Abstract
Osteonecrosis of the jaw (ONJ) following the use of bisphosphonates has become of increased interest in the scientific community, due in particular to its as-yet-unsolved pathogenesis. An experimental model of ONJ was induced in normal male rats [alendronate (ALN); 1 mg/Kg/day; n = 10] and matched controls (saline solution; n = 10). After 60 days of drug treatment, all animals were subjected to extractions of the left first lower molars and were euthanized at 3 and 28 days postsurgery. The following analyses were performed: (i) descriptive and quantitative (scores) histological evaluation, (ii) stereometry of distal sockets and (iii) biochemical measurement of C-telopeptide cross-linked collagen type I (CTX) and bone-specific alkaline phosphatase (BALP). The results showed that 28 days postsurgery the animals treated with ALN had areas of exposed and necrotic bone, associated with significant infection, especially in the interalveolar septum area and crestal regions, compared with controls. The levels of CTX, BALP and bone volume, as well as the degrees of inflammation and vascularization, were significantly reduced in these animals. Therefore, analysis of the data presented suggests that ALN therapy is associated with the development of osteonecrosis in the jaws of rodents after tooth extraction.
Keywords: alendronate, osteonecrosis, tooth extraction
Bisphosphonates (Bps) are synthetic analogues of inorganic pyrophosphate that have strong properties for the suppression of osteoclast-mediated bone resorption. They have been widely used to stabilize the bone loss occasioned by bone disorders, such as osteoporosis and Paget's disease (Rogers et al. 2000). However, since 2003, increasing reports of osteonecrosis of the jaw (ONJ) induced by Bps have been described in the literature.
Several hypotheses concerning the pathogenesis of ONJ induced by Bps have been proposed, but none has been accepted completely. However, analysis of data retrieved from clinical studies suggests that some cofactors may play a relevant role in the development of these lesions, even in the presence of ONJ with no obvious comorbidity factors (Ruggiero et al. 2004; Marx et al. 2005).
Within this context, many investigators have attempted to reproduce this pathological condition in experimental animals. Indeed, models of ONJ-like lesions have been published in the literature, being developed by the association of Bps therapy, tooth extraction and other cofactors, such as concomitant steroid use (Sonis et al. 2009; Bi et al. 2010; Lopez-Jornet et al. 2010), vitamin D deficiency (Hokugo et al. 2010) or increased socket damage (Biasotto et al. 2010). However, the great number of variables could reduce the reliability of these models.
The purpose of this study was to develop a suitable experimental model of ONJ-like lesions by the association of only Bps therapy and tooth extraction, without other variable factors. Furthermore, we aimed to discuss critically relevant features that could justify the strong relationship between ONJ induced by Bps and tooth extraction.
Materials and methods
Animals and reagents
The study was approved by the Ethics in Animal Research Committee of the School of Dentistry of Araraquara (UNESP, Brazil) (protocol number 18/2009). It included 20 male rats (Rattus norvegicus, albinus, Holtzmann), each weighing around 200 g (8 weeks). These animals were kept in a special room at São Paulo State University, UNESP, School of Dentistry of Araraquara, and maintained on a 12:12 h light/dark cycle (lights on at 7:00 am) at 23 ± 2°C with ad libitum access to a standard laboratory diet and water.
The alendronate (ALN) was purchased from ALCON Laboratory (São Paulo, SP, Brazil). The drug was dissolved in sterile physiological saline (0.9% NaCl) and diluted to the given concentration.
Experimental design and surgical procedures
After a 3-day acclimatization period, animals were randomly assigned in two experimental groups: AL (n = 10), including animals treated with daily subcutaneous doses of ALN (1 mg/kg) (Jee et al. 2010), or control (CTL) (n = 10), comprised of animals subjected to sterile physiological saline, following the same schedule as AL group.
After 60 days of the pharmacologic therapy, all animals were subjected to left lower first molar (M1) extraction under general anaesthesia by a combination of ketamine chloridrate (Ketamina Agener; Agener União Ltda, São Paulo, SP, Brazil; 0.08 mL/100 g body weight) and xylazine 2% (Rompum; Bayer S.A., São Paulo, SP, Brazil; 0.04 mL/100 g body weight).
The same professional performed the tooth extractions with the same technique in all animals. Initially the rats were placed in a dorsal position and fixed in a special device. The surrounding gingivae were carefully detached from the lower first molars with a dental explorer. Then, with a Hollenback Carver, the tooth was luxated and separated into two segments (mesial and distal) that were removed with a forceps adapted around the cervical line of the segments.
After the surgical procedure, all animals received an intramuscular dose of antibiotic (Pentabiótico®; Wyeth-Whitehall Ltda, São Paulo, Brazil – 0.1 mg/Kg) and anti-inflammatory Ketoflex (ketoprofen 1.0%, 0.03 mL/rat). Animals were euthanized by anaesthesia overdose at 3 and 28 days after tooth extractions, and the ALN or sterile physiological saline administration protocol was maintained until the animals’ death.
Specimen processing
All tissue blocks were immersed directly in 10% buffered formalin fixative solution for 48 h. After that five specimens of each group/period were subjected to routine histological processing for descriptive and stereometric evaluation. All specimens were decalcified in tetrasodium-EDTA aqueous solution (0.5 M, pH 7.4) for 2–3 months, under agitation at room temperature. All specimens were then processed and included in paraffin blocks. Serial 4-μm sections were obtained in the buccolingual direction, stained with haematoxylin and eosin, and referred for evaluation by light microscopy (Leica DM1200M; Leica Microsystems, Wetzlar, Hessen, Germany).
Histological and stereometric analyses
One board-certified oral pathologist blinded to the group assignments performed these analyses in three distinct moments to minimize discrepancy in the scores (kappa index = 0.76). The histological endpoints were evaluated at four fields in each section (cervical and apical thirds of the mesial and distal sockets) using magnifications of 100×, 200×, 400× and 1000×. It included the degree of infection (bacteria proportion and extension of biofilm organization) and bone necrosis, which was defined as areas of empty osteocytic lacunae and heavily eroded surfaces. The inflammation was evaluated according to quantity and quality based on the density, type and distribution of inflammatory cell infiltrate (Sonis et al. 2009). Qualitatively, the inflammation was classified in acute (predominance of neutrophils infiltration), chronic (predominance of mononuclear cells infiltration, such as macrophages and lymphocytes) and mixed (presence of both cells of acute and chronic process in similar proportions). Furthermore, the degree of vascularisation (number of vessels) was also evaluated. These parameters were scored on a four-point scale: 0 (absent; 0%), 1 (mild; ≤10%), 2 (moderate; >10% and ≤50%) and 3 (increased; >50%).
The stereometric analysis was performed with Leica Application software suite 3.8.0 (Leica Microsystems LTD). The measurements were performed at the distal root of the left M1 at the regions of interest (ROIs) that included three different areas inside the socket. Initially, a standard ROI was determined by identification of a quadrangular area extending from the level of the cemento-enamel junction (CEJ) of the left second mandibular (M2) to the apical end level of the M2 and between the mesial and distal alveolar bone surfaces. This quadrangular area was divided into three equal ROIs (1, 2 and 3) (Figure 1).
Figure 1.
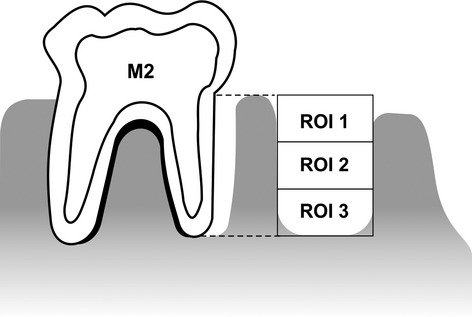
Schematic view of the regions of interest (ROIs) for stereometric analysis. The measurements were taken within the distal root of the left M1 at the ROIs that included three different areas inside the sockets. Initially, a standard ROI was determined by a quadrangular area extending from the level of the cemento-enamel junction (CEJ) of the left second mandibular (M2) to the apical end level of the M2 and between the mesial and distal alveolar bone surfaces. This quadrangular area was subsequently divided into three equal ROIs (1, 2 and 3).
The variables analysed included the percentage of the root socket filled with bone tissue (BV) in each ROI, following the nomenclature and abbreviations recommended by the American Society for Bone and Mineral Research (Parfitt 1988). Bone volume (%) represented bone volume (mm3) per total tissue volume (mm3). All analyses were performed at a magnification of 100×, and three measurements were taken for each specimen, to complete the stereometric analysis. The distance between the selected sections was 50 μm.
Assessment of bone turnover biochemical markers
Blood samples were collected on the day of sacrifice by cardiac puncture and centrifuged for plasma separation. The levels of serum collagen type 1 cross-linked C-telopeptide [collagen type I (CTX); sensitivity of 50 pg/ml; specificity to rats without significant cross-reactivity or interference; intracoefficient of variation (CV) = 6.34% and inter-CV = 8.49%] and bone-specific alkaline phosphatase (BALP; sensitivity of 20 μU/ml; specificity for rats without significant cross-reactivity or interference; intra-CV = 7.16% and inter-CV = 9.28%) were determined by enzyme-linked immunosorbent assay (ELISA) kits (CUSABIO BIOTECH CO., Ltd, Wuhan, China).
Statistical analysis
Data were initially subjected to the Kolmogorov–Smirnov normality test. We then performed comparisons among groups and periods for non-parametric data, using the Kruskal–Wallis test (non-parametric data), followed by Dunn's multiple-comparison test or analysis of variance (anova), then Tukey tests for parametric data. The data were evaluated by means of graphpad prism 5.0 (GraphPad Software, San Diego, CA, USA), and statistical significance was set at P < 0.05 with 95% confidence intervals.
Results
Body weight gain
All animals showed decrease in body weight 3 days after tooth extraction compared with baseline. At 7 and 14 days, both groups exhibited a tendency to increase in weight compared with the 3-day period, and at 28 days after tooth extractions all animals showed a slight decrease in the mean body weight compared with the 14-day measurements. No significant differences were observed between CTL and AL groups at any period (Figure 2).
Figure 2.
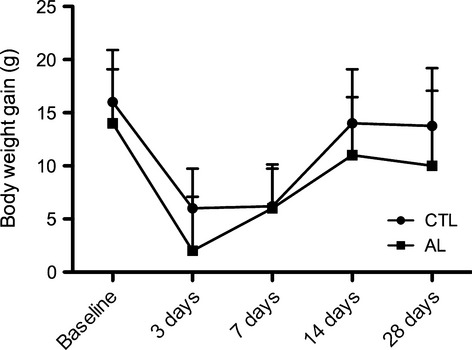
Mean of body weight gain (g) of groups AL and CTL after surgical procedure. Baseline represents the mean of the body weight gain (g) on the day of teeth extractions. All animals tended to a decrease in body weight gain after 3 days of teeth extractions compared with baseline. At 7 and 14 days, both groups showed a tendency to an increase in the measures compared with 3-day period, and at 28 days after tooth extractions, all animals presented a slight decrease in the mean body weight gain compared with 14-day measures. No significant differences were found between CTL and AL groups in any periods.
Clinical features
Twenty-eight days after tooth extraction all animals of the AL group showed partial epithelial coverage, exhibiting areas of bone exposure. In contrast, control animals showed total epithelial coverage, without signs of inflammation (Figure 3). Furthermore, four animals in the AL group exhibited areas of exposed bone around the upper incisors, which were associated with coronal fractures (Figure 4). These fractures were diagnosed by clinical examination that revealed a complete loss in tooth continuity involving the crown of upper incisors.
Figure 3.
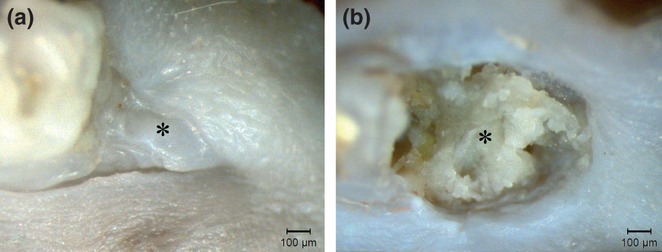
Clinical aspects of a lower first molar (M1) alveolar socket 28 days after tooth extraction. Animals treated with sterile physiological saline (a) presented a full epithelial lining on the alveolar socket, with no signs of inflammation or infection (asterisk). In contrast, animals treated with alendronate (b) exhibited significant areas of exposed bone with a marked impairment of alveolar socket re-epithelialization (asterisk).
Figure 4.
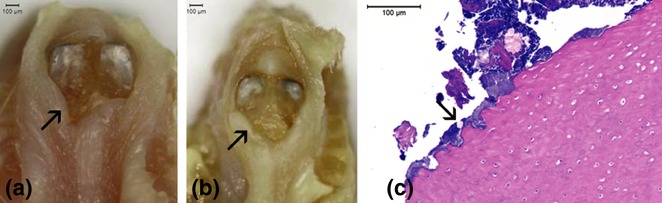
Clinical aspects of bone lesions around upper incisors of animals treated with alendronate (a, b). Some animals of the AL group presented, at euthanasia, areas of exposed bone surrounding the upper incisors (arrow). Of note, these lesion areas were associated, in all animals, with coronal fractures. Histological sections of these lesions (c) showing areas of bone necrosis and infection (arrow; original magnification × 100).
Histological features
Three days after tooth extraction, all groups exhibited areas of necrotic bone and infection, especially at the interalveolar septum area (Figure 5). However, animals treated with ALN presented significantly increased necrotic bone at 28 days (P < 0.01), at the interalveolar septum and crest areas, while control animals displayed a statistically significant decrease in the same period (P < 0.01) (Figure 6 and 7a). Furthermore, the CTL animals also exhibited statistically significantly decreased infection at 28 days (P < 0.001), while the AL group did not (Figure 7b). At this period, these features (bone necrosis and infection) were also observed around the upper incisors area of some animals treated with ALN (Figure 4c).
Figure 5.
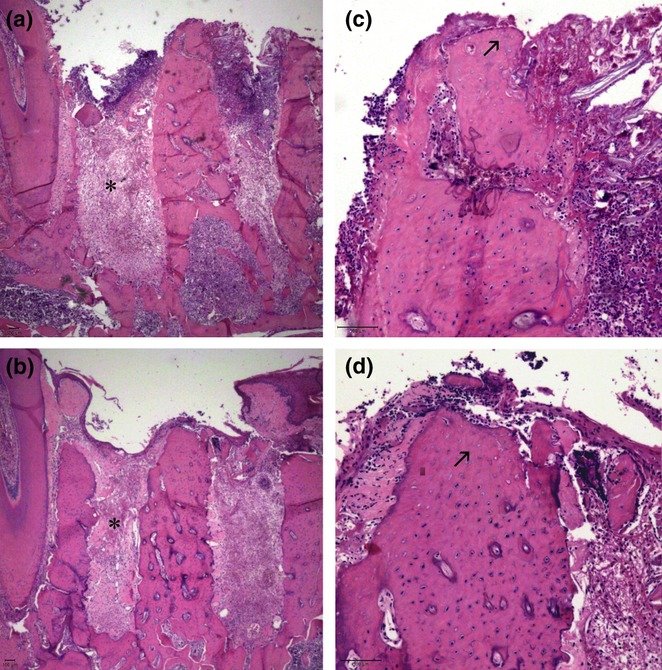
Histological sections of tooth sockets 3 days after left lower first molar extraction and stained with haematoxylin and eosin. Both the CTL (a) and AL (b) groups presented granulation tissue formation at this time (asterisk; original magnification × 25), as well as empty osteocyte lacunae at superior areas of the inter-radicular septum (arrow) (c, CTL; d, AL; original magnification × 100).
Figure 6.
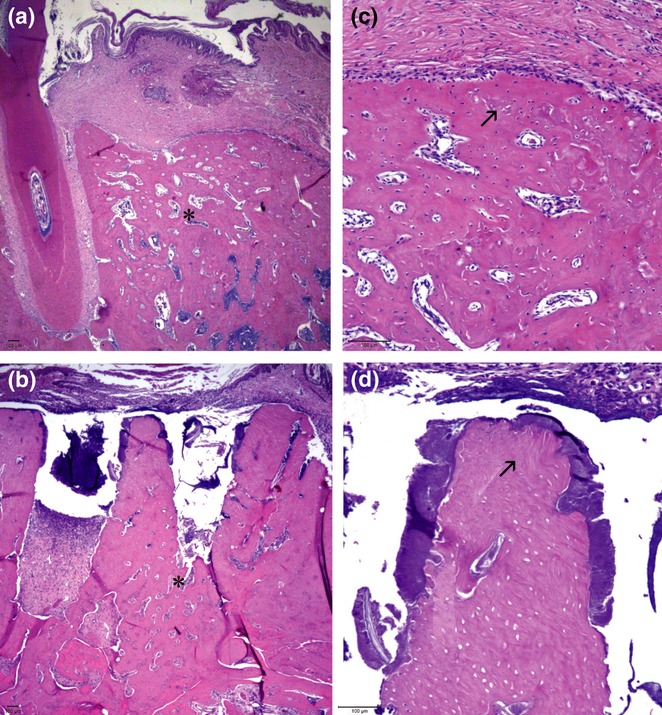
Histological sections of tooth sockets 28 days after left lower first molar extraction and stained with haematoxylin and eosin. (a) Animals in the CTL group presented complete alveolar bone regeneration, while animals in the AL group (b) exhibited only slight bone formation, restricted to the apical socket area (asterisk; original magnification × 25). The animals treated with alendronate (d) demonstrated retention of the inter-radicular septum, which was associated with bone necrosis and infection, while in the CTL (c) group, this area was completely remodelled, with signs of bone necrosis (arrow; original magnification × 100).
Figure 7.
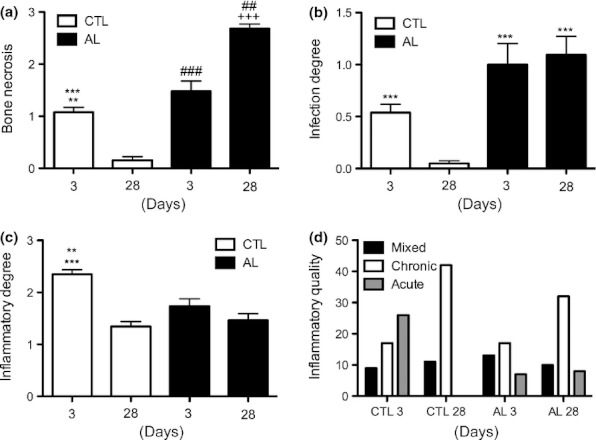
The effects of alendronate (ALN) therapy on bone necrotic levels (a), degrees of infection (b), degree (c) and quality (d) of inflammation at 3 and 28 days after tooth extraction. (a) Animals treated with ALN developed a higher proportion of bone necrosis compared with matched controls (**P < 0.01 in relation to CTL 28 days; ##P < 0.01 in relation to AL 3 days; ***P < 0.001 in relation to AL 28 days; ###P < 0.001 in relation to CTL 28 days and AL 28 days; +++P < 0.001 in relation to CTL 28 days). (b) Animals treated with ALN presented an increased degree of infection compared with matched controls (***P < 0.001 in relation to CTL 28 days). (c) At 3 days after tooth extraction, animals in the CTL group presented a higher degree of inflammatory response compared with all groups and periods (***P < 0.001 in relation to CTL 28 days and AL 3 days; **P < 0.01 in relation to AL 28 days).
Regarding inflammation, animals in the control group exhibited scores significantly higher at 3 days compared with those in the AL group (P < 0.01), and the scores decreased markedly at 28 days only in control animals (P < 0.001), while no statistical changes were observed in the AL group (Figure 7c). Furthermore, while inflammation was predominantly acute at 3 days in control animals, it was mainly chronic in the AL group in the same time. At 28 days, both groups presented characteristic chronic inflammation (Figure 7d).
No important differences in vascularization were found between the groups at 3 days. However, at 28 days, animals treated with ALN presented significantly less vascularization compared with the control animals (P < 0.01). Furthermore, while this process increased from 3 to 28 days in control animals (P < 0.05), no differences were observed in the AL group in the same periods (Figure 8a).
Figure 8.
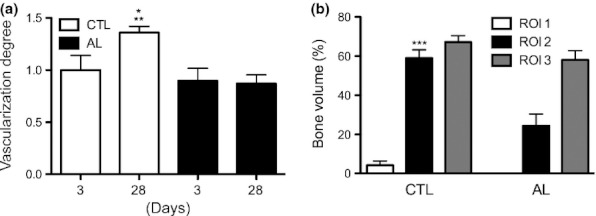
Degree of vascularization (a) and bone volume (%) (b) in experimental groups. (a) At 28 days after tooth extraction, animals in the CTL group presented the highest levels of vascularization compared with all groups and periods (*P < 0.05 in relation to CTL 3 days and AL 3 days; **P < 0.01 in relation to AL 28 days). (b) The region of interest 2 (ROI 2) of CTL animals exhibited a statistically significant increase in bone volume compared with the ROI 2 of animals treated with alendronate (***P < 0.001 in relation to ROI 2 AL). Furthermore, no significant differences were found in other ROIs.
Regarding the BV count, we observed that animals of the AL group showed rates significantly lower than those in the control group at ROI 2 (P < 0.001), while no statistically significant differences were found in ROIs 1 and 3 (Figure 8b).
Bone metabolism markers
Animals treated with ALN had the lowest values of BALP at both 3 (P < 0.05) and 28 days (P < 0.001) when compared with control animals. This same tendency was observed for CTX levels (P < 0.05) (Figure 9).
Figure 9.
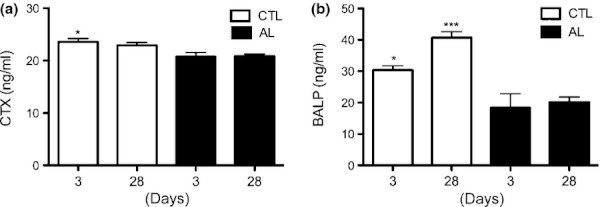
Levels of collagen type I (CTX) (a) and bone-specific alkaline phosphatase (BALP) (b) in experimental groups at 3 and 28 days after tooth extractions. In the CTX analysis (a), animals in the CTL group presented levels statistically significantly higher at 3 days compared with all periods for group AL (*P < 0.05 in relation to 3 and 28 days AL). At 28 days, no significant differences were found between groups and periods. In the BALP analysis (b), at 3 days, significant differences were found between the CTL and AL groups (*P < 0.05 in relation to 3 days AL), and at 28 days, animals in CTL exhibited increased levels of BALP compared with all periods for the AL group (***P < 0.001 in relation to AL periods).
Discussion
The development of animal models of pathological conditions has several advantages for the study of disease. As can be seen with the increasing reports of ONJ induced by Bps from around the world, many efforts have been made to obtain experimental models of these lesions. Only few papers describe the development of bone lesions associated with Bps and trigger agents (Bi et al. 2010; Aghaloo et al. 2011) while most of the other studies are associated with many different variables, including Bps therapy, tooth extraction, concomitant steroid use (Sonis et al. 2009), vitamin D deficiency (Hokugo et al. 2010) or increased damage to the alveolar socket (Biasotto et al. 2010). Thus, in an attempt to increase the model's reliability, we reduced the number of variables and demonstrated the development of experimental ONJ-like lesions using only the combination of Bps therapy and tooth extraction.
Alendronate is a Bps that has been widely used to stabilize the bone loss occasioned by osteoporosis (Rogers et al. 2000). Based on the bioavailability of ALN – about 1% by oral route and almost 100% following subcutaneous (sc) administration (Lin 1996) – the rat models of osteoporosis have been using sc dosages ranging from 30 to 70 μg/kg/week (Fuchs et al. 2007; Aguirre et al. 2010; Nakao et al. 2011; Wang et al. 2011). In fact, these doses are significantly lower compared with the one used in our study (7000 μg/kg/week); however, our first purpose was to establish a model of bone turnover suppression in healthy animals, based on the theory that, with reduced turnover, we would observe an increase in the numbers and sizes of matrix necrosis areas in the jaws (Allen & Burr 2008).
Thus, aiming to create a favourable environment for the development of ONJ, we prescribed long-term therapy with high non-toxic dosages of ALN (Lin 1996), based on the following assumptions: (i) the magnitude of the reduction in bone turnover is closely related to the total Bps dose administered over a long period of time (Chapurlat & Delmas 2006); (ii) the bone absorption of Bps follows a linear pattern until the administration of intravenous doses of 5 mg/kg (Lin 1996), with no evidence of bone saturation or pharmacological interference with subsequent doses (Porras et al. 1999) and c) the rapid metabolic rate of rats, particularly young rats (Lin et al. 1992).
Indeed, our findings of the lowest levels of CTX and BALP, which are markers of bone metabolism (Pagani et al. 2005), in the AL group confirm the suppression of bone metabolism. Furthermore, the relevance of the Bps treatment schedule used in our study is supported by the findings of transient impairment (Hikita et al. 2009; Aguirre et al. 2010) or even the promotion of the healing action of Bps (Jee et al. 2010) when lower cumulative dosages were associated with tooth extraction, in contrast to the prolonged bone lesions observed in our study. Therefore, the Bps schedule seems to be closely related to ONJ pathogenesis.
Although ONJ induced by Bps can occur spontaneously (Conte-Neto et al. 2011), most of the reports have been associated with surgical procedures (Ruggiero et al. 2009). In this study we opted to use tooth extraction as the trigger agent, based on the fact that analysis of data retrieved from clinical and in vivo studies (Thumbigere-Math et al. 2009; Treister et al. 2009; Bi et al. 2010; Lopez-Jornet et al. 2010) suggested a strong relationship between tooth extraction and ONJ induced by Bps. Some authors have reported that this kind of bone necrosis may be related to the impairment of bone capacity to accommodate the increasing demand for healing that is required in situations of tissue trauma such as surgical procedures and infection (Woo et al. 2005).
Indeed, our study confirmed that tooth extraction appears to be relevant to the development of ONJ-like disease. Of note, we also demonstrated some bone lesions around upper incisors of ALN-treated animals that, curiously, were present only in the animals with coronal tooth fractures. Based on the fact that these animals constantly chewed their cages, some hypothesis can be stated. Regarding to the tooth fractures, it is possible that the chewing habits represent periods of cyclic and sustained loading, which may have increased the risks of cracks that can lead to tooth fractures due to failure of mechanisms related to fatigue that are well-recognized as pathways of dental fractures (Kruzic & Ritchie 2008).
In relation to the bone lesions, it is known that when occlusal forces exceed the adaptive capacity of the periodontal tissue, injuries may occur and the compromised bone is usually resorbed (Gher 1998). However, with the use of Bps, this damaged bone is not replaced by vital tissue, which could lead to the accumulation of bone lesions. Furthermore, the treatment with Bps results in increased bone density, especially after long-term use with high dosages, which also makes the tissue more prone to microcrack initiation (Currey et al. 1996). Taken together, these factors could act in favour of the ONJ lesions development according to the bone suppression theory and supporting the role of trauma as an ONJ trigger factor (Woo et al. 2005).
In contrast, considering the environment and dynamics of alveolar socket healing, we believed that, besides trauma, other factors, such as infection and ischaemic conditions, might also explain the strong relationship between tooth extraction and ONJ induced by Bps. This is based on the potential of Bps to impair several physiological events that occur after tooth extraction, including inflammation (Breuil & Euller-Ziegler 2006), angiogenesis, resorption activity and epithelial migration (Kobayashi et al. 2010). Therefore, Bps not only impairs tissue scaffold re-establishment, but also leaves the organism more vulnerable to occasional harmful effects of micro-organisms and their by-products.
In this study, we demonstrated that Bps therapy is associated with an important impairment of vascularization in the later stages of alveolar healing. These findings can be reasonably explained by the inhibitory effects of Bps on angiogenesis (Fournier et al. 2002; Kobayashi et al. 2010), which is of particular interest considering that ONJ lesions could be a result of ischaemic changes to tissues (El-Salem et al. 2011).
In contrast, Aguirre et al. (2010), using low doses of ALN, observed only a transient impairment of angiogenesis after tooth extraction. These differences indicate that Bps could exert a dose-dependent effect on vascularization, and no anti-angiogenic effect is observed with clinical dosing regimens (Biver et al. 2010). Furthermore, considering that at 3 days no differences in the degree of vascularization were found in either group, the 8-week pretreatment with ALN did not seem to interfere significantly with angiogenesis, at least in this phase.
It is well-known that inflammation is a natural step in the wound-healing process, being important for the removal of contaminating micro-organisms (Guo & Dipietro 2010). Indeed, after tooth extraction, we showed that the exposed bone, especially at the inter-radicular region, naturally became infected, regardless of the administration of Bps. However, in the AL group, this infection was present even in later periods.
Possible explanations for these features may be related to the changes in the inflammatory response induced by Bps. Some authors have stated that Bps may exert anti-inflammatory effects, decreasing the release of pro-inflammatory cytokines (Breuil & Euller-Ziegler 2006) and depressing the innate immune system for a prolonged time because of an impairment of neutrophils (Kuiper et al. 2011). Indeed, our findings demonstrated that the inflammatory response at 3 days was chronic instead of acute and significantly lower in animals treated with ALN. This is of special concern, as neutrophils are the first line of defence against micro-organisms, and a critical concentration of these cells is required for the effective killing of bacteria (Li et al. 2002).
In the context of infection the dynamics of osteoclast activity during alveolar socket healing seems to be relevant. Soon after tooth extraction a high degree of resorption takes place within the sockets to remove necrotic bone and bone debris (Lin et al. 1994). In this way, the inhibitory effects of Bps on osteoclasts, especially at the interdental alveolar area, allow for bone exposure to an environment rich in bacterial toxins, inflammatory cytokines and oxidative stress (Aghaloo et al. 2011). Indeed, we observed a high degree of bone necrosis and infection at the interdental alveolar area. Of note, necrotic bone fragments themselves can act as avascular foci for further bacterial adherence (Rodner et al. 2003), which could also be facilitated by Bps, as it has been demonstrated that ONJ could result from increased bacterial adhesion to bone coated with these drugs (Kos 2011).
Another condition that could act in favour of infection is the soft tissue impairment observed in animals treated with ALN, given the essential role of epithelial coverage protection against oral bacterial infection (Kawahara et al. 1998). After tooth extraction there is naturally a high demand for oral epithelial cell migration, because healing occurs by secondary intention. This aspect is of special concern in the field of Bps, because these drugs inhibit the migration of oral epithelial cells (Landesberg et al. 2008; Kobayashi et al. 2010). In contrast, considering that the exposed bone areas were mainly located close to the infection, the potential impairment of bacteria and their by-products and their effects on soft tissue healing should not be taken for granted, because infection can also impair epithelial cell growth (Pollanen et al. 1997).
Within this context a relevant point to be discussed is the diagnostic criteria for ONJ induced by Bps. The persistence of bone exposure in the oral cavity for at least 2 months has been recognized as an established diagnostic criterion (Ruggiero et al. 2009), and this concept has been extended to animal models of ONJ-like lesions (Bi et al. 2010; Biasotto et al. 2010; El-Salem et al. 2011). However, given the inherent metabolic differences between species, we believe that clinical human concepts might not be applicable to animals.
Although the alveolar healing phases are similar in both humans and rats, they occur more rapidly in rodents than in humans (Bodner et al. 1993), lasting about one-third of the time required for human healing (Okamoto & de Russo 1973). Therefore, the 4-week bone exposure in rats observed in this study could be considered a clinical equivalent concept of ONJ induced by Bps in rats. To support this assumption, studies have shown transient impairment of Bps in alveolar socket healing, and these effects have not been extended beyond 14 days (Aguirre et al. 2010).
The effects of Bps on osteogenesis are not yet well-established in the literature. Our findings demonstrated that, 28 days after tooth extraction, the ALN-treated animals showed the lowest levels of bone volume at the ROI2. These features are in accordance with our outcomes of lower levels of BALP in the ALN group, which is in agreement with authors who found inhibitory effects of ALN over osteogenesis (Kobayashi et al. 2010).
In contrast, other authors did not find evidence of the direct effects of Bps on the ability of osteoblasts to produce bone matrix in vivo (Allen et al. 2006; Feher et al. 2010), which supports our results of similar bone volume levels at apical regions in both groups. Therefore, it is also possible that Bps indirectly reduced bone volume, delaying the initial steps of wound healing, because osteogenesis begins at the apical region, where statistically significant differences were not observed between groups, as previously stated.
In conclusion, we believe that infection may be closely related to lesion development. Indeed, the environment of tooth extraction in the field of Bps treatment is favourable to the development of infection, for several reasons: (i) lower inflammation response and degree of vascularization; (ii) increased bacterial adhesion to bone coated with Bps and (iii) maintenance of exposed bone to the oral cavity because of resorption and epithelial coverage impairment, which could act as a substrate for bacterial growth.
Within the limitations of this study, our results clearly demonstrated that the association of ALN therapy and tooth extraction was able to induce ONJ-like lesions in rodents. Further studies are necessary to investigate whether lower dosages of Bps, compatible with therapeutic doses, could also be related to the development of bone lesions.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aghaloo TL, Kang B, Sung EC, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J. Bone Miner. Res. 2011;26:1871–1882. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010;16:674–685. doi: 10.1111/j.1601-0825.2010.01677.x. [DOI] [PubMed] [Google Scholar]
- Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J. Oral Maxillofac. Surg. 2008;66:987–994. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MR, Follet H, Khurana M, Sato M, Burr DB. Antiremodeling agents influence osteoblast activity differently in modeling and remodeling sites of canine rib. Calcif. Tissue Int. 2006;79:255–261. doi: 10.1007/s00223-006-0031-5. [DOI] [PubMed] [Google Scholar]
- Bi Y, Gao Y, Ehirchiou D, et al. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am. J. Pathol. 2010;177:280–290. doi: 10.2353/ajpath.2010.090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasotto M, Chiandussi S, Zacchigna S, et al. A novel animal model to study non-spontaneous bisphosphonates osteonecrosis of jaw. J. Oral Pathol. Med. 2010;39:390–396. doi: 10.1111/j.1600-0714.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Biver E, Vieillard MH, Cortet B, Salleron J, Falgayrac G, Penel G. No anti-angiogenic effect of clinical dosing regimens of a single zoledronic acid injection in an experimental bone healing site. Bone. 2010;46:643–648. doi: 10.1016/j.bone.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Bodner L, Kaffe I, Littner MM, Cohen J. Extraction site healing in rats. A radiologic densitometric study. Oral Surg. Oral Med. Oral Pathol. 1993;75:367–372. doi: 10.1016/0030-4220(93)90153-u. [DOI] [PubMed] [Google Scholar]
- Breuil V, Euller-Ziegler L. Bisphosphonate therapy in rheumatoid arthritis. Joint Bone Spine. 2006;73:349–354. doi: 10.1016/j.jbspin.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Chapurlat RD, Delmas PD. Drug insight: bisphosphonates for postmenopausal osteoporosis. Nat. Clin. Pract. Endocrinol. Metab. 2006;2:211–219. doi: 10.1038/ncpendmet0121. [DOI] [PubMed] [Google Scholar]
- Conte-Neto N, Bastos AS, Spolidorio LC, Marcantonio RA, Marcantonio E., Jr Oral bisphosphonate-related osteonecrosis of the jaws in rheumatoid arthritis patients: a critical discussion and two case reports. Head Face Med. 2011;7:1–7. doi: 10.1186/1746-160X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey JD, Brear K, Zioupos P. The effects of ageing and changes in mineral content in degrading the toughness of human femora. J. Biomech. 1996;29:257–260. doi: 10.1016/0021-9290(95)00048-8. [DOI] [PubMed] [Google Scholar]
- El-Salem K, Ababneh B, Rudnicki S, et al. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve. 2011;44:877–881. doi: 10.1002/mus.22205. [DOI] [PubMed] [Google Scholar]
- Feher A, Koivunemi A, Koivunemi M, et al. Bisphosphonates do not inhibit periosteal bone formation in estrogen deficient animals and allow enhanced bone modeling in response to mechanical loading. Bone. 2010;46:203–207. doi: 10.1016/j.bone.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Fournier P, Boissier S, Filleur S, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544. [PubMed] [Google Scholar]
- Fuchs RK, Shea M, Durski SL, Winters-Stone KM, Widrick J, Snow CM. Individual and combined effects of exercise and alendronate on bone mass and strength in ovariectomized rats. Bone. 2007;41:290–296. doi: 10.1016/j.bone.2007.04.179. [DOI] [PubMed] [Google Scholar]
- Gher ME. Changing concepts. The effects of occlusion on periodontitis. Dent. Clin. North Am. 1998;42:285–299. [PubMed] [Google Scholar]
- Guo S, Dipietro LA. Factors affecting wound healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S. Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J. Bone Miner. Metab. 2009;27:663–672. doi: 10.1007/s00774-009-0090-6. [DOI] [PubMed] [Google Scholar]
- Hokugo A, Christensen R, Chung EM, et al. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J. Bone Miner. Res. 2010;25:1337–1349. doi: 10.1002/jbmr.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee JH, Lee W, Lee BD. The influence of alendronate on the healing of extraction sockets of ovariectomized rats assessed by in vivo micro-computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;110:e47–e53. doi: 10.1016/j.tripleo.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Kawahara D, Hashimoto K, Takashima Y, Ong JL. Morphologic studies on the biologic seal of titanium dental implants. Report I. In vitro study on the epithelialization mechanism around the dental implant. Int. J. Oral Maxillofac. Implants. 1998;13:457–464. [PubMed] [Google Scholar]
- Kobayashi Y, Hiraga T, Ueda A, et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J. Bone Miner. Metab. 2010;28:165–175. doi: 10.1007/s00774-009-0128-9. [DOI] [PubMed] [Google Scholar]
- Kos M. Bisphosphonates promote jaw osteonecrosis through facilitating bacterial colonisation. Med. Hypotheses. 2011;77:214–215. doi: 10.1016/j.mehy.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Kruzic JJ, Ritchie RO. Fatigue of mineralized tissues: cortical bone and dentin. J. Mech. Behav. Biomed. Mater. 2008;1:3–17. doi: 10.1016/j.jmbbm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, Forster C, Sun C, Peel S, Glogauer M. Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br. J. Pharmacol. 2011;165:532–539. doi: 10.1111/j.1476-5381.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landesberg R, Cozin M, Cremers S, et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J. Oral Maxillofac. Surg. 2008;66:839–847. doi: 10.1016/j.joms.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Karlin A, Loike JD, Silverstein SC. A critical concentration of neutrophils is required for effective bacterial killing in suspension. Proc. Natl Acad. Sci. USA. 2002;99:8289–8294. doi: 10.1073/pnas.122244799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- Lin JH, Chen IW, Duggan DE. Effects of dose, sex, and age on the disposition of alendronate, a potent antiosteolytic bisphosphonate, in rats. Drug Metab. Dispos. 1992;20:473–478. [PubMed] [Google Scholar]
- Lin WL, McCulloch CA, Cho MI. Differentiation of periodontal ligament fibroblasts into osteoblasts during socket healing after tooth extraction in the rat. Anat. Rec. 1994;240:492–506. doi: 10.1002/ar.1092400407. [DOI] [PubMed] [Google Scholar]
- Lopez-Jornet P, Camacho-Alonso F, Molina-Minano F, Gomez-Garcia F, Vicente-Ortega V. An experimental study of bisphosphonate-induced jaws osteonecrosis in Sprague-Dawley rats. J. Oral Pathol. Med. 2010;39:697–702. doi: 10.1111/j.1600-0714.2010.00927.x. [DOI] [PubMed] [Google Scholar]
- Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Nakao S, Minamide A, Kawakami M, Boden SD, Yoshida M. The influence of alendronate on spine fusion in an osteoporotic animal model. Spine (Phila Pa 1976) 2011;36:1446–1452. doi: 10.1097/BRS.0b013e3181f49c47. [DOI] [PubMed] [Google Scholar]
- Okamoto T, de Russo MC. Wound healing following tooth extraction. Histochemical study in rats. Rev. Fac. Odontol. Aracatuba. 1973;2:153–169. [PubMed] [Google Scholar]
- Pagani F, Francucci CM, Moro L. Markers of bone turnover: biochemical and clinical perspectives. J. Endocrinol. Invest. 2005;28:8–13. [PubMed] [Google Scholar]
- Parfitt AM. Bone histomorphometry: standardization of nomenclature, symbols and units (summary of proposed system) Bone. 1988;9:67–69. doi: 10.1016/8756-3282(88)90029-4. [DOI] [PubMed] [Google Scholar]
- Pollanen MT, Overman DO, Salonen JI. Bacterial metabolites sodium butyrate and propionate inhibit epithelial cell growth in vitro. J. Periodontal Res. 1997;32:326–334. doi: 10.1111/j.1600-0765.1997.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Porras AG, Holland SD, Gertz BJ. Pharmacokinetics of alendronate. Clin. Pharmacokinet. 1999;36:315–328. doi: 10.2165/00003088-199936050-00002. [DOI] [PubMed] [Google Scholar]
- Rodner CM, Browner BD, Pesanti E. Chronic osteomyelitis. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, editors. Skeletal Trauma. Philadelphia, PA: WB Saunders; 2003. pp. 483–506. [Google Scholar]
- Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J. Oral Maxillofac. Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw – 2009 update. Aust. Endod. J. 2009;35:119–130. doi: 10.1111/j.1747-4477.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009;45:164–172. doi: 10.1016/j.oraloncology.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Thumbigere-Math V, Sabino MC, Gopalakrishnan R, et al. Bisphosphonate-related osteonecrosis of the jaw: clinical features, risk factors, management, and treatment outcomes of 26 patients. J. Oral Maxillofac. Surg. 2009;67:1904–1913. doi: 10.1016/j.joms.2009.04.051. [DOI] [PubMed] [Google Scholar]
- Treister N, Sheehy N, Bae EH, Friedland B, Lerman M, Woo S. Dental panoramic radiographic evaluation in bisphosphonate-associated osteonecrosis of the jaws. Oral Dis. 2009;15:88–92. doi: 10.1111/j.1601-0825.2008.01494.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang P, Tang PF, Chan KM, Li G. Alendronate (ALN) combined with osteoprotegerin (OPG) significantly improves mechanical properties of long bone than the single use of ALN or OPG in the ovariectomized rats. J. Orthop. Surg. Res. 2011;6:34. doi: 10.1186/1749-799X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SB, Hande K, Richardson PG. Osteonecrosis of the jaw and bisphosphonates. N. Engl. J. Med. 2005;353:99–102. [PubMed] [Google Scholar]


