Abstract
Vascular endothelial growth factor C (VEGF-C) is a crucial regulator of the development of lymphatic vessels and is involved in the lymph node metastasis of cancer. The levels of VEGF-C expression and lymphatic vessel density (LVD) in 128 gastro-oesophageal junction adenocarcinoma (GEJA) tissues were examined by immunohistochemistry and analysed for their association with clinicopathological features and disease-free survival. We found that 75.0% of tumour samples displayed strong immunoreactivity to VEGF-C. The levels of VEGF-C expression in the tumour tissues were associated with the stages of the clinical tumours and the lymph node metastasis status, but not with the age, gender and the size and type of tumours in the cohort. Similarly, LVD, as evaluated by anti-D2-40 staining, was also associated with the clinical stages of GEJA. The values of LVD were positively correlated with the levels of VEGF-C expression in these samples (r = 0.3760, P = 0.0001). High levels of VEGF-C expression and high values of LVD were associated with shorter periods of disease-free survival (DFS) in patients with GEJA (P < 0.001). In addition, GEJA at N1 and N2 stages, at T4 stage, chemotherapy after surgery, high levels of VEGF-C expression and lower marginal resection were independent factors for the prognosis of DFS in patients with GEJA. Our data indicate that VEGF-C may promote the lymphangiogenesis and lymphatic metastasis of GEJA and that VEGF-C may be a valuable biomarker for the diagnosis of lymphatic metastasis and a prognostic factor of the survival of patients with GEJA.
Keywords: disease-free survival rate, gastro–oesophageal junction adenocarcinoma, lymphangiogenesis, lymphatic vessel density, prognosis, vascular endothelial growth factor C
Gastro-oesophageal junction adenocarcinoma (GEJA) is defined as tumours occurring within 5 cm proximal or distal to the gastro-oesophageal junction and is one of the most lethal malignant tumours worldwide. Tumour metastasis to regional lymph nodes is a common step in the progression of cancer, and the extent to which lymph nodes are affected is a major determinant for the staging and prognosis of many kinds of cancers. Mounting evidence suggests that tumour lymphangiogenesis, which is defined as the formation of new tumour-associated lymphatic vessels, promotes lymphatic tumour metastasis and plays an important role in the progression of numerous malignant tumours, including cutaneous melanoma (Dadras et al. 2003), pancreatic endocrine tumour (Rubbia-Brandt et al. 2004), colorectal carcinoma (Omachi et al. 2007), gastric carcinoma (Gao et al. 2009) and breast cancer (Cunnick et al. 2002). Vascular endothelial growth factor C (VEGF-C) is associated with lymphangiogenesis (Jeltsch et al. 1997; Oh et al. 1997). The mature VEGF-C can bind and activate its receptors of VEGFR3 (Flt-4) and VEGFR2 (Joukov et al. 1996). Physiologically, VEGF-C is a specific ligand for the VEGFR3 and is expressed mainly by mesenchymal cells adjacent to the VEGFR3-expressing lymphatic endothelia in the heart, placenta, ovary, small intestine, thyroid gland, prostate and mammary duct epithelium (Joory et al. 2006), and VEGFR3 is predominantly expressed in the endothelium of lymphatic vessels (Kaipainen et al. 1995). The engagement of VEGFR3 by VEGF-C can activate the downstream signalling and promote endothelial cell growth, survival and lymphangiogenesis, thereby regulating the permeability of blood vessels (Su et al. 2007). VEGF-C promotes lymphangiogenesis in different organs in a paracrine manner. A previous study has shown that overexpression of VEGF-C selectively induces the proliferation of lymphatic endothelial cells and the hyperplasia of the lymphatic vasculature (Kukk et al. 1996). VEGF-C has been shown to promote tumour lymphangiogenesis and lymph node metastasis in animal models of tumours and humans (Bunone et al. 1999; Tsurusaki et al. 1999; Su et al. 2006). Increased levels of VEGF-C are associated with intratumoral and peritumoral lymphangiogenesis and tumour spread to regional lymph nodes (Akagi et al. 2000; Xiang et al. 2009). Moreover, VEGF-C is a potential prognostic factor for patients with colorectal, gastric, cervical and ovarian carcinomas (Ueda et al. 2002; Nishida et al. 2004), and VEGF-C is a significant independent factor for the poor prognosis of patients with gastric cancer (Yonemura et al. 1999). However, little is known about the levels of VEGF-C expression in GEJA tissues and how the levels of VEGF-C expression are associated with the clinical pathogenesis of GEJA. Whether and how the levels of VEGF-C expression could be related to the degrees of lymphangiogenesis in GEJA have not been explored. In addition, it is unclear whether VEGF-C expression and lymphagiogenesis in GEJA tissues are associated with the survival of patients with GEJA.
In this study, tissue from 128 patients with GEJA was examined. The levels of VEGF-C expression and the degree of lymphagiogenesis in their GEJA tissues were characterised and their potential association with clinicopathological features of GEJA was investigated and correlated with in the disease-free survival (DFS) in these patients. Our data indicate that higher levels of VEGF-C and lymphagiogenesis in GEJA tissues were associated with particular clinical stages of GEJA and DFS in these patients. Furthermore, VEGF-C appeared to be an independent prognostic factor of the survival of patients with GEJA.
Material and methods
Patients and tumour samples
A total of 128 patients with GEJA were recruited at the inpatient service of the Department of General Surgery of Cancer Hospital of Shantou University Medical College, China between October 2000 and October 2001.
Individual patients with GEJA were diagnosed, according to the practice guidelines for gastric cancer of NCCN, and confirmed by histological examination of tumour sections. All of the patients were subjected to radical resection by oesophago-gastric anastomosis via left thoracotomy for obvious invasive tumours or to simple resection via laparotomy for localized and minimal invasive tumours. These patients were followed for 6 years, except for seven patients who missed appointments or discontinued treatment. There were 106 men and 22 women with a mean age of 60.0 years (range 35–81) at the time of surgery. Of the 128 studied cases, 103 received only the surgical treatment, and 25 received both surgery and post-operative adjuvant therapy consisting of chemotherapy and other medications. Immediately after resection the primary tumour samples were collected and embedded in paraffin for histological diagnosis and immunohistochemical study.
Individual tumour tissues were staged, according to the criteria for histological classification proposed by the Union for International Cancer Control (UICC, 2002). The demographic and clinicopathological features of the patients, including age, sex, tumour size, histological classification, differentiation degree and tumour stage, are summarized in Table 1.
Table 1.
Correlation between VEGF-C expression, LVD and various clinicopathologic parameters
| Clinicopathological parameters | Cases | VEGF-C expression (MOD) | t(F)-value | P-value | LVD | t(F)-value | P-value |
|---|---|---|---|---|---|---|---|
| Age (year) | |||||||
| <60 | 65 | 0.197 ± 0.079 | t = −0.944 | 0.347 | 14.649 ± 5.898 | t = −1.044 | 0.298 |
| >60 | 63 | 0.211 ± 0.085 | 15.752 ± 6.051 | ||||
| Gender | |||||||
| Male | 106 | 0.199 ± 0.079 | t = −1.431 | 0.155 | 15.106 ± 6.003 | t = −0.358 | 0.721 |
| Female | 22 | 0.226 ± 0.092 | 15.609 ± 5.968 | ||||
| Tumour size | |||||||
| <5 cm | 64 | 0.200 ± 0.073 | t = −0.565 | 0.573 | 14.816 ± 5.922 | t = −0.811 | 0.419 |
| >5 cm | 64 | 0.208 ± 0.091 | 15.676 ± 6.040 | ||||
| Gross type | |||||||
| Ulcer-Infiltrative type | 62 | 0.205 ± 0.081 | 15.229 ± 6.206 | ||||
| Ulcer type | 50 | 0.190 ± 0.074 | F = 1.923 | 0.129 | 14.272 ± 5.319 | F = 1.53 | 0.21 |
| Infiltrative type | 9 | 0.258 ± 0.111 | 17.889 ± 7.347 | ||||
| Other type | 7 | 0.223 ± 0.091 | 17.971 ± 5.866 | ||||
| Differentiation degree | |||||||
| Well and moderate | 51 | 0.197 ± 0.080 | 14.451 ± 5.889 | t = 1.307 | 0.255 | ||
| Poor | 77 | 0.208 ± 0.084 | t = 0.651 | 0.421 | 15.683 ± 6.021 | ||
| Clinical stage | |||||||
| I + II | 34 | 0.173 ± 0.070 | 12.018 ± 4.316 | ||||
| III | 61 | 0.210 ± 0.084 | F = 3.821 | 0.025 | 16.049 ± 6.028 | F = 7.419 | 0.001 |
| IV | 33 | 0.225 ± 0.083 | 16.879 ± 6.275 | ||||
| T stage | |||||||
| T2 + T3 | 85 | 0.194 ± 0.078 | t = −1.869 | 0.064 | 14.624 ± 5.979 | t = −1.521 | 0.131 |
| T4 | 43 | 0.223 ± 0.087 | 16.316 ± 5.879 | ||||
| N stage | |||||||
| N0 | 42 | 0.178 ± 0.077 | t = −2.515 | 0.013 | 12.319 ± 4.096 | t = −4.022 | 0.001 |
| N1 + N2 | 86 | 0.216 ± 0.082 | 16.651 ± 6.073 | ||||
VEGF-C, vascular endothelial growth factor C; MOD, mean optical density; LVD, lymphatic vessel density.
Ethical Approval
Written informed consent was obtained from individual patients, and the study was approved by the Ethics Committee of Shantou University.
Immunohistochemistry analysis
The surgical specimens were fixed in 10% formalin solution and embedded in paraffin. The paraffin-embedded GEJA tissue sections (3 μm in thickness) were deparaffinised in xylene and rehydrated using a series of graded ethanol solutions. The tissue sections were treated with 0.3% H2O2 in methanol for 20 min at room temperature for blocking endogenous peroxidase activity and subjected to antigen retrieval. After being washed with phosphate-buffered saline (PBS) and blocked with 10% normal rabbit sera in PBS, the sections were incubated with goat polyclonal anti-VEGF-C antibodies (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a mouse monoclonal anti-D2-40 antibody (1:100, Santa Cruz Biotechnology) overnight at 4 °C. Goat and mouse IgG were used as negative controls respectively. The bound antibodies were detected with biotinylated rabbit anti-goat antibodies (Zhongsan Jinqiao Biotechnology, Beijing, China) for 20 minutes at room temperature and visualized using peroxidase-conjugated streptavidin and diaminobenzidine (DAB, Zhongsan Jinqiao Biotechnology), followed by counterstaining with Mayer's haematoxylin. The sections were imaged under a Leica DMRXA2 microscope equipped with Leica IM50 software (DC500; Leica, Wetzlar, Germany). A total of five fields per single sample excluding non-tumour and necrotic areas were randomly selected and imaged for anti-VEGF-C staining (magnification ×400) in a blinded manner. The immunostaining was quantitatively analysed for a value of mean optical density (MOD), defined as the number of optical pixels in a given imaging area, using Image-pro® Plus 6.0 (Media Cybernetics, Inc. Bethesda, MD, USA).
Similarly, the most vascularized tumoral areas determined by strong anti-D2-40 staining were identified under low magnification. The number of immunostained lymphatic vessels in five strong anti-D2-40 staining areas at 200× magnification was counted. The mean number of lymphatic vessels in individual tumour samples was calculated as the LVD in a blinded manner.
Statistical analysis
Data are expressed as mean ± SEM, and all statistical analyses were performed by spss version 17.0 software (SPSS for Windows, Inc., Chicago, IL, USA). The differences in the MOD and LVD values among multiple groups were analysed by one-way anova and post hoc Bonferroni test. The difference in the MOD and LVD values between two groups was analysed by two-tailed Student's paired t-test. The potential correlation between the levels of VEGF-C expression and the values of LVD was analysed by the Spearman rank correlation test. The DFS curves were generated using the Kaplan–Meier method and analysed by the log-rank test. Independent factors in the value of predicting lymph node metastasis were analysed by a multivariate analysis using the Cox stepwise regression model. A P value of <0.05 was considered statistically significant.
Results
Various levels of VEGF-C are expressed in the tumour tissues from patients with GEJA
To determine VEGF-C expression and LVD in tumour tissues, tissue from a total of 128 patients with GEJA was examined and the levels of VEGF-C expression characterized by immunohistochemistry. While most tissue specimens were positive for anti-VEGF-C staining, some tumour samples were negative for anti-VEGF-C staining. Thus, 96 of 128 specimens (75%) displayed strong immunoreactivity for VEGF-C. The levels of VEGF-C expression varied the different tumour samples. The mean MOD value was 0.20 ± 0.08 in these samples. As shown in Figure 1, positive staining for VEGF-C was found in the cytoplasm of the tumour cells. The levels of VEGF-C expression were significantly higher in GEJA tissues from clinical stage IV than from the other clinical stages (0.225 ± 0.083 vs. 0.210 ± 0.084 or vs. 0.173 ± 0.070, P = 0.025, Table 1). Furthermore, the MOD values of VEGF-C expression were also markedly greater in GEJA at stages of N1 + N2 than at stage N0 (0.216 ± 0.082 vs. 0.178 ± 0.077, P = 0.013). However, there was no significant difference in the levels of VEGF-C expression between the GEJA tissues from men and women, young and old and patients with a tumour greater or <5 cm in a dimension, with or without ulceration, as well as the degree of differentiation. Therefore, the levels of VEGF-C expression were associated with the clinical stages of the tumours and the status of lymph node metastasis, but independent of age, gender and the size and type of tumour in this population.
Figure 1.
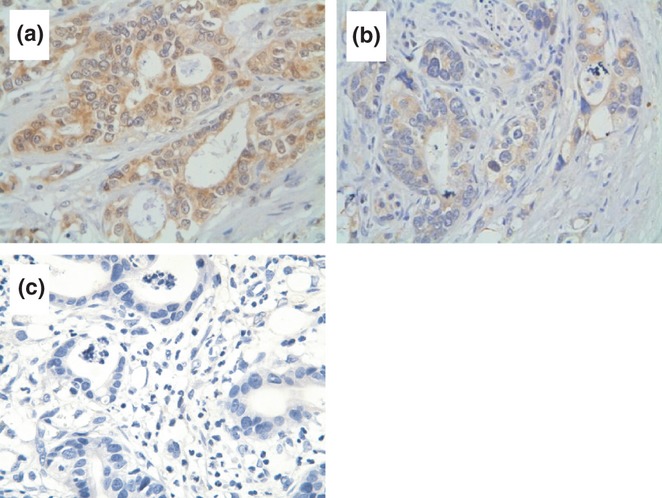
Immunohistochemical analysis of Vascular endothelial growth factor C (VEGF-C) expression in gastro-oesophageal junction adenocarcinoma (GEJA) tissues. The levels of VEGF-C expression in GEJA tissues were characterized by immunochemistry using anti-VEGF-c antibodies. Data shown are representative images (magnification ×400) of positive and negative GEJA tissue sections from 128 patients. (a) Strong immunoreactivity of anti-VEGF-C. (b) Moderate immunoreactivity of anti-VEGF-C. The yellow-brown staining was mainly identified in the cytoplasm of tumour cells. (c) Negative control.
Various levels of LVD display in GEJA tissues
It is well known that VEGF can promote angiogenesis and cancer metastasis, and GEJA usually metastasizes to local lymph nodes and other organs through lymphatic vessels (Bunone et al. 1999; Tsurusaki et al. 1999; Swisher et al. 2000; Su et al. 2006). The D2-40 is expressed by lymphatic endothelial cells and has been widely used for the evaluation of LVD (Weidner 1995). To determine the contents of lymphatic vessels in tumours, we characterized D2-40 expression in the GEJA tissues by immunohistochemistry using anti-D2-40 antibody. All of the tissue specimens were positive for anti-D2-40 staining. As shown in Figure 2, positive staining of anti-D2-40 was predominantly exhibited on lymphatic endothelial cells, and various levels of D2-40 expression were detected in different tumour samples with an average LVD value of 15.20 ± 5.98 per field. Further stratification analyses indicated that the LVD values in tumours at clinical stages III or IV were significantly greater than that at stages I and II (0.210 ± 0.084, 0.225 ± 0.083 vs. 0.173 ± 0.070, P < 0.001, Table 1). Similarly, the LVD values in tumours at stages of N1 and N2 were also greater than that at stage N0 (0.216 ± 0.082 vs. o.178 ± 0.077, P < 0.001). However, there was no significant difference in the LVD values between tumours from different genders, ages, tumour sizes, tumour types, different degrees and T stages in these samples. Thus, the contents of lymphatic vessels in tumours were associated with clinical stages of GEJA, but were independent of age, gender, tumour size and differentiation degrees. The LVD values were positively correlated with the MOD values in these tumour samples (r = 0.3760, P = 0.0001, Figure 3), as determined by Spearman rank correlation analysis. Additionally, the LVD values were significantly greater in tumours with high levels of VEGF-C expression than in those with low levels of VEGF-C expression (16.38 ± 0.78 vessels/field vs. 13.66 ± 0.69 vessels/field; P < 0.01).
Figure 2.
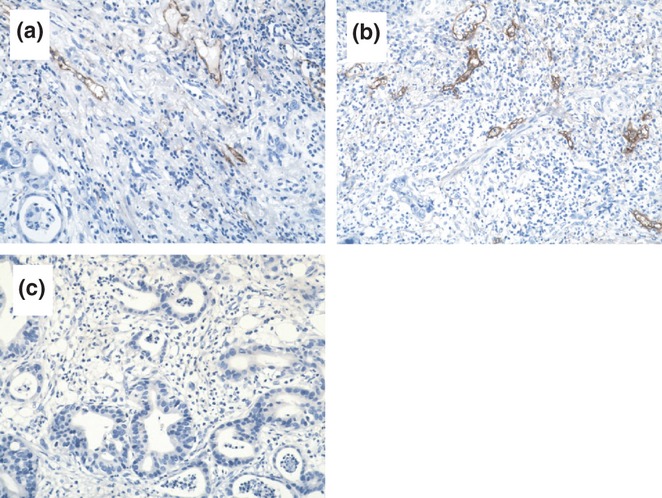
Immunohistochemical analysis of D2-40 expression in GEJA tissues. The levels of D2-40 expression in gastro-oesophageal junction adenocarcinoma (GEJA) tissue sections were characterized by immunohistochemistry using an anti-D2-40 monoclonal antibody. Data shown are representative images (magnification ×200) of 128 samples. (a) Strong immunoreactivity of anti-D2-40 antibody. (b) Moderate immunoreactivity of anti-D2-40 antibody. The D2-40 was mainly expressed in the cytoplasm and membrane of lymphatic endothelial cells. (c) Negative staining with control IgG.
Figure 3.
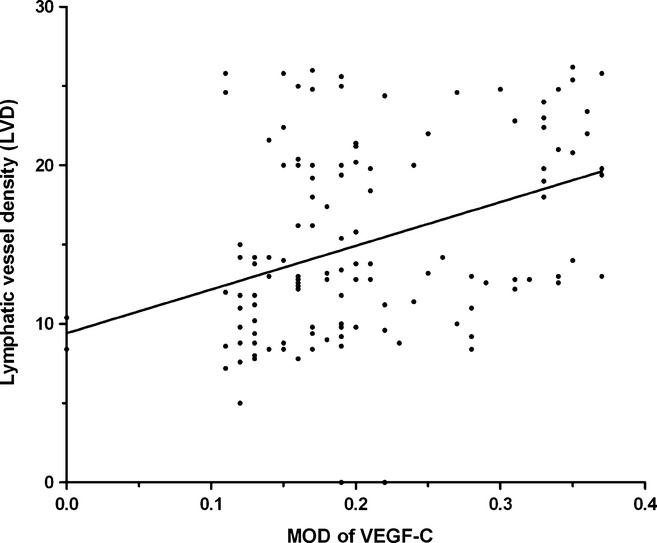
Correlation analysis of the levels of vascular endothelial growth factor C (VEGF-C) with the lymphatic vessel density (LVD) in GEJA tissues. Individual MOD and LVD values were analysed for the potential correlation by the Spearman rank correlation analysis. Data shown are individual values of 128 patients with GEJA. The levels of VEGF-C expression in GEJA were significantly correlated with the values of LVD (r = 0.3760, P < 0.0001).
The levels of VEGF-C expression and LVD are inversely correlated with the survival of patients with GEJA
Of the 128 patients, 121 patients were followed for up to 6 years and the mean follow-up period was 45 months. During this observation period, 39 patients progressed and 82 patients died. The median survival period of all patients was 27 months (range: 1–77 months). Further stratification of patients revealed that GEJA patients with low levels of VEGF-C expression (a MOD value of <0.18) survived significantly longer than those with high levels of VEGF-C expression (a MOD value of >0.18), as determined by log-rank test (median DFS: 51.1 ± 3.35 vs. 28.9 ± 3.16 months, P = 0.001, Figure 4a). Similarly, stratification of patients indicated that patients with high LVD GEJA (a LVD value >13) had a DFS period significantly shorter than those with low LVD tumours (a LVD value <13, median DFS: 31.1 ± 3.14 vs. 49.0 ± 3.56 months, P < 0.001, Figure 4b). These data indicated that high levels of VEGF-C expression and high values of LVD were associated with shorter periods of DFS in patients with GEJA.
Figure 4.
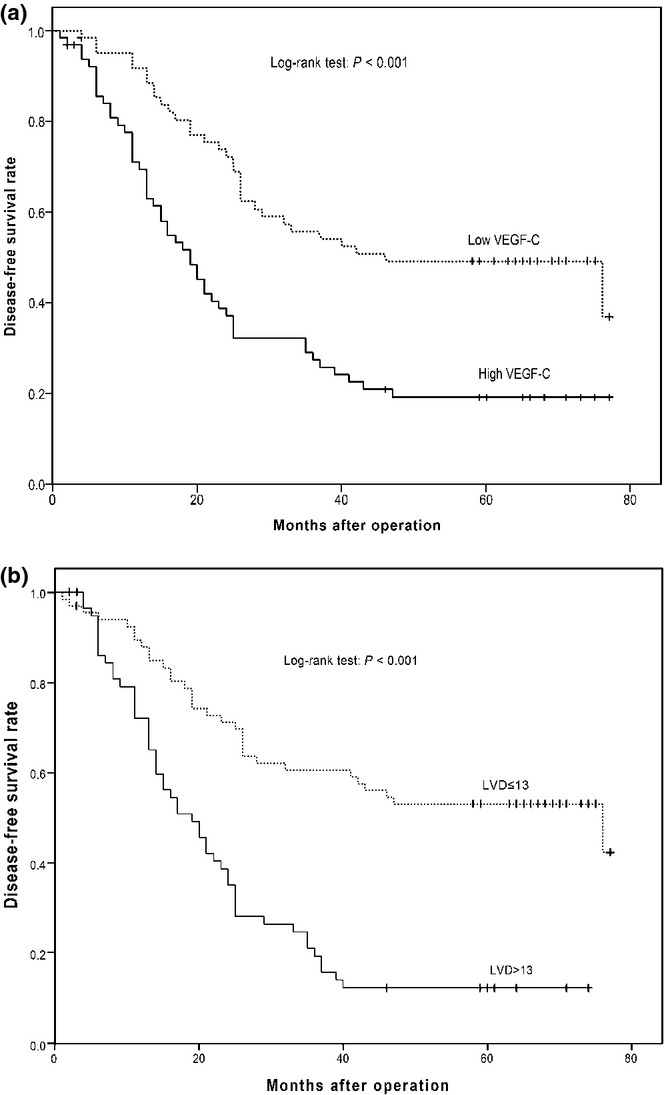
Stratification analysis of disease-free survival (DFS) in patients with gastro-oesophageal junction adenocarcinoma (GEJA). The patients were stratified, according to the levels of VEGF-C expression or lymphatic vessel density (LVD) values, and the DFS curves were estimated using the Kaplan–Meier method, followed by log-rank analysis. (a) The levels of vascular endothelial growth factor C (VEGF-C) expression are associated with DFS. There were 65 patients with high levels of VEGF-C expression in their tumour tissues and 63 patients with low levels of VEGF-C expression in their tumour tissues. (b) The LVD-associated DFS. Patients with high levels of LVD (solid line, n = 61) had a DFS period significantly shorter than those with low LVD (dotted line, n = 67, P < 0.001).
COX multivariate survival analysis of factors for DFS in patients with GEJA
To understand factors that could affect DFS in patients with GEJA, we performed a multivariate Cox regression analysis of patients’ age and sex, histological type, TNF stage, tumour size, VEGF-C expression, LVD, total number of lymph nodes affected, lymph node metastasis, treatment method (surgery only or surgery + chemotherapy) and the distance between tumour and resection margin. We found that individual patients with GEJA at N1 and N2 stages (P < 0.0001), at T4 stage (P = 0.046), with surgery and chemotherapy (P = 0.003), with high levels of VEGF-C (P < 0.0001) and lower marginal resection (P < 0.0001) were associated with a shorter DFS (Table 2). Therefore, GEJA at N1 and N2 stages, at T4 stage, chemotherapy after surgery, high levels of VEGF-C expression and lower marginal resection were independent factors for the prognosis of DFS in patients with GEJA.
Table 2.
The multivariate Cox regression analysis for DFS in 128 patients with GEJA
| 95.0% CI for Exp(B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variants | B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper |
| N Stage | 1.156 | 0.279 | 17.145 | 1 | <0.0001 | 3.176 | 1.838 | 5.489 |
| VEGF-C | 1.25 | 0.246 | 25.87 | 1 | <0.0001 | 3.491 | 2.156 | 5.652 |
| T Stage | 0.477 | 0.239 | 3.998 | 1 | 0.046 | 1.612 | 1.009 | 2.573 |
| Chemotherapy | −0.946 | 0.313 | 9.105 | 1 | 0.003 | 0.388 | 0.21 | 0.718 |
| Lower Resection margins | −0.291 | 0.081 | 12.81 | 1 | <0.0001 | 0.748 | 0.638 | 0.877 |
VEGF-C, vascular endothelial growth factor C; MOD, mean optical density; LVD, lymphatic vessel density; GEJA, gastro-oesophageal junction adenocarcinoma; DFS, disease-free survival.
Discussion
The status of lymph node metastasis is valuable for the prognosis of patients with GEJA. The accuracy of current methods for the diagnosis of lymph node metastasis, such as computed tomography and ultrasonography, is low (Rasanen et al. 2003). VEGF-C is a crucial regulator of the development of lymphatic vessels and the lymph node metastasis of tumours in animal models (Skobe et al. 2001; Karkkainen et al. 2004). In the present study, we examined the levels of VEGF-C expression in 128 GEJA tissue specimens and found variable levels of VEGF-C expression in 75.0% of GEJA specimens. Vascular endothelial growth factor C was expressed predominantly in the cytoplasm of tumour cells, and the average MOD value was 0.20 ± 0.08 in these samples. Further analysis revealed that the levels of VEGF-C expression were associated positively with the clinical stages and pathological degrees of GEJA in this population. However, the levels of VEGF-C expression were independent of age, gender of patients and the size and degrees of tumour differentiation in this population. These data further suggest that VEGF-C participates in the pathogenesis of GEJA, possibly for the lymph node metastasis of GEJA. Previous studies have shown that the levels of VEGF-C expression were positively correlated with the degrees of lymph node metastasis in other types of human cancers (Kabashima et al. 2001; Amioka et al. 2002; Takahashi et al. 2002; Su et al. 2006). Our data extend previous findings and support the notion that VEGF-C may participate in the pathogenic process of many types of solid tumours, particularly in gastrointestinal cancers. Therapeutic strategies to knock down VEGF-C expression may inhibit the lymph node metastasis and pathogenesis of GEJA.
We further examined the levels of lymphatic vessels in the GEJA tissue samples and found that all of the GEJA tissue sections were positive for anti-D2-40 staining, indicating the presence of lymphatic vessels in these specimens. Quantitative analysis revealed various levels of LVD in these specimens and that the values of LVD were positively associated with the clinical stages and pathogenic degrees of tumours, but not with age and gender of the patients, the size and differentiation degrees of tumours in this population. In addition, the values of LVD were positively correlated with the levels of VEGF-C expression in these tumour specimens, further indicating the importance of VEGF-C in the development of lymphatic vessels in GEJA tissues. Our data are consistent with a recent finding that the levels of VEGF-C expression in tumour cells are correlated with the contents of lymphangiogenesis in an animal model (Skobe et al. 2001). Given that the contents of lymphatic vessels in GEJA are crucial for the local lymph node and long distant metastasis of GEJA, the values of LVD may be valuable for the prediction of lymph node metastasis in GEJA.
In this study, we found that the levels of VEGF-C expression and the values of LVD were negatively associated with the length of DFS in this population. This, together with a positive correlation between the levels of VEGF-C expression and the values of LVD, suggests that either the levels of VEGF-C expression or the values of LVD may be a valuable predictor of the prognosis of patients with GEJA. Previous studies have demonstrated that the levels of VEGF-C expression are important for the prognosis of patients with ovarian and cervical cancer (Ueda et al. 2002; Nishida et al. 2004) and may help in predicting the beneficial effect of surgical therapy for patients with gastric cancers (Kabashima et al. 2001; Amioka et al. 2002; Takahashi et al. 2002). VEGF-C is a specific factor promoting lymphangiogenesis (Su et al. 2007), functioning as a ligand for tyrosine kinase receptor VEGFR-3 (Joukov et al. 1996). VEGF-C is expressed mainly in mesenchymal cells with the similar temporal and spatial pattern of VEGFR-3 in lymphatic endothelial cells. It is possible that VEGF-C expressed by tumour cells promotes lymphangiogenesis through interaction with its receptor in a paracrine mode in the tumours. Conceivably, VEGF-C may interact with its receptor on lymphatic endothelial cells and promote the formation of lymphatic vessels, leading to the lymphatic metastasis of GEJA. Therefore, our finding may provide new insights into the lymphatic metastasis of GELA.
Vascular endothelial growth factor C has been found to be an independent prognostic marker in colorectal, gastric, cervical and ovarian carcinomas (Ueda et al. 2002; Nishida et al. 2004). Another study indicates that VEGF-C is a significantly independent factor for the poor prognosis in patients with gastric cancer (Yonemura et al. 1999). We performed a multivariate Cox regression analysis of the factors contributing to a shorter DFS, including patients’ age and sex, histological type, TNF stage, tumour size, VEGF-C expression, LVD, total number of lymph nodes affected, lymph node metastasis, treatment method (surgery only or surgery + chemotherapy) and the distance between tumour and resection margin. We found that GEJA at N1 and N2 stages, at T4 stage, chemotherapy after surgery, high levels of VEGF-C expression and lower marginal resection were independent factors for the prognosis of DFS in patients with GEJA. Therefore, the levels of VEGF-C expression may be a valuable aid in the diagnosis of patients with lymph node metastatic GEJA.
In summary, our data indicated that the levels of VEGF-C expression were associated with the clinical stages and pathogenic degrees of GEJA and were positively correlated with the values of LVD in these specimens. Furthermore, the levels of VEGF-C expression and the values of LVD in these GEJA tissues were inversely associated with the lengths of DFS in patients with GEJA, and the levels of VEGF-C expression were one of the independent factors for the prognosis of patients with GEJA. We recognized that our study had limitations of a small sample size and the lack of molecular mechanisms by which VEGF-C promotes the lymphangiogenesis in GEJA. However, our novel data indicate that the levels of VEGF-C expression and the values of LVD in GEJA may be valuable for the diagnosis of lymphatic metastasis of GEJA. Our findings may provide new insights into the development of lymphatic metastasis of GEJA.
Acknowledgments
Grant support: This research was supported by grants from the National Nature and Science Foundation of China (NO. 81171994 and NO. 81071736), Nature and Science Foundation of Guangdong Province of China (NO. 10151503102000029) and Science Foundation of Cancer hospital, Shantou University Medical College for young scientist (NO. 2009A002). We would like to thank scientists from the Medjaden, a professional publication service company, for their help in proofreading the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Akagi K, Ikeda Y, Miyazaki M, et al. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br. J. Cancer. 2000;83:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amioka T, Kitadai Y, Tanaka S, et al. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur. J. Cancer. 2002;38:1413–1419. doi: 10.1016/s0959-8049(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am. J. Pathol. 1999;155:1967–1976. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Lymphangiogenesis and breast cancer metastasis. Histol. Histopathol. 2002;17:863–870. doi: 10.14670/HH-17.863. [DOI] [PubMed] [Google Scholar]
- Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am. J. Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Zhou GY, Zhang QH, et al. Lymphangiogenesis in gastric carcinoma correlates with prognosis. J. Pathol. 2009;218:192–200. doi: 10.1002/path.2523. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Joory KD, Levick JR, Mortimer PS, Bates DO. Vascular endothelial growth factor-C (VEGF-C) expression in normal human tissues. Lymphat. Res. Biol. 2006;4:73–82. doi: 10.1089/lrb.2006.4.73. [DOI] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- Kabashima A, Maehara Y, Kakeji Y, Sugimachi K. Overexpression of vascular endothelial growth factor C is related to lymphogenous metastasis in early gastric carcinoma. Oncology. 2001;60:146–150. doi: 10.1159/000055312. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- Nishida N, Yano H, Komai K, Nishida T, Kamura T, Kojiro M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer. 2004;101:1364–1374. doi: 10.1002/cncr.20449. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Jeltsch MM, Birkenhager R, et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- Omachi T, Kawai Y, Mizuno R, et al. Immunohistochemical demonstration of proliferating lymphatic vessels in colorectal carcinoma and its clinicopathological significance. Cancer Lett. 2007;246:167–172. doi: 10.1016/j.canlet.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Rasanen JV, Sihvo EI, Knuuti MJ, et al. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann. Surg. Oncol. 2003;10:954–960. doi: 10.1245/aso.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L, Terris B, Giostra E, Dousset B, Morel P, Pepper MS. Lymphatic vessel density and vascular endothelial growth factor-C expression correlate with malignant behavior in human pancreatic endocrine tumors. Clin. Cancer Res. 2004;10:6919–6928. doi: 10.1158/1078-0432.CCR-04-0397. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Su JL, Yang PC, Shih JY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Su JL, Yen CJ, Chen PS, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer. 2007;96:541–545. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher SG, Pisters PW, Komaki R, Lahoti S, Ajani JA. Gastroesophageal junction adenocarcinoma. Curr. Treat. Options Oncol. 2000;1:387–398. doi: 10.1007/s11864-000-0066-1. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kono K, Itakura J, et al. Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology. 2002;62:121–127. doi: 10.1159/000048257. [DOI] [PubMed] [Google Scholar]
- Tsurusaki T, Kanda S, Sakai H, et al. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br. J. Cancer. 1999;80:309–313. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Terai Y, Yamashita Y, et al. Correlation between vascular endothelial growth factor-C expression and invasion phenotype in cervical carcinomas. Int. J. Cancer. 2002;98:335–343. doi: 10.1002/ijc.10193. [DOI] [PubMed] [Google Scholar]
- Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res. Treat. 1995;36:169–180. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Zeng Z, Tang Z, et al. Increased expression of vascular endothelial growth factor-C and nuclear CXCR4 in hepatocellular carcinoma is correlated with lymph node metastasis and poor outcome. Cancer J. 2009;15:519–525. doi: 10.1097/PPO.0b013e3181c6aa6b. [DOI] [PubMed] [Google Scholar]
- Yonemura Y, Endo Y, Fujita H, et al. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin. Cancer Res. 1999;5:1823–1829. [PubMed] [Google Scholar]


