Abstract
Racemization is one of the most abundant modifications in long-lived proteins. It has been proposed that the accumulation of such modifications over time could lead to changes in tissues and ultimately human age-related diseases. Serine is one of the main amino acids involved in racemization; however, the site of D-Ser in any aged protein has yet to be reported. In this study, racemization of two residues, Ser 59 and Ser 62, has been demonstrated in an unstructured region of the small heat shock protein, αA-crystallin. αA-crystallin is also the most abundant structural protein in the human lens. D-Ser increased linearly with age in normal lenses, until it accounted for approximately 35% of the Ser at both sites by the age of 75 years. In agreement with a possible role in human age-related disease, levels were significantly higher in cataract lenses. It is likely that such prevalent age-related changes contribute to the denaturation of α-crystallin, and therefore its ability to act as a chaperone. Racemization of amino acids, such as serine, in flexible regions of long-lived proteins, could be associated with the development of human age-related conditions such as cataract.
Keywords: accelerated aging, protein denaturation, blindness, cataract, human lens
Introduction
It is likely that degradation of proteins that either do not turnover,1, 2 or are long lived,3–5 is a significant contributor to the debilitating conditions associated with human aging.6–8 One of the most common of these age-related conditions is cataract, where the normally transparent lens becomes opaque. Age-related cataract remains the major cause of blindness. The accumulation of post-translational modifications (PTMs) to lens proteins over time is a likely cause of age-related cataract; however, the identification of the PTMs, and which of them are of most importance for cataract etiology, remains to be elucidated. Racemization is one of the most abundant changes. The best known involves Asp and Asn residues which are each susceptible to cyclization followed by ring opening of the succinimide to yield D-isomers as well as isoAsp residues.9
Ser is another amino acid that is prone to racemization. D-Ser has been detected in hydrolysates of brain3, 10 and cartilage11 from adult humans and the levels increase with age. High levels of D-Ser have also been found in proteins isolated from human lenses.12 The levels were second only to those of D-Asp/Asn and increased markedly with age. Unlike Asp/Asn, the mechanism for racemization of Ser in proteins13 is unknown although studies with free amino acids, and their derivatives, suggest that amino acids with hydroxy-containing side chains are more susceptible to loss of the α proton. To date, the localization of D-Ser in any intact human protein has not been reported. In this study, we describe the characterization of D-Ser in the chaperone protein, αA-crystallin from adult human lenses. Two sites in the protein were identified and both D-Ser residues were located in an unstructured region of the protein.
Results
Identification of D-Ser in tryptic peptides
When investigating the liquid chromatography/mass spectrometry (LC/MS) profiles of tryptic digests of proteins from human lenses, multiple peaks were observed for one Ser-containing peptide derived from αA-crystallin, which corresponds to residues 55–65. The MS/MS spectra of these four peptide peaks were very similar and we proposed that this could be owing to racemization of Ser within this peptide. This tryptic peptide (TVLDSGISEVR) contains two Ser residues at positions 59 and 62. To investigate if indeed Ser was racemized, peptides were synthesized which contained all possible combinations, that is L-Ser (59)/ L-Ser (62), D-Ser (59)/ L-Ser (62), L-Ser (59)/ D-Ser (62) and D-Ser (59)/ D-Ser (62).
At the retention times of the four Ser isoforms of interest, other abundant crystallin peptides also eluted and this complicated direct nano-LC/MS analysis. In particular, versions of the same tryptic peptide (αA-crystallin 55–65) with different isomeric versions of Asp 5814 eluted at similar retention times by capillary LC and had similar MS/MS spectra. To permit unambiguous MS/MS identification of the D-Ser peptides, and to enable their quantification, a two-stage purification procedure was developed. This involved an initial analytical high-performance liquid chromatography (HPLC) separation as shown in Figure 1(a). Under the conditions shown in Figure 1(a), the L-isoAsp (58) tryptic peptide eluted at 42.3 min, the D-Asp (58) peptide eluted at 46.0 min, and the D-isoAsp (58) peptide eluted at 55.8min (Supporting Information Fig. 1).
Figure 1.
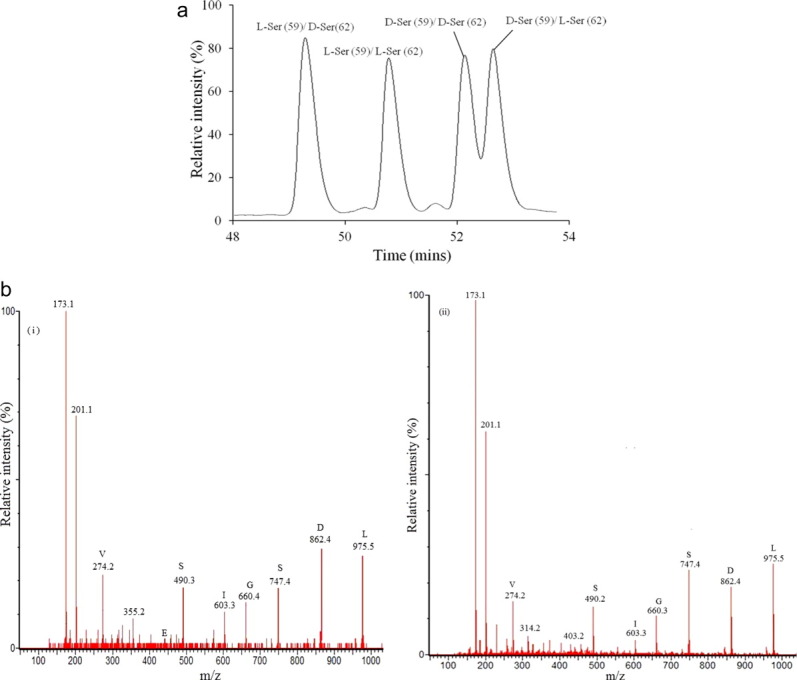
(a) Analytical HPLC trace showing the separation of the four isoforms of the αA-crystallin tryptic peptide (55–65) TVLDSGISEVR. Peptide standards were synthesized which contained all possible Ser combinations, that is L-Ser (59)/ D-Ser (62); L-Ser (59)/ L-Ser (62); D-Ser (59)/D-Ser (62); D-Ser (59)/L-Ser (62) (b). (i) MS/MS spectrum of one of the four peaks (the peak eluting at 52.2 min in (a) obtained by LC/MS of the tryptic digest of a 40-year-old human cataract lens. This peptide corresponds to D-Ser (59)/D-Ser (62). (ii) MS/MS spectrum of the synthetic L-Ser (59)/ L-Ser (62) peptide. Analytical HPLC was performed prior to LC/MS/MS. MS/MS spectra of the other three Ser isoforms from the lens protein digest were almost identical. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
HPLC peaks from the tryptic digests of lens proteins were collected at the elution positions of the four Ser peptide standards as shown in Figure 1(a) and then each was analyzed separately by capillary LC/MS. After analytical HPLC, a single peak for each peptide was observed by LC/MS at m/z of 588.2. Using this approach, fractions were obtained that enabled unequivocal identification of the D-Ser peptides. This methodology showed that all forms of the D-Ser peptides were present in older normal lenses. Representative MS/MS spectra of one synthetic (L-Ser (59)/L-Ser (62)) peptide, and one of the peptides isolated from the tryptic digest of lens proteins is shown in Figure 1(b). All peptide isoforms displayed almost identical LC MS/MS spectra.
Interestingly, nanospray MS/MS analysis of the four isomeric Ser peptide standards revealed slight, but reproducible, differences in the intensities of some fragment ions that could be used as an additional tool to characterize the peptides (data not shown). Comparison of the MS/MS spectra of synthetic standards with those of peaks eluting at the expected positions of each isomer from the lens digests confirmed the identification of individual lens peptides.
Quantification of D-Ser as a function of age and cataract
LC/MS showed that both Ser 59 and Ser 62 were racemized in αA-crystallin from human lenses. Examination of the ion intensities enabled relative quantification of D-Ser in the various forms of the αA-crystallin peptide as a function of age in normal lenses, as well as a comparison with age-matched cataract lenses (Fig. 2).
Figure 2.
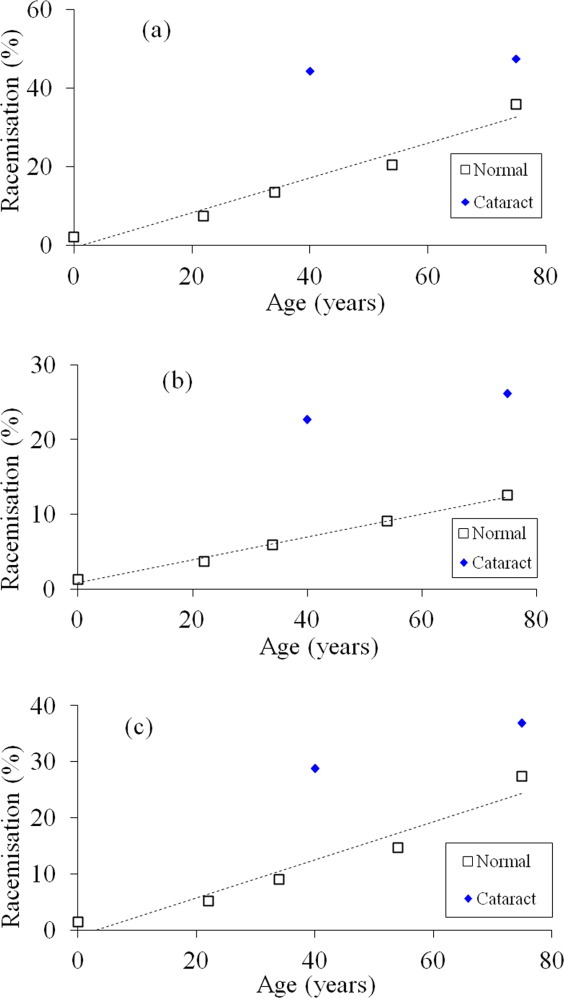
Racemization of L-Ser to D-Ser at Ser 59 and Ser 62 in αA-crystallin as a function of age in normal and cataract lenses (symbols: □ Normal ♦ cataract). (a) Total D-Ser. The peak intensities of the D-Ser (59)/L-Ser (62), L-Ser (59)/D-Ser (62), and D-Ser (59)/ D-Ser (62) peaks were combined and the sum expressed as a function of the total ion abundance for the αA-crystallin peptide TVLDSGISEVR (normal; y = 0.443x ( 0.477, R2 = 0.955, two-sided P 0.004). Mann–Whitney U test, two-sided P = 0.0286. (b) D-Ser 59. The peak intensities of the D-Ser (59)/L-Ser (62) and D-Ser (59)/D-Ser (62) peaks were combined and the sum expressed as a percentage of the total ion abundance for the αA-crystallin peptide, TVLDSGISEVR (normal; y = 0.153x (0.794, , R2 = 0.994, two-sided P = 0.0002). Mann–Whitney U test, two-sided P = 0.0286. (c) D-Ser 62. The peak intensities of the L-Ser (59)/D-Ser (62) and D-Ser (59)/ D-Ser (62) peaks were combined and the sum expressed as a percentage of the total ion abundance for the αA-crystallin peptide, TVLDSGISEVR (normal; y = 0.4x 1.05, R2 = 0.936, two-sided P = 0.007). Mann–Whitney U test, two-sided P = 0.0286. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The inversion of L-Ser to D-Ser at both sites as a function of age for normal and cataract lenses (i.e., the sum of D-Ser (59)/L-Ser (62), L-Ser (59)/D-Ser (62), and D-Ser (59)/D-Ser (62) isoforms) is shown in Figure 2(a). It can be seen from the graph that there is an age-dependent increase in the combined content of D-Ser in the αA-crystallin present in normal lenses. The amount of D-Ser present at these two sites in αA-crystallin from a 75-year-old lens accounted for (35% of the total. Remarkably, the levels of D-Ser from age-matched cataract lenses were much higher [Fig. 2(a)]. At the age of 40 years, the amount of Ser racemization in this region of the protein amounted to 44% in cataract lenses compared to 17% in normal lenses. The level of D-Ser in a 75-year-old cataract lens (48%) was only slightly elevated compared with that from the younger cataract lenses.
As all four Ser isomers were separated, it was possible to compare the rates of inversion at the two sites.
Racemization of Ser 59
Racemization of Ser 59 was calculated using the relative content of TVLD(D-Ser)GI(L-Ser)EVR plus TVLD(D-Ser)GI(D-Ser)EVR as a function of the total amount of TVLDSGISEVR.
In normal lenses, the amount increased linearly, reaching a level of (9% by the age of 75 years [Fig. 2(b)]. The amount present in cataract lenses was significantly higher, being more than double the values found in normal lenses at young and older ages.
Racemization of Ser 62
Racemization of Ser 62 was calculated using the relative content of TVLD(L-Ser)GI(D-Ser)EVR plus TVLD(D-Ser)GI(D-Ser)EVR as a function of the total amount of TVLDSGISEVR.
Racemization levels of Ser 62 were greater than for Ser 59, reaching 24% by the age of 75 years [Fig. 2(c)]. Once again, there was a large difference in the extent of racemization at this site in the cataract lenses. The amount of D-Ser present at this site in αA-crystallin from 40-year-old cataract lenses was more than double that of age-matched normals.
Racemization of both Ser 62 and Ser 59
Racemization at both sites was calculated using the relative content of TVLD(D-Ser)GI(D-Ser)EVR as a function of the total amount of TVLDSGISEVR. Evidence for racemization of Ser at both sites was found [Fig. 3(c)]; however, the content was less than that for the single sites [Fig. 3(a,b)]. In normal human lenses, racemization of both Ser 59 and Ser 62 in αA-crystallin accounted for approximately 4–5% of the total by the age of 75 years [Fig. 3(c)]. As was found with the individual Ser 59 and Ser 62 sites, the amount of the di-D-Ser peptide was significantly higher in cataract lenses. For example, the amount of the D-Ser/D-Ser peptide was 16% of the total in the 75-year-old cataract lens; more than three times the level in the age-matched control.
Figure 3.
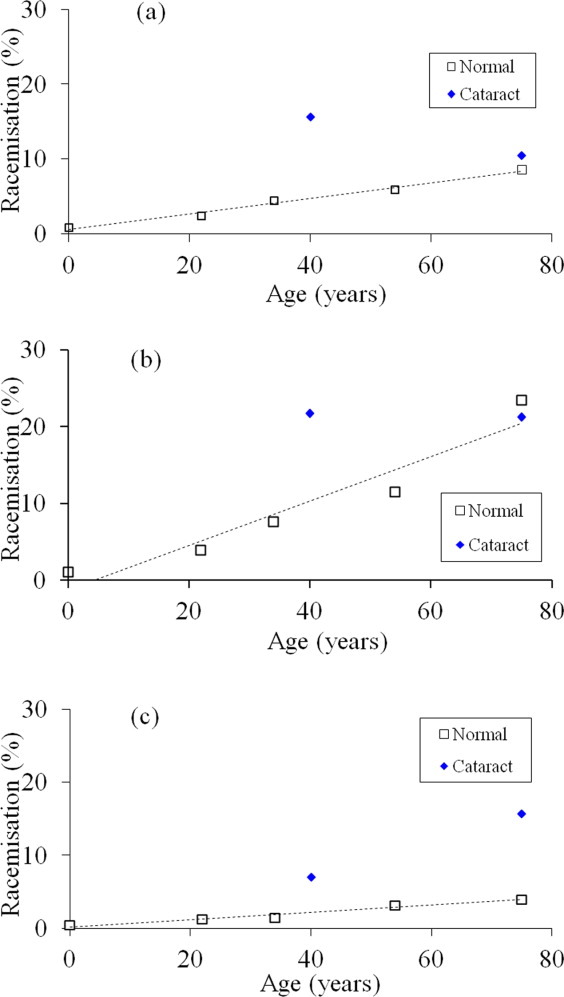
Individual D-Ser variants of the αA-crystallin peptide, TVLDSGISEVR as a function of age, in normal and cataract lens proteins. In each case, the peak intensities were expressed as a percentage of the total ion abundance for TVLDSGISEVR (symbols: □ Normal ♦ Cataract ). (a) D-Ser (59)/ L-Ser (62) (normal; y = 0.103x + 0.576, R2 = 0.987, two-sided P = 0.0007). Mann–Whitney U test, two-sided P = 0.743. (b) L-Ser (59)/ D-Ser (62) (normal; y = 0.29x ( 1.27, , R2 = 0.918, two-sided P = 0.0103). Mann–Whitney U test, two-sided P = 0.886. (c) D-Ser (59)/D-Ser (62) (normal; y = 0.0495x ( 0.218, R2 = 0.96, two–sided P = 0.0034). Mann–Whitney U test, two-sided P = 0.0286. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Racemization of Ser 62 and Ser 59 in cataract lenses
The contents of the other individual isomers (i.e., D-Ser (59)/L-Ser (62) and L-Ser (59)/D-Ser (62) peptides) are also shown in Figure 3(a,b).
The graphs were illuminating as they revealed similar patterns. In both cases, the amount of the particular isomer increased linearly with age in normal lenses. The rate of racemization of Ser 62 seemed to be more than twice as fast as that of Ser 59: approximately 1.6% per decade for D-Ser (59)/L-Ser (62) [Fig. 3(a)] and 3.9% per decade for L-Ser (59)/D-Ser (62) [Fig. 3(b)]. The increased rate of racemization of Ser 62 may relate to its location at the junction of a β-sheet with a random coil or solvent accessibility (Fig. 4).
Figure 4.
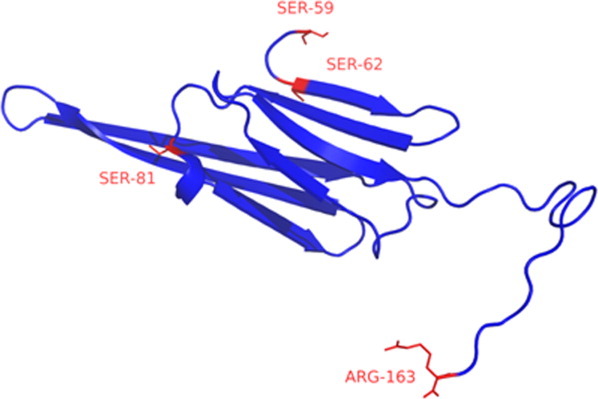
The structure of bovine αA-crystallin showing the locations of Ser 59 and Ser 62.33, 47 The structure of the C-terminal portion of the protein (residues, 59–163) was obtained from the Protein Data Bank (PDB ID: 3L1E) (http://www.rcsb.org/pdb/home/home.do). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
For both Ser 59 and Ser 62, the levels of D-Ser in the 40-year-old cataract lenses were considerably elevated compared with the comparable normal levels; however, this difference had largely disappeared when the 75-year-old cataract lenses were compared to their normal counterparts [Figure 3(a,b)]. One reason may be that in the case of the older cataract lenses, the single D-Ser-modified protein had been converted into doubly racemized αA-crystallin and this is supported by the graph shown in Figure 3(c). Such a pattern would be consistent with an accelerated aging of Ser at these sites in the case of cataract lenses.
Discussion
In this manuscript, the localization of D-Ser residues within the sequence of an intact human protein has been identified for the first time. These sites of racemization were found in the chaperone protein, αA-crystallin. This small heat shock protein15 binds to proteins as they unfold in the process forming large soluble aggregates.16–18 The homologous small heat shock protein, αB-crystallin, is also in the lens and it may act as a chaperone in other organs as it is present in tissues such as heart, as well as the brain15, 19 where it is upregulated in various neurological diseases such as Alzheimer's disease20 and Parkinson's disease.21
The amount of D-Ser in proteins, as determined by amino acid analysis, increases with age in the human lens,12 articular cartilage,11 dentin,11 and brain.3 The mechanism for racemization of Ser in proteins is not known; however, it likely involves direct abstraction of the α proton from the Ser residue followed by reprotonation. Studies with amino acids indicate that Ser22 and Thr lose their α protons more readily than those of other side chains. In addition, metals may facilitate this abstraction as Zn (II), Cu (II), Co (II), and Pd (II) enhance the racemization of L-Ser and L-Thr.23
The levels of racemized Ser at both sites increased with age until they represented approximately 35% of the combined total Ser at residues 59 and 62 in normal lenses by the 8th decade [Fig. 2(a)]. Interestingly, both D-Ser residues are located in one part of αA-crystallin that corresponds to an unstructured region as shown in Figure 4. This region appears to be susceptible to age-related modification, as Asp-5814 is also more highly racemized than other Asp residues in this protein from older lenses.
To determine whether other Ser residues in αA-crystallin were racemized, the remainder of the protein was examined. Proteomic analysis is restricted in this case owing to the uneven distribution of Ser in αA-crystallin, and the size of the tryptic peptides. No evidence for D-Ser was found in HFS81PEDLTVK, which is located within a β-sheet (Fig. 4). The flexible C-terminal peptide of αA-crystallin24 may also show racemization of Ser; however, this tryptic peptide contains 3 Ser, making analysis complicated. Other crystallins were also examined for evidence of Ser racemization using the same strategy as that used to identify the D-Ser (59)/D-Ser (62), that is multiple peaks with the same mass and MS/MS spectra in tryptic digests. One other possible site of significant racemization appears to be Ser 89 in γS-crystallin, which is in the flexible linker region between the two domains.25 This preliminary finding suggests that racemization of Ser residues in unstructured regions is favored over racemization in structured parts, such as β-sheets. This conclusion is in agreement with Asn deamidation/Asp racemization data from the previous studies.26, 27
With regard to the possible impact of the amino acid inversions on protein function, the unstructured portion of this small heat shock protein that includes Ser 59 and Ser 62 is localized adjacent to the α-crystallin domain of α-crystallin and mutations in this region typically result in a decrease in chaperone function.28–30 Furthermore, several point mutations within αA-crystallin are associated with human cataract formation,31, 32 including one (R49C)32 close to the region of interest. Despite the fact that both Ser residues are located close together in a flexible region of the protein, Ser 62 was found to racemize at approximately double the rate of Ser 59. This finding serves to illustrate that our knowledge of the factors responsible for amino acid racemization in proteins is limited. Indeed as shown in Figure 4, Ser 62 is the first residue of the β-sheet of the α-crystallin domain33 and so, on this basis, it is unlikely that secondary structure is the only factor responsible for determining racemization.
There is little data on the effect of Ser racemization on protein function in other biological systems. Considerable quantities of D-Ser, as well as D-Asp, are present in β-amyloid from human brain10, 34 and one site, D-Ser 26 has been characterized that alters the properties of the peptide.35 Replacement of L-Ser 46 by D-Ser in the toxin of the funnel-web spider increased its toxicity 5-fold.36 In chicken eggs, the properties of the egg white alter with time of storage, and this has marked effects on its cooking properties. The crystal structure of ovalbumin from older eggs showed that three D-serine residues were present and that they were located in a flexible region of the protein.37, 38 By analogy, in human lenses racemization of Ser at positions 59 and 62 may cause significant changes to the structure of αA-crystallin, and could diminish its ability to act as a chaperone.30, 39
Changes to α-crystallin in human lenses have been linked to the development of presbyopia40 where lenses become much stiffer with age, until near focussing becomes impossible. With a decreased chaperone capacity, aggregation of other proteins in the lens would take place more readily as the unfolding intermediates would be less likely to be intercepted by αA-crystallin18, 41, 42 and this may exacerbate the formation of cataract.43–45 Thus, the rate of racemization of Ser at these sites could determine the rate of cataract formation. If this hypothesis was correct, and racemization of Ser in α-crystallin was implicated in human cataract etiology by effectively reducing the amount of functional chaperone available, one may hypothesize that in cataract lenses the levels of racemization would be higher than in the age-matched normal lenses. Indeed, this was observed [Fig. 2(a–c)]. Furthermore, a comparison of the graphs of the individual D-Ser peptides was consistent with an ongoing process of inversion, as well as a more rapid racemization of Ser at both the 59 and the 62 positions in cataract lenses compared with age-matched normals (Fig. 3); however, more cataract lenses would need to be examined to confirm this observation.
Age-related alterations to unstructured regions may well be a general feature of old proteins. Such parts appear to be more susceptible to PTMs than comparable residues located within structured portions.26, 27 This may be owing to the flexibility of residues within these regions that allows access to a larger range of conformational states, and thereby favoring processes such as inversion.
Materials and Methods
Lens dissection and digestion of proteins
Normal human lenses were obtained from the Sydney Eye Bank, with ethical approval from the University of Sydney and fetal lenses from the Prince of Wales Hospital, Randwick, NSW, Australia. Cataract lenses, aged 40 and 75 years, were obtained from the K.T. Seth Eye Hospital, Rajkot, Gujarat, India. For all lenses, the nucleus was separated from the cortex by coring through the visual axis with a 4.5-mm internal diameter trephine precooled at (20°C. A cold scalpel was used to remove (0.5 mm of newly synthesized material from each end of the core and the nuclear regions of the lenses analyzed as described previously.27 Briefly, tissues were homogenized in 7M guanidine hydrochloride 100 mM Tris, pH 7.0 (2 mg/mL), and centrifuged (16,000g, 10 min). A 20-fold molar excess of dithiothreitol was added and incubated for an hour at 37°C. The solution was adjusted to pH 8.0 with NaOH, a 40-fold molar excess of iodoacetamide (Sigma Aldrich, USA) was added, and incubated in the dark (30 min, 37°C). Alkylated protein was diluted (1:20) into 100 mM Tris pH 8.0 and then digested with 1:100 trypsin (Promega) at 37°C for 16 h. Peptides were desalted using a C18 Zip tip (Millipore, USA) and freeze dried.
Peptide standards
The four isoforms of serine in TVLDSGISEVR, which correspond to tryptic peptides 55–65 of human αA-crystallin, were synthesized by Peptide 2.0 (Chantilly, USA) with either L-serine or D-serine at positions 59 and 62. Each peptide was dissolved in formic acid (FA):HFBA:H2O (1:0.05:98.85 v/v) and analyzed by LC/MS. Optimized LC conditions that allowed separation of the four isoforms were used to chromatograph human lens digests. Confirmation of the elution times of the αA-crystallin peptides in digests was carried out by spiking in 1 pmol of the standard peptides.
Analytical HPLC
Peptides were dissolved in H2O:trifluoroacetic acid (TFA) (99.9:0.1) and run on a HPLC system consisting of two Shimadzu (Kyoto, Japan) LC-10Ai pumps, and a Shimadzu UV–visible detector (Model SPD-10A). Separation of the four isoforms of serine was achieved using a 5 μm, C18 (4.6 × 250 mm) column (Phenomenex, Torrance, USA). Samples (20 μL) were injected and a linear gradient (10% B to 30% B in 115 min) run at a flow rate of 1 mL/min, where buffer B is acetonitrile (ACN):TFA (99.9:0.1 v/v).
Liquid chromatography/mass spectrometry
Digested peptides were separated by nano-LC using a Cap-LC auto-sampler system (Waters, Milford, MA). Samples (1 μL) were concentrated and desalted onto a micro C18 precolumn (500 μm ( 2 mm, Michrom Bioresources, Auburn, CA) with H2O:ACN:HFBA (97.5:2:0.05 v/v) with a flow rate of (350 nL/min (mobile phase A from pump A [H2O:FA (99.9:0.1 v/v]). After a 4-min wash, the precolumn was automatically switched (Valco 10 port valve, Houston, TX) into line with a fritless analytical column containing C18 reverse phase packing material with packing material (Michrom Bioresources, Auburn, CA) of 5 μm particle size, 200 Å, 75 μm inner diameter, 360 μm outer diameter, and (10 cm long manufactured according to Gatlin.46 Peptides were eluted using a linear gradient of H2O:ACN:FA (97.9:2:0.1 v/v) to H2O:CH3CN:FA (54.9:45:0.1 v/v) at (200 nL/min over 30 min. The analytical column was connected via a fused silica capillary ((10 cm; inner diameter = 25 μm, outer diameter = 360 μm) to a low-volume tee (Upchurch Scientific, Silsden, United Kingdom) where a high voltage (2400 V) was applied and the column tip positioned (0.5 cm from the Z-spray inlet of an Ultima API hybrid Q-TOF tandem mass spectrometer (Micromass, Manchester, United Kingdom). Positive ions were generated by electrospray and the QTof operated in data-dependent acquisition mode. A Tof MS survey scan was acquired (m/z = 350–1700, 1 s) and the two largest multiply charged ions were sequentially selected by Q1 for MS/MS analysis. Argon was used as collision gas and an optimum collision energy chosen (based on charge state and mass). Tandem mass spectra (MS-MS) were accumulated for up to 2 s (m/z = 50–2000). Peak lists were generated by MassLynx (version 4.0 SP4, Micromass) using the Mass Measure program.
Data analysis
The triply charged ions for the αA-crystallin-derived peptides were extracted and the intensities from the extracted ion chromatogram (XIC) determined using MassLynx. Peak areas of specific peptides were calculated using a mean smoothing method (Number of smooths: 2, window size [scans]: (3). The MS/MS spectrum of each peptide was matched to the XIC, ensuring the peak area used corresponded to that of the matched peptide and not an isobaric peptide. For αA-crystallin, all forms of the peptide [(L-Ser (59)/L-Ser (62); D-Ser (59)/L-Ser (62); L-Ser (59)/D-Ser (62), and D-Ser (59)/D-Ser (62)] were summed and modification for each one was expressed as a % of the total peak area. Simple linear regression analysis and Mann–Whitney U tests were performed as described previously.12
Conclusions
Old proteins undergo a range of modifications that appear to be mainly the result of the intrinsic instability of certain amino acids. In this study, racemization of two Ser residues has been demonstrated in an unstructured region of the small heat shock protein, αA-crystallin. As such modifications were abundant, it is likely that these age-related changes contribute to perturbation of the structure of the protein, and therefore its ability to act as a chaperone.
Racemization appears to be the single most abundant modification in the lifelong proteins isolated from older normal human lenses,12 and cataract lenses have significantly higher levels of D-Ser than age-matched normal lenses. In this study, it has been shown that racemization of Ser 59 and Ser 62 in αA-crystallin is in part responsible for this. Racemization of amino acids such as serine, particularly in flexible regions of long-lived proteins, could be associated with the development of cataract, and potentially other age-related conditions, in man.
Supplementary material
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallins reveal proteins without carbon turnover throughout life. PLoS One. 2008;1:1529–1531. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro S, Endicott S, Province M, Pierce J, Campbell E. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapira R, Chou CHJ. Differential racemization of aspartate and serine in human myelin basic protein. Biochem Biophys Res Commun. 1987;146:1342–1349. doi: 10.1016/0006-291x(87)90797-2. [DOI] [PubMed] [Google Scholar]
- 4.Ritz-Timme S, Collins MJ. Racemization of aspartic acid in human proteins. Ageing Res Rev. 2002;1:43–59. doi: 10.1016/s0047-6374(01)00363-3. [DOI] [PubMed] [Google Scholar]
- 5.Gineyts E, Cloos PA, Borel O, Grimaud L, Delmas PD, Garnero P. Racemization and isomerization of type I collagen C-telopeptides in human bone and soft tissues: assessment of tissue turnover. Biochem J. 2000;345:481–485. [PMC free article] [PubMed] [Google Scholar]
- 6.Truscott RJW. Macromolecular deterioration as the ultimate constraint on human lifespan. Ageing Res Rev. 2011;10:397–403. doi: 10.1016/j.arr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Truscott RJW, Zhu X. Presbyopia and cataract: a question of heat and time. Prog Retinal Eye Res. 2010;29:487–499. doi: 10.1016/j.preteyeres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Cloos P, Christgau S. Non-enzymatic covalent modifications of proteins: mechanisms, physiological consequences and clinical applications. Matrix Biol. 2002;21:39–52. doi: 10.1016/s0945-053x(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 9.Geiger T, Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987;262:785–794. [PubMed] [Google Scholar]
- 10.Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. Beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stabler TV, Byers SS, Zura RD, Kraus VB. Amino acid racemization reveals differential protein turnover in osteoarthritic articular and mensical cartilages. Arthritis Res Ther. 2009;11:34–42. doi: 10.1186/ar2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooi M, Truscott R. Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age. 2011;33:131–141. doi: 10.1007/s11357-010-9171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulus H. Protein splicing and related forms of protein autoprocessing. Ann Rev Biochem. 2000;69:447–496. doi: 10.1146/annurev.biochem.69.1.447. [DOI] [PubMed] [Google Scholar]
- 14.Fujii N, Matsumoto S, Hiroki K, Takemoto L. Inversion and isomerization of Asp-58 residue in human [alpha]A-crystallin from normal aged lenses and cataractous lenses. Biochim Biophys Acta. 2001;1549:179–187. doi: 10.1016/s0167-4838(01)00258-8. [DOI] [PubMed] [Google Scholar]
- 15.Derham BK, Harding JJ. Alpha crystallin as a molecular chaperone. Prog Retinal Eye Res. 1999;18:463–509. doi: 10.1016/s1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- 16.Rao PV, Huang Q, Horwitz J, Zigler JS. Evidence that alpha crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim Biophys Acta. 1995;1245:439–447. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 17.Nicholl I, Quinlan R. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwaki T, Kume IA, Goldman JE. Cellular distribution of alpha b crystallin in non-lenticular tissues. J Histochem Cytochem. 1990;38:31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- 20.Lowe J, McDermott H, Pike I, Speudlove I, Landon M, Mayer R. Alpha B-crystallin expression in non-lenticular tissues and selective presence in ubiquinated inclusion bodies in human disease. J Pathol. 1992;166:61–68. doi: 10.1002/path.1711660110. [DOI] [PubMed] [Google Scholar]
- 21.Iwaki T, Wisniewski T, Akiko I, Corbin E, Tomokane N, Tateishi J, Goldman JE. Accumulation of alpha B-crystallin in central nervous system glia and neurons in pathologic conditions. Am J Pathol. 1992;140:345–256. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GG, Reddy GV. Effect of the side chain on the racemization of amino acids in aqueous solution. J Organic Chem. 1989;54:4529–4535. [Google Scholar]
- 23.Sudhakar Reddy G, Vanita Reddy G, Gill Smith G. The effect of Ni(II), Zn(II), Cu(II), Co(II) and Pd(II) ions on racemisation of hydroxy α-amino acids. Inorganica Chim Acta. 1989;166:55–58. [Google Scholar]
- 24.Carver JA, Aquilina JA, Truscott RJW, Ralston GB. Identification by 1H NMR spectroscopy of flexible C-terminal extensions in bovine lens α-crystallin. FEBS Lett. 1992;311:143–149. doi: 10.1016/0014-5793(92)81386-z. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Delaglio F, Wyatt K, Wistow G, Bax A. Solution structure of gammaS-crystallin by molecular fragment replacement NMR. Protein Sci. 2005;14:3101. doi: 10.1110/ps.051635205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wearne SJ, Creighton TE. Effect of protein conformation on rate of deamidation: ribonuclease A. Proteins. 1989;5:8–12. doi: 10.1002/prot.340050103. [DOI] [PubMed] [Google Scholar]
- 27.Hooi MYS, Raftery MJ, Truscott RJW. Racemisation of two proteins over our lifespan. Deamidation of asparagine 76 in γS crystallin is greater in cataract than in normal lenses across the age range. Invest Opthal Vis Sci. 2012;14:3554–3561. doi: 10.1167/iovs.11-9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muchowski PJ, Wu GJS, Liang JJN, Adman ET, Clark JI. Site-directed mutations within the core “α-crystallin” domain of the small heat-shock protein, human αB-crystallin, decrease molecular chaperone functions. J Mol Biol. 1999;289:397–411. doi: 10.1006/jmbi.1999.2759. [DOI] [PubMed] [Google Scholar]
- 29.Sharma KK, Kaur H, Kester K. Functional elements in molecular chaperone α-crystallin: identification of binding sites in αB-crystallin. Biochem Biophys Res Commun. 1997;239:217–222. doi: 10.1006/bbrc.1997.7460. [DOI] [PubMed] [Google Scholar]
- 30.Derham BK, van Boekel MAM, Muchowski PJ, Clark JI, Horwitz J, Hepburne-Scott HW, de Jong WW, Crabbe MJC, Harding JJ. Chaperone function of mutant versions of αA- and αB-crystallin prepared to pinpoint chaperone binding sites. Eur J Biochem. 2001;268:713–721. doi: 10.1046/j.1432-1327.2001.01929.x. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Ding L, Phan KB, Cheng C, Xia C-, Gong X, Horwitz J. Mechanism of cataract formation in αA-crystallin Y118D mutation. Invest Ophthal Vis Sci. 2009;50:2919–2926. doi: 10.1167/iovs.08-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay D, Andley U, Shiels A. Cell death triggered by a novel mutation in the αA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 33.Laganowsky A, Benesch JLP, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19:1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapira R, Austin GE, Mirra SS. Neuritic plaque amyloid in Alzehimer's disease is highly racemized. J Neurochem. 1988;50:69–74. doi: 10.1111/j.1471-4159.1988.tb13231.x. [DOI] [PubMed] [Google Scholar]
- 35.Kubo T, Kumagae Y, Miller C, Kaneko I. β-Amyloid rcemized at the Ser26 residue in the brains of patients with Alzheimer disease: implications in the pathogenesis of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:248–259. doi: 10.1093/jnen/62.3.248. [DOI] [PubMed] [Google Scholar]
- 36.Heck SD, Siok CJ, Krapcho KJ, Kelbaugh PR, Thadeio PF, Welch MJ, Williams RD, Ganong AH, Kelly ME, Lanzetti AJ, Gray WR, Phillips D, Parks TN, Jackson H, Ahlijanian MK, Saccomano NA, Volkmann RA. Functional consequences of posttranslational isomerization of Ser46 in a calcium channel toxin. Science. 1994;266:1065–1068. doi: 10.1126/science.7973665. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Maeda M, Yamasaki M, Mikami B. Protein-engineering study of contribution of conceivable D-Serine residues to the thermostabilization of Ovalbumin under alkaline conditions. Chem Biodiv. 2010;7:1634–1643. doi: 10.1002/cbdv.200900305. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki M, Takahashi N, Hirose M. Crystal structure of S-ovalbumin as a non-loop-inserted thermostabilized Serpin form. J Biol Chem. 2003;278:35524–35530. doi: 10.1074/jbc.M305926200. [DOI] [PubMed] [Google Scholar]
- 39.McHaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48:3828–3837. doi: 10.1021/bi900212j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heys KR, Friedrich MG, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 41.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 42.Meehan S, Berry Y, Luisi B, Dobson CM, Carver JA, MacPhee CE. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem. 2004;279:3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz J, Bova MP, Ding L, Haley DA, Stewart PL. Lens α-crystallin: function and structure. Eye. 1999;13:403–408. doi: 10.1038/eye.1999.114. [DOI] [PubMed] [Google Scholar]
- 44.Cheng C, Xia C-, Huang Q, Ding L, Horwitz J, Gong X. Altered chaperone-like activity of α-crystallins promotes cataractogenesis. J Biol Chem. 2010;285:41187–41193. doi: 10.1074/jbc.M110.154534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santhoshkumar P, Raju M, Sharma KK. αA-crystallin peptide 66SDRDKFVIFLDVKHF80 accumulating in aging lens impairs the function of α-crystallin and induces lens protein aggregation. PLoS One. 2011;6:e19291. doi: 10.1371/journal.pone.0019291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatlin CL, Kleemann GR, Hays LG, Link AJ, Yates JR. Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography-microspray and nanospray mass spectrometry. Anal Biochem. 1998;263:93–101. doi: 10.1006/abio.1998.2809. [DOI] [PubMed] [Google Scholar]
- 47.Bagnéris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal structures of [alpha]-crystallin domain dimers of [alpha]B-crystallin and Hsp20. J Mol Biol. 2009;392:1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


