Software). A Friedman repeated measures test followed by Duncan's post-test was used to determine within-group differences over time. Statistically differing time points were then tested against the control condition using a Mann–Whitney rank-sum test.
was fitted to dose–response relationships. Differences between groups were tested using Student's t-test or anova with P < 0.05 as the threshold for significance. Behavioural scoring was analysed using SigmaPlot (v11.0, Systat Software). A Friedman repeated measures test followed by Duncan's post-test was used to determine within-group differences over time. Statistically differing time points were then tested against the control condition using a Mann–Whitney rank-sum test.
Results
Basic properties of cultured vestibular neurons
Under phase contrast microscopy, low-density cultures of vestibular ganglion neurons had refringent somata with diameters from 10 to 25 µm. All recorded neurons had intact, long interconnected neural processes. Neurons had resting membrane potentials of −49.06 ± 0.45 mV (n= 116, range −45 to −68 mV), with few cells displaying low-frequency spontaneous activity. Depolarizing current pulses (1 s, 200 pA) elicited two types of discharge volleys as previously reported (Iwasaki et al., 2008; Kalluri et al., 2010). Transient discharges comprised only one to four action potentials, while sustained discharge patterns (Figures 1A,B, 2A,B and 3A) consisted of 10 to 83 action potentials (mean 28.0 ± 1.8, n= 98). In the absence of drug application, these discharge patterns were stable for up to 40 min in response to repeated stimulation. The mean inter-spike intervals were 19.43 ± 0.36 ms (n= 89) and 19.58 ± 0.79 ms (n= 24) for, respectively, transient and sustained units (no significant difference). We analysed pharmacological effects on sustained discharging neurons since test compounds almost completely inhibited the firing in transient discharging neurons regardless of the concentrations used. Following the application of histamine receptor antagonists, the mean inter-spike interval was not significantly altered and the basic characteristics of the initial spike (amplitude, time to peak, half width, rise and decay time constants) remained unchanged (data not shown).
Figure 3.
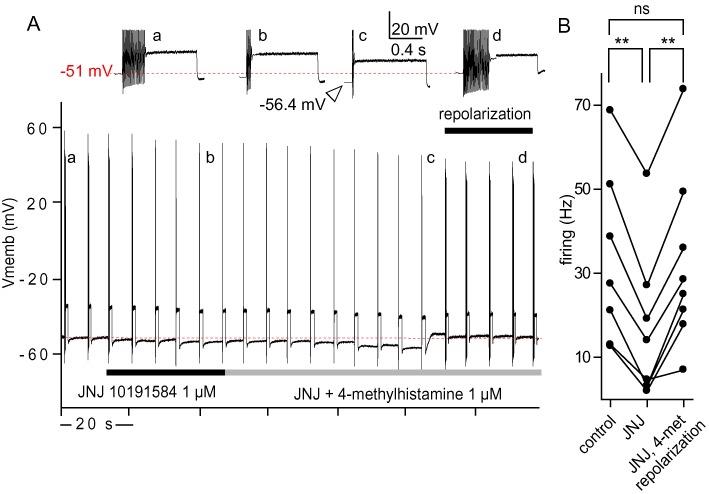
Competitive effect of the selective H4 receptor agonist 4-methylhistamine. (A) Representative traces of current-clamp recordings showing the inhibitory effect of 1 µM JNJ 10191584 (b) followed by application of 1 µM JNJ 10191584 plus 1 µM 4-methylhistamine. To compensate for the hyperpolarization induced by 4-methylhistamine, subsequent repolarization restored the membrane potential to its initial value (c). Trains of action potentials were elicited by 1 s, 200 pA depolarizing pulses applied every 6.5 s. Inserts present enlarged trains before (a), during JNJ 10191584 (b) application and after combined 1 µM JNJ 10191584 plus 1 µM 4-methylhistamine with repolarization. (B) JNJ 10191584 significantly reduced neuronal firing (**P < 0.001, n= 8, paired t-test), while additional application of 4-methylhistamine abolished the firing inhibition when the membrane potential shift was restored to its initial value by repolarization (P > 0.2, n= 8, paired t-test). Dashed red lines show the initial membrane potential.
Effects of H3 and H3/H4 receptor antagonists on the excitability of vestibular primary neurons
As the expression of H3 receptors was previously reported in mammalian vestibular primary neurons (Tritto et al., 2009), we tested the effect of betahistine on depolarization-evoked discharges. Betahistine application reduced the number of action potentials evoked by depolarizing current injections in a dose-dependent manner (Figures 1A), with 10 µM betahistine not affecting the discharges, while a concentration of 10 mM completely silenced the neurons. All the effects of betahistine were rapidly reversed following wash (Figure 1A). Betahistine affected both the membrane potential and evoked discharges of vestibular neurons. Between 10 µM and 2 mM, betahistine induced a slight membrane hyperpolarization, while a strong depolarization of the membrane potential occurred above the 2 mM concentration (Figure 1A,C). Due to the strong depolarization of the neurons at high concentrations (resulting in a depolarization-block of firing due to sodium current inactivation), a dose–response relationship could not be established for all concentrations applied (Figure 4). However, using the data obtained with concentrations 10 µM to 2 mM, a Hill equation gave the best fit of dose–response inhibition curve with an IC50 of 118 µM and a maximal efficacy of 47% firing inhibition.
Figure 1.
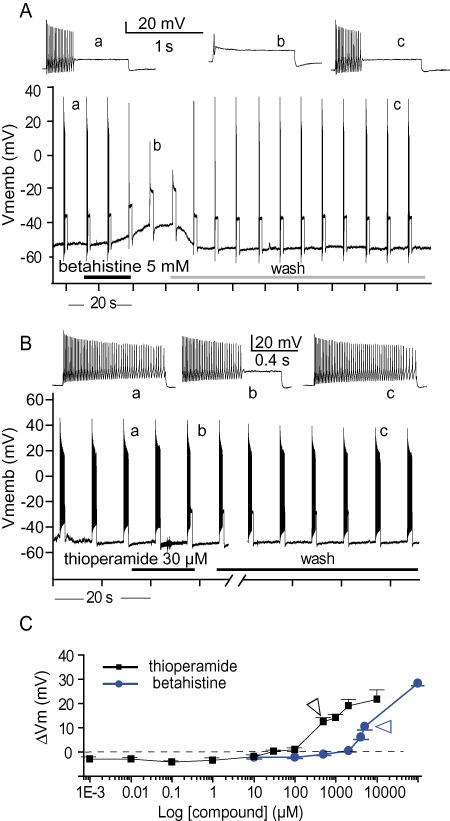
Effects of H3 and H3/H4 receptor antagonists on the firing properties of vestibular neurons. (A,B) Representative traces of current-clamp recordings. Trains of action potentials were elicited by 1 s, 200 pA depolarizing pulses applied every 6.5 s. (A) Sustained evoked discharges blocked by 5 mM betahistine. Betahistine induced a large reversible depolarization of 11.3 mV. Inserts present enlarged trains before betahistine application (a), during (b) and after wash (c). (B) Sustained evoked discharges inhibited by 30 µM thioperamide. Inserts present enlarged trains before (a) and during (b) thioperamide application. (C) Absolute variations of membrane potential elicited by betahistine and thioperamide. The two compounds induced a strong depolarization of the neurons at concentrations above 2 mM and 30 µM, respectively. Data represent means ± SEM from 3 to 21 separate experiments. Arrowhead indicates the concentration inducing 90% firing inhibition.
Figure 4.
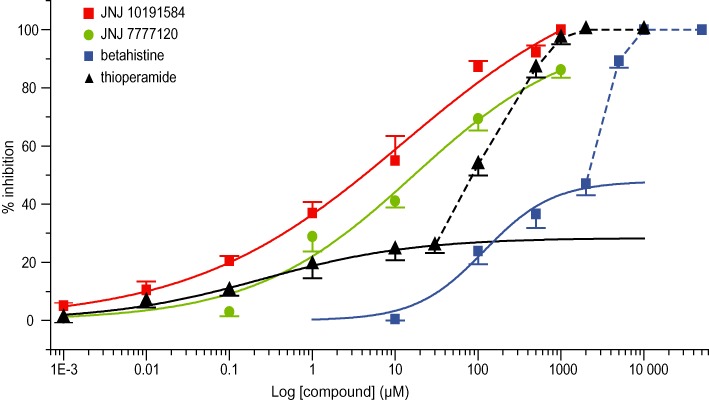
Dose–response relationships for the firing inhibition induced by applications of the histamine antagonists. Red and green curves represent Hill fits of JNJ 10191584 and JNJ 7777120 with IC50 of 9.8 and 16.7 µM, respectively. Betahistine and thioperamide induced a firing decrease with a break around 2 mM and 30 µm, respectively, coincident with the commencement of strong neuronal depolarization-induced firing block. Solid blue curve represent the Hill fit of betahistine between 10 µM and 2 mM, with IC50 of 118 µM and maximal efficacy of 47%. Solid black curve represents the Hill fit of thioperamide between 1 nM and 30 µM, with IC50 of 250 nM and maximal efficacy of 28%. Data represent means ± SEM from 5 to 17 separate experiments.
Subsequently, thioperamide, an antagonist of both H3 and H4 receptors (Gbahou et al., 2006), was tested (Figure 1B). Thioperamide also inhibited the discharge volley following depolarizing current injection in a concentration-dependent manner. At millimolar concentrations, thioperamide completely blocked the discharges (97% inhibition at 1 mM). The effects of thioperamide were only partially reversible following wash, especially at high concentrations. Thioperamide affected the resting membrane potential in two ways. At low concentrations (1 nM to 30 µM), this compound elicited only a slight hyperpolarization, while higher concentrations led to a strong depolarization of the neurons. Similar to betahistine, the thioperamide-induced strong depolarization of neurons did not allow us to establish a dose–response relationship for the inhibition of evoked firing for concentrations above 30 µM (Figure 4). Between 1 nM and 30 µM, the best fit of the dose–response inhibition relationship by a single Hill equation gave an IC50 of 250 nM and a maximal efficacy of 28% firing inhibition. The breaks in the membrane potential (Figure 1C) and dose–response curves (Figure 4) suggested the presence of two sites of action for both betahistine and thioperamide.
Effects of the selective H4 receptor antagonists, JNJ 10191584 and JNJ 7777120 on the excitability of vestibular primary neurons
Due to the pronounced inhibitory effect of thioperamide on depolarization-evoked firing in vestibular primary neurons at lower concentrations, we investigated the effect of applications of the selective H4 receptor antagonists. The highly selective H4 receptor antagonist JNJ 10191584 (Terzioglu et al., 2004; Venable et al., 2005) was applied at concentrations ranging from 1 nM to 1 mM. Control experiments determined that the JNJ 10191584 solvent (DMSO) at 2% concentration in the extracellular medium did not affect either the membrane potential or the evoked firing of vestibular neurons (data not shown). JNJ 10191584 reduced the number of action potentials fired by vestibular primary neurons in response to depolarizing current steps in a reversible manner (Figure 2A). The inhibition of firing was concentration-dependent (Figure 4) with 1 nM JNJ 10191584 reducing the action potential number in a volley by 5%, while 500 µM inhibited the evoked activity by 92%. Total inhibition was reached at 1 mM, with maximal efficacy of 100% firing inhibition. Initial firing was completely restored after wash (Figure 2A). A single Hill equation gave the best fit of dose–response inhibition curve with an IC50 of 9.8 µM (Figure 4). Although application of JNJ 10191584 induced a slight hyperpolarization of the membrane potential at all concentrations tested, there was no significant difference between concentrations (anova, P > 0.2) (Figure 2C). For comparison, we tested the effect of another H4 receptor antagonist JNJ 7777120 (Jablonowski et al., 2003; Thurmond et al., 2004) (Figure 2B) at concentrations between 10 nM and 1 mM. Like JNJ 10191584, JNJ 7777120 induced a slight membrane hyperpolarization and reduced evoked action potential firing in a concentration-dependent manner (Figure 4). The best fit of the dose–response inhibition relationship by a single Hill equation gave an IC50 of 16.7 µM. Comparisons between the two H4 receptor antagonists revealed no significant difference in the dose–response relationship (two-way anova, P > 0.5) indicating a similar efficacy of the two compounds.
Figure 2.
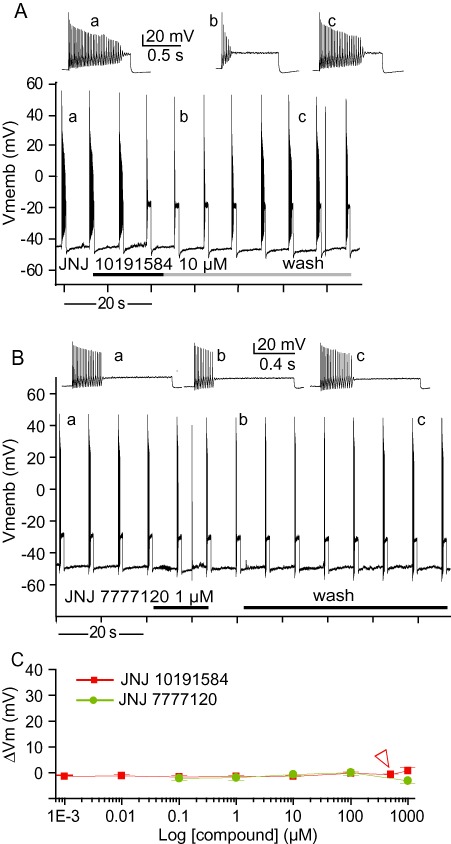
Effects of H4 receptor antagonists on the firing properties of vestibular neurons. (A,B) Representative traces of current-clamp recordings. Trains of action potentials were elicited by 1 s, 200 pA depolarizing pulses applied every 6.5 s. (A) Representative traces showing the reversible effects of 10 µM JNJ 10191584. Inserts present enlarged trains before JNJ 10191584 application (a), during (b) and after wash (c). (B) Representative traces showing the reversible effects of 1 µM JNJ 7777120. Inserts present enlarged trains before (a) and during (b) JNJ 7777120 application. (C) Absolute variations in membrane potential elicited by JNJ 10191584 and JNJ 7777120. The two compounds did not significantly affect the resting membrane potential at any of the concentrations applied. Data represent means ± SEM from 3 to 16 separate experiments. Arrowhead indicates the point of 90% firing inhibition.
To confirm the specificity of the H4 receptor antagonists, we next determined the effect of the potent and selective H4 receptor agonist 4-methylhistamine (Lim et al., 2005). Depolarization-evoked activity was first inhibited with 1 µM JNJ 10191584 (decreasing the firing by 37%). A subsequent switch to a solution containing both 1 µM 4-methylhistamine and 1 µM JNJ 10191584 induced a hyperpolarization (–5.6 ± 0.9 mV, n= 14). When this membrane hyperpolarization was compensated for by a repolarization to its initial value (Figure 3A), the action potential firing induced was not significantly different from the control condition (P > 0.2, n= 8, Figure 3B). These effects were similarly observed when the two compounds were applied at 0.1 µM (data not shown). Hence, 4-methylhistamine counteracted the effect of JNJ 10191584.
Expression of H4 receptors in vestibular primary neurons
To determine the expression of mRNA corresponding to H3 and H4 receptors, we used conventional RT-PCR experiments performed on vestibular ganglia as well as in spleen (used as a positive control) dissected from adult rats. Figure 5A illustrates the structure of the H4 receptor gene with lengths of exons and introns shown in base pairs and the position of the primers used in our study. We confirmed the expression of H3 receptors in both tissues (data not shown) and revealed for the first time the simultaneous expression of H4 receptors (Figure 5B). For both the spleen and the vestibular ganglia samples, the size of the bands observed in gels corresponded to the predicted sizes of the amplified products: 477 bp for H4 receptor and 502 bp for GAPDH used as a positive control for the PCR reaction. A second band at 313 bp representing a splice variant where exon 2 (164 bp) is fully truncated (as confirmed by the direct sequencing of the band) could be observed in vestibular ganglia. Although not present in the gel shown in Figure 5B, a similar band was often observed with PCR products from spleen samples. As expected, no bands were seen in our negative controls (RT-) confirming the purity of our RNA samples. Interestingly, although a single PCR was enough to observe the expression of H3 receptors in samples from vestibular ganglia, a second amplification of the first PCR product (using nested primers) was systematically necessary to reveal the expression of H4 receptors.
Behavioural recovery from vestibular deficits induced in vivo
The effects of histamine receptor antagonists on experimentally-induced vestibular deficits were tested in two different animal models. Firstly, a bilateral lesion of the peripheral vestibular system by i.p. injection of IDPN elicited pronounced vestibular deficits. For all the rats tested, we determined a total vestibular deficit score that reached a maximum between 4 and 7 days post-IDPN injection (n= 35). At this stage, rats displayed behaviour stereotypical of vestibular deficits (Llorens et al., 1993). Rats were circling, walking backwards and head-bobbing when exploring, displayed ventral bending during tail-hanging and failed to rectify a supine position in the air – righting or contact inhibition reflex tests. Vestibular deficit scores were normalized to their baseline pretreatment values for analysis. We first established the effect of the H3 receptor antagonist (betahistine) and H3/H4 histamine receptor antagonist (thioperamide) on vestibular deficit symptoms. Using previously described effective doses of these compounds, 3 mg·kg−1 and 30 mg·kg−1, respectively (Tighilet et al., 2005), the vestibular deficits were determined over a period from 30 min to 96 h after drug injections. Neither a single i.p. dose of betahistine nor thioperamide significantly reduced the vestibular deficits (Figure 6A,B).
Figure 6.
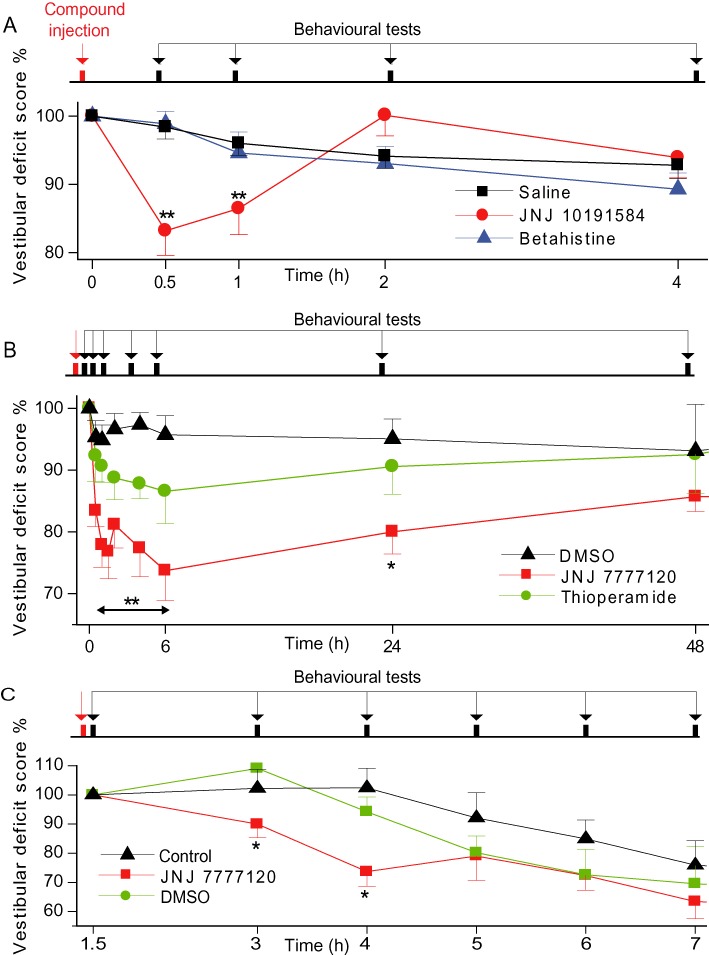
Effects of the histamine antagonists on the induced vestibular deficits. (A) Bilateral vestibular lesions were induced by single i.p. administration of IDPN. Mean value (± SEM) of the vestibular scores following a single injection of JNJ 10191584 (time = 0 h) or betahistine compared with their specific vehicle (DMSO and 0.9% NaCl saline). Vestibular deficits were significantly reduced 30 min and 1 h after JNJ 10191584 administration, while betahistine had no effect. P-values were computed by using a Dunn's test and are indicated as follows **P < 0.01 as compared with vehicle-treated animals, (n= 6–18 per group). (B) Bilateral vestibular lesions induced by single i.p. administration of IDPN. Mean value (± SEM) of the vestibular scores following a single injection of JNJ 7777120 (time = 0 h) or thioperamide compared with their specific vehicle (DMSO). Vestibular deficits were significantly reduced over 24 h after JNJ 7777120 administration, while thioperamide and vehicle had no effect. P-values were computed by using a Dunn's test and are indicated as follows: *P < 0.05, **P < 0.01 as compared with DMSO-treated animals, (n= 4–12 per group). (C) Unilateral vestibular lesion induced by three transtympanic 100 µL injections of 25 mM kainate. Mean value (± SEM) of the vestibular scores following single injection of JNJ 7777120 (time = 0 h) compared with its specific vehicle (DMSO). Vestibular deficits were significantly reduced 1 and 2 h after JNJ 7777120 administration at t= 2 h while DMSO had no effect. P-values were computed by using a Dunn's test and is indicated as follows *P < 0.05 as compared with control animals (n= 6–10 per group).
We then tested the effect of a single i.p. injection of both H4 receptor antagonists on vestibular deficits. Control experiments with the JNJ compound vehicle DMSO revealed no significant effect on vestibular deficits (P > 0.7; Figure 6B). JNJ 10191584 (30 mg·kg−1) and JNJ 7777120 (20 mg·kg−1) clearly reduced the induced vestibular deficits. JNJ 10191584 significantly reduced the vestibular deficit by 16.8 ± 3.6% and 13.5 ± 3.8% at, respectively, 30 min and 1 h post-injection (n= 8) (Figure 6A). These recoveries were statistically significant compared to the vehicle control (P < 0.01 at both 30 min and 1 h post-injection) and betahistine (P < 0.001 and P < 0.01 at 30 min and 1 h post-injections, respectively, n= 7 and n= 15). The effect was transient, as the treatment differences disappeared after 2 h (P > 0.05). Conversely, the reduction in vestibular deficits following JNJ 7777120 treatment (compared to vehicle) occurred as rapidly as 30 min post-injection (17.3 ± 2.5%, n= 8) reaching a maximum at 6 h (26.3 ± 4.8%, n= 8) and lasting up to 24 h post-injection (P < 0.01; Figure 6B).
To ensure that the potential for reducing vestibular deficit symptoms was not model-specific, the effect of JNJ 7777120 was tested in a unilateral, excitotoxic insult model of vestibular deficits induced by three transtympanic 100 µL injections of 25 mM kainate. Following kainate injections at 0 and 1 h, the baseline deficit score characterizing symptoms (bobbing, circling, head tilt, tail hanging and air righting reflexes) was determined at 1.5 h. A single i.p. injection of JNJ 7777120 (20 mg·kg−1) or vehicle (DMSO) was subsequently administered simultaneously with the final transtympanic kainate injection at 2 h. JNJ 7777120 administration abolished the increase in vestibular deficit symptoms observed for controls and DMSO-treated rats at 3 h (Figure 6C) with a reduction of vestibular deficits lasting until 5 h. This reduction was significant at 3 h (P < 0.05, 23.6% mean reduction vs. DMSO) and 4 h (P < 0.01, 21.1% mean reduction vs. DMSO). The vehicle (DMSO) treatment was not significantly different from untreated controls at any time point.
Discussion and conclusions
In this study we demonstrated that antagonism of H4 histamine receptors leads to strong reversible inhibition of evoked action potential firing. Furthermore, to our knowledge, this is the first time evidence has been obtained showing that systemic in vivo administration of H4 receptor antagonists effectively reduce the vestibular behavioural deficits induced by peripheral injury. Our RT-PCR experiments in vestibular ganglia confirmed the presence of H3 receptor mRNA and demonstrate, for the first time, the expression of H4 receptors.
Electrophysiological recordings demonstrated that highly selective H4 receptor antagonists reversibly decrease the depolarization-evoked firing in a concentration-dependent manner. At millimolar concentrations, they completely abolished the neuronal discharge without affecting the resting membrane potential. The specific functional involvement of H4 receptors in vestibular primary neuron physiology is strongly supported by the abolishment of these effects by the selective H4 receptor agonist 4-methylhistamine (Lim et al., 2005). The firing inhibition induced by the H3 receptor antagonist betahistine at more elevated concentrations may also depend on a low affinity for the H4 receptor. Indeed, our results reporting an IC50 of 118 µM are consistent with the Ki of 81 µM (pEC50 > 100 µM), previously reported for betahistine on human H4 receptors in binding experiments (Deml et al., 2009). At higher concentrations, the depolarizing effect of betahistine could possibly result from an agonistic action on the H1 receptor (Arrang et al., 1985), which is involved in membrane depolarization (for review, see Brown et al., 2001). Thioperamide, a combined H3/H4 receptor antagonist, presents weak affinity at H1 receptors (Arrang et al., 1987) but remarkably displays the effects of both JNJ compounds and betahistine, depending on concentration. This suggests that both thioperamide and betahistine work through a combination of effects on both H4 and H3 receptors, with firing inhibition mediated by the H4 receptor and depolarization by the H3 receptor and depolarization block potentially through H1 receptors.
Due to the presence of both H3 and H4 receptors in primary neurons, the specificity of the applied antagonists is crucial for data interpretation. The IC50 presently determined for the H4 receptor antagonists in vestibular neurons (IC50 of 9.8 and 16.7 µM for respectively JNJ 10191584 and JNJ 7777120) differs notably from the affinities reported in binding studies from different species. For example, the Ki determined for human and rat H4 receptors were, respectively, 31 and 50 nM for JNJ 10191584 (Lim et al., 2010). However, JNJ 7777120 has yielded an IC50 higher than 300 nM in the regulation of adrenocorticotropic hormone release from AtT-20 cells (Meng et al., 2008) and in eosinophil chemotactic response (Ling et al., 2004). In both cases, the efficacy constants are at least 75 times higher than the Ki of 4 nM reported from binding studies. Furthermore, H4 receptor antagonists have been shown to modulate the excitability of CNS and peripheral neurons in the micromolar range (up to 10 µM), by means of either depolarization or hyperpolarization (Breunig et al., 2007; Connelly et al., 2009). H4 receptor antagonists display low affinity for other histamine receptors (>1000-fold difference) (Zhang et al., 2007), especially H3 receptors (Thurmond et al., 2004). Any effect of the H4 receptor antagonists applied on H3 receptors is thus highly unlikely, since our results were obtained by application of low concentrations of H4 receptor antagonists on vestibular neurons (e.g. initial effect of JNJ 10194584 began at 1 nM).
The specific mechanism underlying H4 receptor antagonist inhibition of neuronal firing remains undetermined and will require further investigation. The H4 receptor, which is constitutively active, is coupled to Gαi/o proteins (Oda et al., 2000) and has been shown to increase intracellular calcium and/or inhibit cAMP (Liu et al., 2001; Zhu et al., 2001). In vestibular neurons, H4 receptor antagonists may directly regulate the evoked firing and/or the synaptic activity by means of intracellular calcium regulations following receptor blockade. Pre-incubation with pertussis toxin could clarify the potential involvement of Gαi/o proteins in the neuronal responses. The pathway involving β-arrestins (Rosethorne and Charlton, 2011) seems improbable since the recorded effects appeared seconds after compound application, while β-arrestins signalling occurs on a timescale of minutes (Charest et al., 2005).
While histamine slightly hyperpolarized neurons at an appropriate concentration (data not shown), H3 and H3/H4 receptor antagonists applied at high concentrations in vestibular neurons led to strong membrane depolarization, resulting in decreased firing and probably increases in intracellular calcium via activation of voltage-dependant N-type calcium channels. Voltage-clamp approaches combined with calcium imaging would allow further study of the underlying mechanisms and possible deleterious effects involved in this response. Another target of H3 receptor activation could be Na+/H+ exchanger inhibition (Silver et al., 2001). Altogether, the complex effects of betahistine on vestibular neuronal activity will require further investigation, highlighted by recent reports of a concentration-dependent role of betahistine as an inverse agonist or agonist of H3 receptors (Gbahou et al., 2010). The membrane depolarization could also modulate the ligand binding, by means of voltage-induced conformational changes of the G-protein coupled receptor as reported for the M2 muscarinic receptor (Navarro-Polanco et al., 2011).
The ability of H4 receptor antagonists to improve the altered vestibular behaviour, following vestibular deficits induced in the rat, confirmed their in vitro effectiveness. Vestibular deficits induced by IDPN are due to inhomogeneous bilateral injuries to hair cells and sensory epithelia (see introduction) resulting in perturbed vestibular inputs in the CNS. The phasic functions, which are carried by the type I hair cells and the dendritic afferents localized in the central part of the receptor epithelia (Baird et al., 1988), are first affected, while tonic inputs brought by type II hair cells and peripheral afferents are less damaged. Moreover, the cristae (transducers of the angular accelerations) are injured before the maculae (encoding the linear acceleration). Altogether, these lesions should lead to massive imbalance of vestibular inputs in the vestibular nucleus and consequently to strong vestibular deficits. Unilateral kainate injections, by also mainly affecting the dynamic functions (Dyhrfjeld-Johnsen et al., 2010), result in similar behavioural vestibular deficits.
Both H4 receptor antagonists reduced the IDPN-induced deficits with maximum effect in less than 1 h, with a similar time-course in the kainate model. The fast alleviation of symptoms induced by the H4 receptor antagonists suggests a direct action on neurons. However, while the present study demonstrates that primary sensory neurons were a target for the H4 receptor antagonists, the possibility that this receptor is involved in second-order vestibular neurons, for the reduction of the induced vestibular deficits, cannot be excluded. Future experiments could address this question by direct application of the antagonists to the first- or second-order vestibular neurons. Regardless of the relative involvement of first- or second-order neuronal targets, the treatment mechanism remains the same, a reduction of the imbalance in vestibular information transmission through an overall reduction in vestibular neuron activity.
Betahistine, the clinical drug of choice for treating vestibular disorders, offers no significant improvement of the behavioural deficits at 30 mg·kg−1 in the present in vivo study. The relatively low doses of betahistine and thioperamide used (according to those tested in other animal models) could explain this lack of effect. While betahistine has some effects on the H4 receptor (Deml et al., 2009), its affinity is several orders of magnitude lower than for the H3 receptor. Together with our demonstration of the low efficacy of betahistine at inhibiting primary vestibular neuron firing without deleterious effects, it is highly unlikely that any clinical effect of betahistine observed is by way of the H4 receptor. Perhaps more importantly, investigations with betahistine in Meniere's populations after unilateral neurectomy (Lacour et al., 2007) demonstrated the requirement for prolonged and repetitive administration (over at least a week) for a significant effect on vertigo symptoms, and it was thought to promote central vestibular compensation. Given the rapid and transient treatment effects of H4 receptor antagonists after a single-dose administration in our in vivo paradigms, it is highly unlikely that there is an overlap in either target or mechanism of action for betahistine and specific H4 receptor antagonists. The two classes of H receptor antagonists, by acting on distinct targets, may induce different physiological outputs: betahistine appears to have a delayed effect mainly on the vestibular compensation (Lacour et al., 2007), while the present study reveals that the JNJ antagonists display an early effect on the acute vertigo-related symptoms.
Although significant, the somewhat milder effect of JNJ 10191584 is consistent with the low bioavailability of this compound (Venable et al., 2005), while its poor pharmacokinetic properties (such as a short half-life) could results in a rapidly declining treatment effect. Conversely, JNJ 7777120 showed a greater potential for treatment of the induced vestibular deficit; this stronger effect is possibly related to the superior bioavailability of JNJ 7777120 (Venable et al., 2005).
The results from our study indicate the role of H4 receptors as the primary receptors involved in effective histaminergic regulation of the excitability of vestibular primary neurons, and clearly demonstrate the potential of the H4 receptor as a major, novel pharmacological target for modulation of peripheral vestibular activity. Since inhibition of the vestibular inputs from vestibular end organs is the main mechanism of vestibulo-depressant drugs (Soto et al., 2001, Chávez et al. 2005), H4 receptor antagonists could rapidly find therapeutic application in the treatment of vestibular disorders.
Acknowledgments
We thank Prof Jordi Llorens for valuable discussions. We express gratitude to Dr Benjamin Delprat for helpful comments and assistance for molecular biology.
Glossary
- IDPN
3,3′-iminodipropionitrile
- JNJ
10191584, 1-[(5-chloro-1H-benzimidazol-2-yl)carbonyl]-4-methylpiperazine
- JNJ
7777120, 1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-ethylpiperazine
Statement of conflict of interest
None.
References
- Arrang JM, Garbarg M, Quach TT, Dam Trung Tuong M, Yeramian E, Schwartz JC. Actions of betahistine at histamine receptors in the brain. Eur J Pharmacol. 1985;111:73–84. doi: 10.1016/0014-2999(85)90115-3. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Lancelot JC, Lecomte JM, Pollard H, Robba A, et al. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Botta L, Mira E, Valli S, Perin P, Zucca G, Valli P. Effects of betahistine on vestibular receptors of the frog. Acta Otolaryngol. 1998;118:519–523. doi: 10.1080/00016489850154658. [DOI] [PubMed] [Google Scholar]
- Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol. 2007;583:731–742. doi: 10.1113/jphysiol.2007.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Brugeaud A, Travo C, Demêmes D, Lenoir M, Llorens J, Puel JL, et al. Control of hair cell excitability by vestibular primary sensory neurons. J Neurosci. 2007;27:3503–3511. doi: 10.1523/JNEUROSCI.5185-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest PG, Terrillon S, Bouvier M. Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6:334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez H, Vega R, Soto E. Histamine (H3) receptors modulate the excitatory amino acid receptor response of the vestibular afferents. Brain Res. 2005;1064:1–9. doi: 10.1016/j.brainres.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol. 2009;157:55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deml KF, Beermann S, Neumann D, Strasser A, Seifert R. Interactions of histamine H1-receptor agonists and antagonists with the human histamine H4-receptor. Mol Pharmacol. 2009;76:1019–1030. doi: 10.1124/mol.109.058651. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Broussy A, Chabbert C, Brugeaud A. 2010. Repeated transtympanic kainate injections as a model of excitotoxic vestibular insult. Society for Neuroscience abstract: 583.7.
- Engelhardt H, Smits RA, Leurs R, Haaksma E, de Esch IJ. A new generation of anti-histamines: Histamine H4 receptor antagonists on their way to the clinic. Curr Opin Drug Discov Devel. 2009;12:628–643. [PubMed] [Google Scholar]
- Gbahou F, Vincent L, Humbert-Claude M, Tardivel-Lacombe J, Chabret C, Arrang JM. Compared pharmacology of human histamine H3 and H4 receptors: structure–activity relationships of histamine derivatives. Br J Pharmacol. 2006;147:744–754. doi: 10.1038/sj.bjp.0706666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbahou F, Davenas E, Morisset S, Arrang JM. Effects of betahistine at histamine H3 receptors: mixed inverse agonism/agonism in vitro and partial inverse agonism in vivo. J Pharmacol Exp Ther. 2010;334:945–954. doi: 10.1124/jpet.110.168633. [DOI] [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- Huppert D, Strupp M, Mückter H, Brandt T. Which medication do I need to manage dizzy patients? Acta Otolaryngol. 2011;131:228–241. doi: 10.3109/00016489.2010.531052. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Chihara Y, Komuta Y, Ito K, Sahara Y. Low-voltage-activated potassium channels underlie the regulation of intrinsic firing properties of rat vestibular ganglion cells. J Neurophysiol. 2008;100:2192–2204. doi: 10.1152/jn.01240.2007. [DOI] [PubMed] [Google Scholar]
- Jablonowski JA, Grice CA, Chai W, Dvorak CA, Venable JD, Kwok AK, et al. The first potent and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem. 2003;46:3957–3960. doi: 10.1021/jm0341047. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Xue J, Eatock RA. Ion channels set spike timing regularity of mammalian vestibular afferent neurons. J Neurophysiol. 2010;104:2034–2051. doi: 10.1152/jn.00396.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour M, Sterkers O. Histamine and betahistine in the treatment of vertigo: elucidation of mechanisms of action. CNS Drugs. 2001;15:853–870. doi: 10.2165/00023210-200115110-00004. [DOI] [PubMed] [Google Scholar]
- Lacour M, van de Heyning PH, Novotny M, Tighilet B. Betahistine in the treatment of Ménière's disease. Neuropsychiatr Dis Treat. 2007;3:429–440. [PMC free article] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Molecular determinants of ligand binding to H4R species variants. Mol Pharmacol. 2010;77:734–743. doi: 10.1124/mol.109.063040. [DOI] [PubMed] [Google Scholar]
- Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004;142:161–171. doi: 10.1038/sj.bjp.0705729. Erratum in: Br J Pharmacol 142:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ma X, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, et al. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol Pharmacol. 2001;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- Llorens J, Demêmes D. 3,3′-Iminodipropionitrile induces neurofilament accumulations in the perikarya of rat vestibular ganglion neurons. Brain Res. 1996;717:118–126. doi: 10.1016/0006-8993(96)00034-0. [DOI] [PubMed] [Google Scholar]
- Llorens J, Rodríguez-Farré E. Comparison of behavioral, vestibular, and axonal effects of subchronic IDPN in the rat. Neurotoxicol Teratol. 1997;19:117–127. doi: 10.1016/s0892-0362(96)00216-4. [DOI] [PubMed] [Google Scholar]
- Llorens J, Demêmes D, Sans A. The behavioral syndrome caused by 3,3′-iminodipropionitrile and related nitriles in the rat is associated with degeneration of the vestibular sensory hair cells. Toxicol Appl Pharmacol. 1993;123:199–210. doi: 10.1006/taap.1993.1238. [DOI] [PubMed] [Google Scholar]
- Martinez DM. The effect of Serc (betahistine hydrochloride) on the circulation of the inner ear in experimental animals. Acta Otolaryngol Suppl. 1972;305:29–47. doi: 10.3109/00016487209122697. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Ma X, Li M, Jia M, Luo X. Histamine H(4) receptors regulate ACTH release in AtT-20 cells. Eur J Pharmacol. 2008;587:336–338. doi: 10.1016/j.ejphar.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Navarro-Polanco RA, Moreno Galindo EG, Ferrer-Villada T, Arias M, Rigby JR, Sánchez-Chapula JA, et al. Conformational changes in the M2 muscarinic receptor induced by membrane voltage and agonist binding. J Physiol. 2011;589:1741–1753. doi: 10.1113/jphysiol.2010.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–36786. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Oda T, Matsumoto S, Masuho Y, Takasaki J, Matsumoto M, Kamohara M, et al. cDNA cloning and characterization of porcine histamine H4 receptor. Biochim Biophys Acta. 2002;1575:135–138. doi: 10.1016/s0167-4781(02)00236-1. [DOI] [PubMed] [Google Scholar]
- Rosethorne EM, Charlton SJ. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits {beta}-arrestin without activating G proteins. Mol Pharmacol. 2011;79:749–757. doi: 10.1124/mol.110.068395. [DOI] [PubMed] [Google Scholar]
- Silver RB, Mackins CJ, Smith NC, Koritchneva IL, Lefkowitz K, Lovenberg TW, et al. Coupling of histamine H3 receptors to neuronal Na+/H+ exchange: a novel protective mechanism in myocardial ischemia. Proc Natl Acad Sci U S A. 2001;98:2855–2859. doi: 10.1073/pnas.051599198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WK, Sankar V, Pfleiderer AG. A national survey amongst UK otolaryngologists regarding the treatment of Meniere's disease. J Laryngol Otol. 2005;119:102–105. doi: 10.1258/0022215053419871. [DOI] [PubMed] [Google Scholar]
- Soto E, Vega R. Neuropharmacology of vestibular system disorders. Curr Neuropharmacol. 2010;8:26–40. doi: 10.2174/157015910790909511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Chávez H, Valli P, Benvenuti C, Vega R. Betahistine produces post-synaptic inhibition of the excitability of the primary afferent neurons in the vestibular endorgans. Acta Otolaryngol Suppl. 2001;545:19–24. doi: 10.1080/000164801750388045. [DOI] [PubMed] [Google Scholar]
- Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD, et al. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Res. 2009;1250:41–48. doi: 10.1016/j.brainres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Terzioglu N, van Rijn RM, Bakker RA, De Esch IJ, Leurs R. Synthesis and structure–activity relationships of indole and benzimidazole piperazines as histamine H(4) receptor antagonists. Bioorg Med Chem Lett. 2004;14:5251–5256. doi: 10.1016/j.bmcl.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung WP, Hofstra CL, Jiang W, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Lacour M. Dose- and duration-dependent effects of betahistine dihydrochloride treatment on histamine turnover in the cat. Eur J Pharmacol. 2005;523:54–63. doi: 10.1016/j.ejphar.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Tritto S, Botta L, Zampini V, Zucca G, Valli P, Masetto S. Calyx and dimorphic neurons of mouse Scarpa's ganglion express histamine H3 receptors. BMC Neurosci. 2009;10:70–76. doi: 10.1186/1471-2202-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable JD, Cai H, Chai W, Dvorak CA, Grice CA, Jablonowski JA, et al. Preparation and biological evaluation of indole, benzimidazole, and thienopyrrole piperazine carboxamides: potent human histamine h(4) antagonists. J Med Chem. 2005;48:8289–8298. doi: 10.1021/jm0502081. [DOI] [PubMed] [Google Scholar]
- Zhang M, Thurmond RL, Dunford PJ. The histamine H(4) receptor: a novel modulator of inflammatory and immune disorders. Pharmacol Ther. 2007;113:594–606. doi: 10.1016/j.pharmthera.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Michalovich D, Wu H, Tan KB, Dytko GM, Mannan IJ, et al. Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol Pharmacol. 2001;59:34–41. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]


