Abstract
Purpose
To study the feasibility, learning curve, and safety of fetoscopy, so that fetal surgery can be confidently performed in ongoing pregnancies.
Methods
Fetoscopy was performed at 12–20 weeks of gestation, in 12 women with fetal congenital malformations and/or for termination of pregnancy, under local anesthesia using fine fetoscopes ranging from 1 to 2-mm diameter. The fetal parts and placenta were examined for clarity of vision, identification, and anomalies.
Results
Fetoscopy required great skill, patience, and extensive use of ultrasound for correct orientation. Visualization was better with endoscope of 2-mm diameter. Laser coagulation of placental vessels using diode laser system was possible in the last two cases. There were no major complications.
Conclusions
Fetal endoscopy is a feasible procedure, safe in experienced hands but has an appreciable learning curve.
Keywords: Fetoscopy, Ultrasound, Endoscope, Laser
Introduction
Prenatal ultrasound and wide implementation of antenatal screening programs allow for the timely diagnosis of many fetal congenital anomalies, which, if left untreated, could be fatal or cause severe disabilities despite optimal postnatal care. Some of them are amenable to intrauterine fetal surgical interventions. In most parts of the world, open fetal surgery is poorly accepted because of its invasiveness and the high incidence of premature labor and rupture of the fetal membranes. In the 1990s, the merger of fetoscopy and advanced video-endoscopic surgery formed the basis for endoscopic fetal surgery. Minimally invasive techniques, “Fetendo (fetal endoscopy)/Fetoscopy” are now real possibilities. This procedure offers access to the placental surface, umbilical cord, and fetus using small endoscopes placed percutaneously through the mother’s abdominal wall, under ultrasound guidance.
Fetoscopy requires special endoscopes with their respective sheaths, cannulas, and additional instruments, designed for the procedure of interest, and most gynecologists are therefore not familiar with them [1]. The technique was first used on women undergoing Medical Termination of Pregnancy (MTP), or to exclude external malformations in ongoing pregnancies [2–4] for an “adequate learning curve” approved by the ethical committees. As with intrauterine prenatal diagnostic procedures like amniocentesis, chorion villus sampling, cord blood sampling, intrauterine fetal blood transfusion and shunts, the procedure of fetoscopy ethically should be “learnt”; expertise and confidence gained—first by observation and then by performing the procedure in MTP cases; and not training directly on ongoing pregnancies, thereby putting the pregnancy and fetus in jeopardy. Studies are going on with regard to the appropriate operating timing, standardization of selection criteria, and multicenter randomized trials are also ongoing to define whether fetal surgery is associated with better outcomes as compared with postnatal treatment [5, 6].
The aim of the study was to establish the feasibility, learning curve of fetoscopy for clear visualization of fetal, placental external features, and congenital malformations in utero, so as to become skilled and confident in the manipulations before embarking on fetal surgery in high-risk ongoing pregnancies.
Methods
Institute ethical clearance was obtained for starting the procedure in the Department. Informed written consent was taken from the couple. Women with pregnancies more than 20 weeks of gestation, with previous uterine scar, medical disorder, Rh negative, or with any complication like leaking or bleeding per vaginum, and oligohydramnios were excluded from the study.
Fetoscopy was attempted in 12 pregnancies with fetal congenital malformations, and/or for MTP) between 12 and 20 weeks of gestation. Fine fetal endoscopes (fetoscopes) of varying and increasing sizes 1 mm/1.2 mm/2 mm were used in different patients.
The women were first sedated with pethidine or calmpose injection in the operation theater, with an Anesthetist as standby. Ultrasound was performed to identify the placental site, and fetal position to mark out the fetoscope entry point for the best visualization of fetus and placenta. Local anesthesia was given over the maternal abdomen and a small 3-mm incision made with fine tip knife. The trocar and cannula were introduced under ultrasound guidance, preferably avoiding the placenta, through the maternal abdomen and uterine wall into the amniotic cavity. The cannula was removed and the fetoscope introduced through the trocar to visualize and examine the fetal face (eyes, lips, nose, and mouth) the limbs (and fingers/toes), the abdominal wall, and umbilical cord insertion, the back, etc. The placenta was examined for placental vessels, or any abnormalities. The clarity of vision, intra-amniotic bleeding, or any procedural problems were noted. Visibility was maintained with continuous saline amnioinfusion system, monitoring the volume infused.
In two, cases, laser coagulation of placental vessels were tried. The placental vessels were identified fetoscopically, and the ones distant from the uterine wall were chosen. Diode laser fiber was introduced through the operating channel of the fetoscope and power switched on. The laser beam was focused on the vessel for few seconds till the vessel was seen to become white and blanched.
All cases had MTP after 48 h with use of vaginal misoprostol (9 cases), oral mifepristone and vaginal misoprostol (2 cases) and intrauterine PGF2 alpha (1 case).
Procedural problems and complications were noted during and after fetoscopy.
Results
Experience of fetoscopy in 12 pregnant women, period of gestation (POG), and position of the placenta are described in Table 1. The very fine 1–1.2 mm fetoscopes used in the initial four cases showed out of focus and hazy view. Visualization of fetal external body parts, umbilical cord, placental sinuses, and vessels were quite clear and satisfactory with endoscopes of 2-mm diameter (Table 2, Figs. 1, 2, 3). It was quite formidable and confusing initially to orient the ultrasound picture to the fetoscopically viewed structures, the right to left sides in utero, distinguishing uterine wall from placental edge and surface, etc. but became easier with increasing number of cases performed and experience—the “learning curve.” Fetoscopy required great skill, patience, and extensive use of ultrasound for correct orientation in the initial 3–4 cases, but dexterity and speed gradually improved, and we could perform the procedure expertly in the last four cases. A summary of the fetal parts visualized in all the cases has been presented in Table 3.
Table 1.
Period of Gestation (POG), position of the placenta at fetoscopy (12 women)
| POG (weeks) | No. of cases | Position of placenta | No. of cases |
|---|---|---|---|
| 9–12 | 1 | Anterior | 8 |
| 12+–14 | 2 | Posterior | 3 |
| 14 +–16 | 2 | Antero—lateral | 1 |
| 16+–18 | 5 | Low lying | – |
| 18+–20 | 2 |
Table 2.
Size of fetoscope and clarity of visualized images
| Visualization | Size of fetoscope | No of cases |
|---|---|---|
| Good visualization of fetal parts | 2 mm, 0° | 8 |
| Fair visualization of fetal parts | 1.2 mm, 30°, 0° | 2 |
| Poor visualization of fetal parts | 1 mm, 0° | 2 |
Fig. 1.
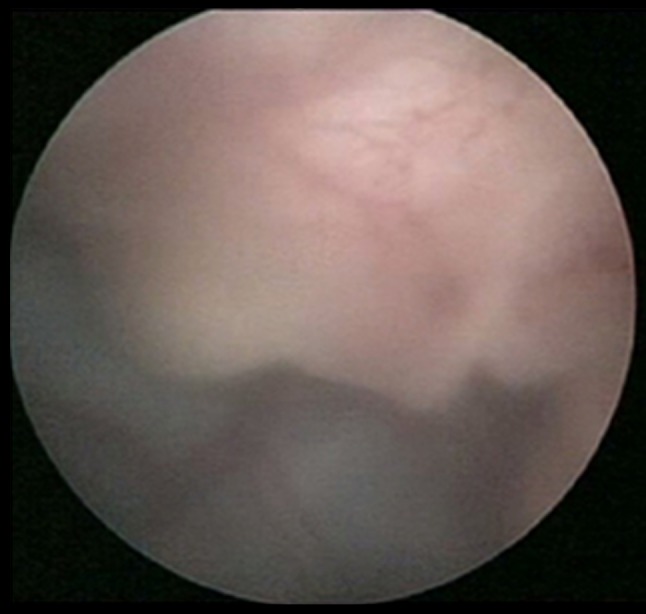
Fetal nose, placenta on fetoscopy
Fig. 2.
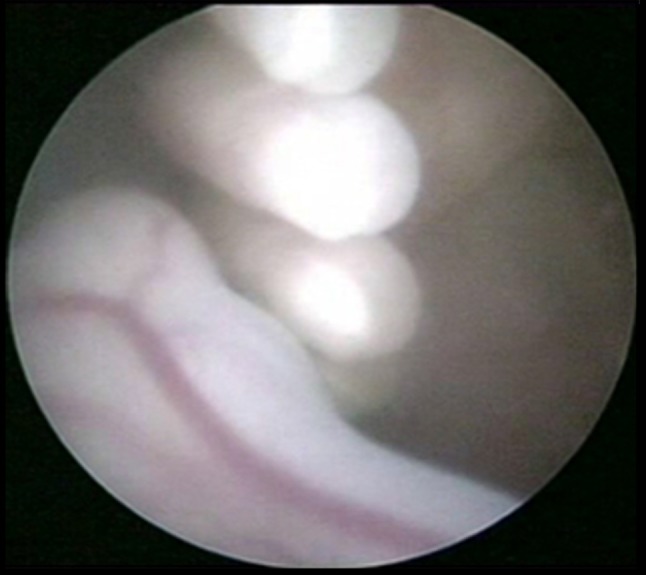
Fetal fingers, ankle on fetoscopy
Fig. 3.
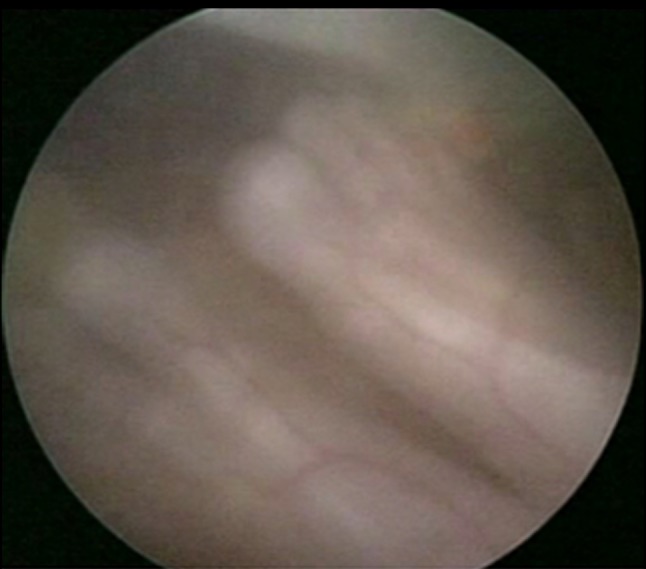
Fetal feet
Table 3.
Summary of visualization of fetal parts in all the cases
| S. No. | Fetal parts visualized | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placenta | Placenta and vessels | Head | Upper limb | Lower limb | Trunk | Nose | Ears | Lips | Eyes | Fingers | Toes | Genitalia | |
| 1. | Posterior | √ | √ | √ | √ | ||||||||
| 2. | Anterior | √ | √ | √ | √ | √ | √ | √ | |||||
| 3. | Anterior | √ | √ | √ | √ | √ | √ | ||||||
| 4. | Anterior | √ | √ | √ | |||||||||
| 5. | Anterior | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 6. | Anterior | √ | √ | ||||||||||
| 7. | Anterior | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 8. | Anterior | √ | √ | ||||||||||
| 9. | Anterior | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 10. | Lateral | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 11. | Anterior | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 12. | Posterior | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
Anterior placenta was a difficult situation for introduction of the fetoscope, visualization of placental vessels, and manipulations. Diode Laser coagulation of placental vessels was possible in the last two cases with accessories for introduction of the laser fiber (Fig. 4).
Fig. 4.
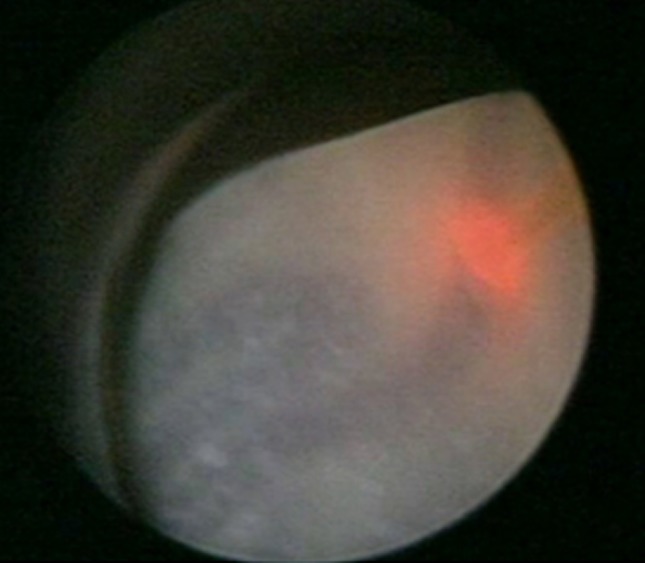
Placental vessel coagulation by diode laser
There were no major complications during the procedure or in the 48-h post procedure such as leaking, uterine contractions, infection, or intrauterine death.
Discussion
Starting fetoscopy in the department, learning and establishing the very skillful and potentially dangerous procedure before performing fetal endoscopic surgery in ongoing pregnancies posed unique challenges: operating theater and personnel requirements, endoscopic techniques, fetoscopic instruments, and technical expertise in the field. We selected the best compromise between image quality and minimal invasiveness and experienced a natural evolution of learning and modification of fetoscopic techniques.
Visualization and observations improved with practice—the oft-quoted “learning curve.” Fetoscopy required great skill, patience, and extensive repeated use of ultrasound for correct orientation of the fetal parts such as mouth, eyes, limbs, and placental position with fetoscope in utero in the initial four cases with finer fetoscopes and in the next 3–4 cases with the 2 mm endoscope. In the last four cases, we performed the procedures quite expertly; in 2 of these, we tried and could perform laser coagulation of placental vessels with precision and without any complications. We have now established the technique of fetal endoscopy, and are quite confident to embark upon fetal surgery by this route.
Technical advances continue to expand the number of genetic disorders that can be diagnosed and even treated in utero. The current methods for prenatal diagnosis are ultrasound, amniocentesis, fetal blood sampling, biopsy of fetal skin, and chorionic villus sampling (CVS). Fetoscopy had been developed which permitted the perinatologist to enter the uterus and obtain tissue sample or to actually view the fetus in the early 1980s [7]. In a few experienced centers in the world, fetoscopy has consolidated its position in fetal medicine, because of a combination of insight into the pathophysiology of selected conditions that are amenable to fetal surgery and the technical innovations in endoscopic equipment. The first clinical fetoscopy were interventions on the umbilical cord and the placenta. In clinical practice, surgical interventions on the placenta, umbilical cord and fetal membranes, laser coagulation of placental vessels in cases of feto-fetal transfusion syndrome, and cord occlusion in monochorionic pregnancies are the most common procedures. Also, for some fetal conditions requiring in utero surgery wherein most experience has been gathered with congenital diaphragmatic hernia. Future developments of fetal endoscopic operations should involve concepts to reduce maternal invasiveness and complications, eventually improving acceptance by parents and doctors [8].
Interest in fetal intervention has become widespread in recent years. Laser therapy for the treatment of severe twin–twin transfusion alone has been the subject of more than 100 peer-reviewed articles in the last 3 years. The outcome of a randomized clinical trial demonstrating that fetoscopic laser coagulation of chorionic plate vessels is the most effective treatment for twin–twin transfusion syndrome (TTTS) has revived interest in endoscopic fetal therapy [9]. Operating on the fetus is another more challenging enterprise. Clinical fetal surgery programs were virtually non-existent in Europe until minimally invasive fetoscopic surgery made such operations clinically possible as well as maternally acceptable. At present, most experience has been gathered with fetal tracheal occlusion as a therapy for severe congenital diaphragmatic hernia. As in other fields, minimally invasive surgery has pushed back boundaries and now allows safe operations to be performed on the fetal patient.
While minimal access seems to solve the problem of preterm labor, all procedures remain invasive, and carry a risk to the mother and a substantial risk of preterm prelabor rupture of the membranes (PPROM). The latter problem may prove to be a bottleneck for further developments, although treatment modalities are currently being evaluated [10]. Reviews of current clinical status and recent advances in endoscopic and open surgical interventions show that in most centers, fetoscopic interventions are widely embraced, compared to open fetal surgery. In terms of surgery on the fetus, an increasingly frequent indication is severe congenital diaphragmatic hernia, as well as myelomeningocele. The efficacy of intrauterine fetal therapeutic intervention is still to be determined as treatment of congenital diaphragmatic hernia, cardiac defects, lower urinary tract obstruction, and sacrococcygeal teratoma.
Fetoscopy has been found to lower the incidence of preterm labor compared to open surgery and very effective for treating several fetal anomalies [11]. Maternal safety is the most crucial. A case of postoperative pulmonary edema in a mother who underwent minimally invasive fetal surgery for the treatment of twin reverse arterial perfusion sequence has been reported. Probably, pulmonary edema resulted from saline irrigating fluid (totaling net 8 L) used during the procedure to facilitate surgical exposure, absorbed intravenously through myometrial venous channels accessed by passage of the operating trocars [12]. Overall maternal safety is high, but rupture of the membranes and preterm delivery remain a problem.
The increasing application of fetal surgery has triggered the interest to embark on fetal surgical therapy, although the complexity as well as the overall rare indications is a limitation to sufficient experience on an individual basis. Significant issues have arisen that affect the availability of these new therapies. Increased exchange between high volume units and collaborative studies—with strictness for self-regulation—is advocated by world-renowned pioneers [13]. Formal training fellowships have yet to be established. The establishment of research networks to evaluate new fetal therapies through randomized clinical trials appears paramount to the advancement of the field [14]. The future of fetoscopic surgical intervention depends on the continued evolution of novel techniques, the elucidation of the pathophysiology and treatment of fetal disorders, and a better understanding of treatment of complications of interventions [15]. Current and probable future applications of fetoscopy are appreciated by this route of fetal access in India. The registration of all experience for prospective data collection should be encouraged, supported by FOGSI; with the primary target being the assessment of maternal safety and fetal improvement.
Conclusion
Fetal endoscopy—“Fetoscopy” is a feasible, safe procedure in the hands of experts in fetal diagnostic and therapeutic techniques. Fetoscopy has been established at our center, and the process was an intense “learning curve,” posing unique requirements, extreme care, precision, and technical expertise, with innovation and refinement. Practice with the new equipment and finer instruments, and proper orientation to the intrauterine environment with ultrasound—fetoscopy—laser coordination was absolutely essential, to become skilled in the procedure before embarking on fetoscopic fetal surgery in high-risk ongoing pregnancies, which we are now ready for.
References
- 1.Klaritsch P, Albert K, Van Mieghem T, Gucciardo L, Done’ E, Bynens B, Deprest J. Instrumental requirements for minimal invasive fetal surgery. BJOG. 2009;116:188–197. doi: 10.1111/j.1471-0528.2008.02021.x. [DOI] [PubMed] [Google Scholar]
- 2.Deprest JA, Flake AW, Gratacos E, Ville Y, Hecher K, Nicolaides K, Johnson MP, Luks FI, Adzick NS, Harrison MR. The making of fetal surgery. Prenat Diagn. 2010;30:653–667. doi: 10.1002/pd.2571. [DOI] [PubMed] [Google Scholar]
- 3.Hobbins JC, Jones OW, Gottesfeld S, Persutte W. Transvaginal ultrasonography and trans-abdominal embryoscopy in the first-trimester diagnosis of Smith-Lemli-Opitz syndrome, type II. Am J Obstet Gynecol. 1994;171:546–549. doi: 10.1016/0002-9378(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 4.Ville Y, Khalil A, Homphray T, Moscoso G. Diagnostic embryoscopy and fetoscopy in the first trimester of pregnancy. Prenat Diagn. 1997;17:1237–1246. doi: 10.1002/(SICI)1097-0223(199712)17:13<1237::AID-PD299>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Kohl T, Hering R, Van de Vondel P, Tchatcheva K, Berg C, Bartmann P, Heep A, Franz A, Müller A, Gembruch U. Analysis of the stepwise clinical introduction of experimental percutaneous fetoscopic surgical techniques for upcoming minimally invasive fetal cardiac interventions. Surg Endosc. 2006;20:1134–1143. doi: 10.1007/s00464-005-0662-z. [DOI] [PubMed] [Google Scholar]
- 6.Rossi AC. Indications and outcomes of intrauterine surgery for fetal malformations. Curr Opin Obstet Gynecol. 2010;22:159–165. doi: 10.1097/GCO.0b013e3283374ab5. [DOI] [PubMed] [Google Scholar]
- 7.Elias S. The role of fetoscopy in antenatal diagnosis. Clin Obstet Gynaecol. 1980;7:73–82. [PubMed] [Google Scholar]
- 8.Gratacós E, Deprest J. Current experience with fetoscopy and the Eurofoetus registry for fetoscopic procedures. Eur J Obstet Gynecol Reprod Biol. 2000;92:151–159. doi: 10.1016/S0301-2115(00)00440-1. [DOI] [PubMed] [Google Scholar]
- 9.Deprest J, Jani J, Lewi L, et al. Fetoscopic surgery: encouraged by clinical experience and boosted by instrument innovation. Semin Fetal Neonatal Med. 2006;11:398–412. Epub 2006; 20. [DOI] [PubMed]
- 10.Yamamoto M, Barki G, Ville Y. Direct visual control on cord coagulation using a fetoscopy-guided bipolar forceps. Description of a new technique. Prenat Diagn. 2010;30:156–158. doi: 10.1002/pd.2419. [DOI] [PubMed] [Google Scholar]
- 11.Peiró JL, Carreras E, Guillén G, Arévalo S, Sánchez-Durán MA, Higueras T, Castillo F, Marhuenda C, Lloret J, Martínez-Ibáñez V. Therapeutic indications of fetoscopy: a 5-year institutional experience. J Laparoendosc Adv Surg Technol A. 2009;19:229–236. doi: 10.1089/lap.2007.0149. [DOI] [PubMed] [Google Scholar]
- 12.Robinson MB, Crombleholme TM, Kurth CD. Maternal pulmonary edema during fetoscopic surgery. Anesth Analg. 2008;107:1978–1980. doi: 10.1213/ane.0b013e3181891201. [DOI] [PubMed] [Google Scholar]
- 13.Deprest JA, Devlieger R, Srisupundit K, Beck V, Sandaite I, Rusconi S, Claus F, Naulaers G, Van de Velde M, Brady P, Devriendt K, Vermeesch J, Toelen J, Carlon M, Debyser Z, De Catte L, Lewi L. Fetal surgery is a clinical reality. Semin Fetal Neonatal Med. 2010;15:58–67. doi: 10.1016/j.siny.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Moise KJ, Jr, Johnson A, Carpenter RJ, Baschat AA, Platt LD. Fetal intervention: providing reasonable access to quality care. Obstet Gynecol. 2009;113(2):408–410. doi: 10.1097/AOG.0b013e318194f250. [DOI] [PubMed] [Google Scholar]
- 15.Sydorak RM, Albanese CT. Minimal access techniques for fetal surgery. World J Surg. 2003;27:95–102. doi: 10.1007/s00268-002-6743-4. [DOI] [PubMed] [Google Scholar]


