Abstract
Bioavailability is a key step in ensuring bioefficacy of bioactive food compounds or oral drugs. Bioavailability is a complex process involving several different stages: liberation, absorption, distribution, metabolism and elimination phases (LADME). Bioactive food compounds, whether derived from various plant or animal sources, need to be bioavailable in order to exert any beneficial effects. Through a better understanding of the digestive fate of bioactive food compounds we can impact the promotion of health and improvement of performance. Many varying factors affect bioavailability, such as bioaccessibility, food matrix effect, transporters, molecular structures and metabolizing enzymes. Bioefficacy may be improved through enhanced bioavailability. Therefore, several technologies have been developed to improve the bioavailability of xenobiotics, including structural modifications, nanotechnology and colloidal systems. Due to the complex nature of food bioactive compounds and also to the different mechanisms of absorption of hydrophilic and lipophilic bioactive compounds, unravelling the bioavailability of food constituents is challenging. Among the food sources discussed during this review, coffee, tea, citrus fruit and fish oil were included as sources of food bioactive compounds (e.g. (poly)phenols and polyunsaturated fatty acids (PUFAs)) since they are examples of important ingredients for the food industry. Although there are many studies reporting on bioavailability and bioefficacy of these bioactive food components, understanding their interactions, metabolism and mechanism of action still requires extensive work. This review focuses on some of the major factors affecting the bioavailability of the aforementioned bioactive food compounds.
Keywords: bioaccessibility, bioavailability, food, phenolics, polyphenols, PUFA
Introduction
Food is for our sustenance, nourishment and enjoyment. More than this, people are increasingly looking to non‐nutrient food components for added benefits from their food which may play a role in health promotion, disease prevention and performance improvement [1]. When considering these properties of food, we aim for long term physiological effects, as opposed to acute effects as in the case of most of the pharmaceutical drugs [2].
The majority of bioactive food compounds responsible for the positive effects on well‐being are predominantly derived from the plant kingdom with some from animal sources. Several epidemiological studies throughout the years have suggested that diets rich in fruits and vegetables promote health and reduce the risk of certain chronic and neurodegenerative diseases [3]. Five portions of fruits or vegetables a day has become a rule of thumb for healthy lifestyle dietary habits [4]. Many of these fruits and vegetables contain polyphenols which are secondary metabolites in plants, and are considered as non‐essential for sustenance of life but potentially contribute to the maintenance of human health [5]. Similar to hydrophilic bioactive molecules, lipophiles derived from various plant and animal sources may also exert beneficial effects on health. Long chain polyunsaturated fatty acids derived from marine animals or plant sources, and carotenoids of plant origin are examples of lipophilic bioactive nutrients. Among the food sources discussed in this review, coffee, tea, citrus fruit and fish oil are included as examples of important ingredients for the food industry. Cereals, dairy products, nuts, red wine and olive oil are also interesting sources of bioactive food compounds, but will not be considered in this review.
In order to exert a health benefit, the compound of interest, whether hydrophilic or lipophilic, needs to withstand food processing, be released from the food matrix post‐ingestion and be bioaccessible in the gastrointestinal tract, undergo metabolism and reach the target tissue of action [2]. In other words, the bioactive compound in question needs to be bioavailable before it can have an effect [6]. It may be considered redundant to study the health effects of dietary bioactive compounds if their bioavailability is not also fully elucidated. Bioavailability is a key step regarding functional foods and health claims related to food components, followed by knowledge of the circulating metabolites, which leads to understanding of the mechanisms of action in relation to the benefit.
Due to the complexity of food compounds, the many factors affecting their transition during digestion, and also to the different mechanisms of absorption of water soluble and lipid soluble molecules, unravelling the bioavailability of food constituents is challenging when compared with pharmaceutical drugs. In addition, understanding, for example, the polyphenol–gut microbiota interactions and gut microbial bioconversion capability will facilitate studies on bioavailability of bioactive food compounds in the host and provide more insight into the health effects of these molecules. Williamson & Clifford [7] reported that since microbial metabolites could be present in very high concentrations, colonic metabolites could be considered as the missing link between the consumption of certain polyphenols and their biological activity.
From a pharmacological perspective, bioavailability is the rate and extent to which the bioactive compound or a drug is absorbed and becomes available at the site of action [8]. From a nutritional perspective, bioavailability is the fraction of a given food that the body can utilize, and is therefore a matter of nutritional efficacy [b9,b10]. Bioavailability addresses several processes such as liberation from a food matrix, absorption, distribution, metabolism and elimination phases (LADME).
The assessment of bioaccessibility and bioavailability of health associated compounds is important for the understanding of the relationship between food and nutrition. The rate and extent of absorption can vary widely between individuals. The inter‐individual variability in bioavailability depends on several key factors including diet, genetic background, gut microbiota composition and activity. Some bioactive food compounds such as polyphenols, are relatively poorly absorbed, the absorption ranging from 0.3% to 43%, and the circulating plasma concentrations of their metabolites can be low [6]. Limited bioavailability hinders the use of bioactive food compounds as functional ingredients [b11,b12]. Only by understanding the mechanisms of absorption of food derived compounds, can their bioavailability be enhanced and thus the potential for greater health benefits be realized.
In this review we focus on the nutrient bioavailability of different bioactive food compounds, polyphenols and PUFAs, two very different classes of compounds that have been reported to contribute to a healthy diet. As groups of bioactive food compounds, they differ greatly in their origin, physicochemical properties and absorption mechanisms, and yet they have similarities with regard to various health related benefits, such as cardio vascular health [13–17]. We also focus on the challenges faced with food matrices, effects of processing, absorption mechanisms, and selected technologies to improve the bioavailability of bioactive food molecules, all in comparison with pharmaceutical drugs.
Bioaccessibility: the first step of bioavailability
Bioaccessibility has been defined as the fraction of a compound which is released from the food matrix in the gastrointestinal lumen and thereby made available for intestinal absorption [b18]. Mastication in the mouth initiates the process and several digestive fluids containing different enzymes continue to break down the food matrix in the stomach and throughout the remainder of the gastrointestinal lumen [b19]. Bioaccessibility is influenced by the composition of the digested food matrix, the synergisms and antagonisms of the different components [b10], but also by physicochemical properties, such as pH, temperature and texture of the matrix [b20]. The digested food is predominantly broken down in the small intestine by bile, pancreatic and other enzymes secreted from the intestinal mucosa [b19]. These digestive aids are crucial for lipid soluble bioactive compounds, such as some vitamins (A, D, E and K), carotenoids and PUFAs. The sequential obligatory steps of lipid digestion prior to absorption are partial gastric hydrolysis, emulsification by bile and further lipolysis by pancreatic lipases. These enzymes release free fatty acids and monoacylglycerol which form micelles so that the lipids can be absorbed across the water barrier and into the intestinal enterocytes [b21–b24].
The caloric content and the volume of the food matrix can cause physiological changes in the gastrointestinal tract which affects the bioaccessibility of digested compounds [b25–b27]. Walsh et al. [b28] observed in an in vitro model that the bioavailability of isoflavonoids from foods containing fat and protein exceeds that of isoflavonoid supplements consumed without food. Brown et al. [b29] showed that both full fat and reduced fat salad dressing improved carotenoid absorption in human subjects when compared with consumption of salad with fat free salad dressing.
Processing of plant foods can influence the bioaccessibility of nutrients, mainly through changes in the plant cell wall structure and properties [b30]. Since plant cell walls are largely resistant to degradation in the upper gut, they represent an important barrier for the release of bioactive compounds. One example is ferulic acid in whole grain wheat, where ferulic acid has a limited bioavailability due to its high binding affinity to polysaccharides. Anson et al. [b31] studied the bioaccessibility of ferulic acid from wheat fractions and breads consumed by human subjects. The authors observed that wheat ferulic acid had a low bioaccessibility (< 1%). However, the bioaccessibility was higher when free ferulic acid was added to flour (∼60%). Also fermentation of wheat prior to baking broke the ferulic acid ester links to fibre, thus releasing ferulic acid and subsequently improving its bioavailability [b32].
By contrast, pharmaceutical drugs can be taken without a meal, keeping the drug dissolution less complicated than the bioaccessibility of a bioactive food compound, since limiting factors are less and orally taken drugs are mostly water soluble. The effects of food on drug bioavailability are usually studied by evaluating the rate and extent of absorption of a drug when administered after a meal vs. no co‐digested meal. Changes observed are related to transit time, luminal dissolution, permeability and systemic availability [8]. Drug dissolution has been extensively studied in the pharmaceutical industry. However, with foods, bioaccessibility has not been investigated to the same extent, although there is a lot of potential for improving bioefficacy via greater liberation of bioactive compounds from food matrices beyond that of the matrix effect per se [5].
Bioavailability
After bypassing the challenge of being released from the food matrix and becoming bioaccessible, bioactive food compounds can be absorbed in the gastrointestinal tract. The absorption of these compounds can be influenced by solubility, interaction with other dietary ingredients, molecular transformations, different cellular transporters, metabolism and the interaction with the gut microbiota, resulting in changes to their bioavailability [b20]. The different solubility of lipophilic and hydrophilic compounds results in different absorption mechanisms [b33]. Before it was thought that dietary lipids pass unaltered through the intestinal wall since structure wise similar lipid particles were found both in the gut and in the systemic and lymphatic circulation. However, lipid bioavailability is not so simple and even today it is not completely elucidated [b34]. Our food contains diverse classes of lipids, the main ones being triacylglycerides, phospholipids, glycolipids, free fatty acids, sterols, vitamins and their precursors [b35]. Owing to the physiology of the small intestine, with the presence of an unstirred water layer across the intestinal barrier, lipid absorption can be compromised [b36]. To overcome the intestinal water barrier, the size of dietary lipid particles is reduced, and after these digestion products form micelles where bile salts and other amphiphilic nutrients act as emulsifiers. Lipases of the gastric juice hydrolyze lipids at the emulsion–water interphase resulting in diacylglycerols and free fatty acids [b34]. Uptake of lipids by the enterocyte is believed to take place through passive diffusion but also through facilitated diffusion via transporters [b37]. Once in the enterocyte, fatty acids are re‐esterified with monoacylglycerols to form triacylglycerols prior to secretion into the lymphatic circulation via triacylglycerol rich lipoproteins also called chylomicrons [b23]. Unlike hydrophilic compounds, lipid soluble compounds are not readily excreted from our body. Lipids are either stored within the liver or re‐excreted into the circulation as lipoproteins and further stored in the adipose tissue.
Hydrophilic compounds, such as polyphenols and most drugs have a more simplistic mechanism of absorption than lipids. Most polyphenols found in foods exist as esters, glycosides or polymers which cannot be absorbed as such [b11]. Enzymatic hydrolysis takes place with most polyphenols at the brush border of small intestine epithelial cells. This liberates the aglycone which can then enter the enterocyte. Aglycones can be liberated also in the enterocyte by cytosolic β‐glucosidase‐mediated hydrolysis [b38,b39]. In the enterocytes flavonoid aglycones can be conjugated by the phase II enzymes, resulting in methylated and/or glucuronidated forms. Some of the metabolites can be effluxed back from the enterocyte into the intestinal lumen by ABC‐transporters [11,38–40]. The absorbed metabolites as well as the flavonoids which escaped conjugation in the enterocyte pass next into hepatocytes via the hepatic portal vein where further conjugation takes place. From the liver, bioactive metabolites can be excreted either into systemic circulation or into the bile. Polyphenol metabolites present in the systemic circulation are finally excreted into urine [b38–b41]. Since a large portion of polyphenols and some larger molecules are not absorbed in the small intestine, these compounds reach the large intestine where they are metabolized by the microbiota into smaller molecules.
The difference and similarities of food bioactives (lipophilic and hydrophilic) and pharmaceutical drugs with regards to bioavailability are highlighted in Figure 1. Taken together, the metabolism of bioactive compounds of foods, as with drugs, affects their physical and chemical properties, which make them more water soluble and more readily excreted at the end of the bioavailability pathway [b42]. This process, also referred to as metabolic detoxication, leads to the near absence of aglycones in the systemic circulation. Previous thinking that the aglycones were the active forms of flavonoids should be re‐considered since the conjugated forms are the ones most likely to express a biological activity [6,11,41,43].
Figure 1.
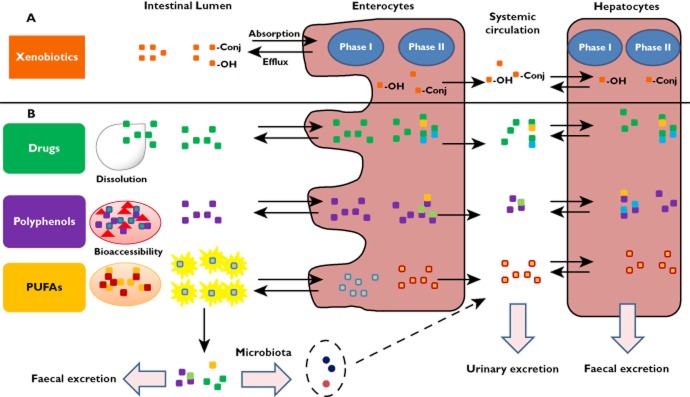
General scheme on the absorption, metabolism and excretion for xenobiotics (A) and examples of compounds discussed in this review (drugs, polyphenols and PUFAs) (B).  , drug;
, drug;  , polyphenols;
, polyphenols;  , other food ingredients;
, other food ingredients;  , micelles;
, micelles;  , PUFAs in form of TAG;
, PUFAs in form of TAG;  , Metabolites (from drugs and polyphenols);
, Metabolites (from drugs and polyphenols);  , microbial metabolites;
, microbial metabolites;  , phase I and phase II metabolites;
, phase I and phase II metabolites;  , PUFA hydrolyzed from TAG;
, PUFA hydrolyzed from TAG;  , PUFA re‐esterified as TAG and released as chylomicron
, PUFA re‐esterified as TAG and released as chylomicron
Factors affecting bioavailability
Structure of bioactive molecules
The molecular structure of a bioactive compound affects its absorption considerably [b44]. For example, it is well known that high molecular weight compounds, such as the oligomeric proanthocyanidins and complex lipids, do not pass through the intestinal cells unless they are firstly broken down [b45]. Also the sugar moiety of flavonoids has been suggested to be an important determinant in their absorption in humans [b46,b47]. Flavonoids attached to β‐glucosides, one of the most predominant forms in nature [b48], can be absorbed to a very small extent as such and/or metabolized by enzymes (e.g. β‐glucosidases and lactase‐phlorizin hydrolase) in the small intestine [42,46,47,49]. However, when flavonoids are attached to an additional rhamnose moiety, as in the case of quercetin from tea, they need to reach the large intestine to have the sugar moieties cleaved off by the intestinal microbiota before absorption [b50,b51].
Moreover, it is not only the chemical structure of bioactive food compounds, but also their isomeric configuration that can affect their absorption. As for some drugs, flavonoids with different stereochemistry exhibit different bioavailability and bioefficacy. This is the case for (–)‐epicatechin and (+)‐catechin bioavailability [b52,b53], the cis‐isomers and all‐trans isomer of lycopene bioavailability [b54], the biological activity of (R/S) equol [b55], and for the metabolism of (R/S) hesperidin [b56,b57]. In the last example, hesperitin‐7‐glucoside was found to have an R : S ratio of 39 : 69 in human plasma and urine samples, suggesting that the S configuration could be more bioavailable [b57]. Another example is that of lycopene, a bioactive carotenoid from tomato, which is present as 95% all‐trans isomer in tomatoes, yet the cis isomers represent around 50% of lycopene in human plasma through a combination of isomerization in the gastrointestinal tract and greater bioavailability of cis‐isomers in crossing the intestinal wall [b58,b59].
Transport mechanisms
The different transport mechanisms which take place in the intestinal lumen are one of the most important factors affecting the bioavailability of ingested food compounds and drugs. These include passive diffusion, facilitated diffusion and active transport. The first two mechanisms involve diffusion towards a concentration gradient through the intestinal cells into the blood circulation. The later mechanism works against the concentration gradient and can result either in the increase of compounds in the blood circulation or in the efflux of the ingredients back to the intestinal lumen [b60].
As many drugs and bioactive food compounds do not have the optimal physicochemical properties necessary for passive diffusion,trans‐membrane transporters are needed for enhancing their permeability. Membrane transporters are involved in two mechanisms related to permeability of compounds, uptake and efflux. Vitamin transporters, GLUT‐family, SGLT‐family and organic anion transporters (OAT1), amongst others, are involved in the uptake of compounds and enhance their transport across the intestine [b61]. The ABC (ATP Binding Cassette) family of transporters, including P‐glycoprotein (P‐gp) and breast cancer resistance protein (BCRP), on the other hand, are examples of efflux mechanisms, and can hinder the bioavailability of drugs and bioactive food compounds [b61]. The activity of different efflux transporters affects the disposition of drugs and bioactive compounds through several different mechanisms, such as limitation of absorption, facilitation of the elimination by secretion into bile and urine, and limitation of distribution to target tissues [b62].
Intestinal transport systems have different selectivity towards different bioactive compounds. The transporters can be blocked by certain nutrients, significantly affecting the bioavailability of other xenobiotics [b61]. It has been reported that competition for transport by organic anion transporters can cause retention of certain drugs in cell culture models, leading potentially to longer plasma half‐lives [b63]. Whitley et al. [b64] studied the ability of organic anion transporters and organic anion‐transporting polypeptides to transport ellagic acid. These authors reported that there is a very high affinity of ellagic acid to organic anion transporters, especially hOAT1, indicating a potential interaction of ellagic acid with drugs such as β‐lactam antibiotics, angiotensin converting enzyme inhibitors, non‐steroidal anti‐inflamatory drugs, antiviral drugs, prostaglandins and diuretics. Itakagi et al. [b65] described the food bioactive compound–drug interaction between ferulic acid and nateglinide (a hypoglycaemic agent) involving a transport system. The concomitant administration of nateglinide with ferulic acid‐rich sources could lead to inhibition of absorption of the drug, reducing its oral bioavailability. Although these examples are from in vitro cell culture models, it has been reported that flavonoids are well known substrates for the ABC family of transporters involved in the efflux mechanism, and affinity with these transporters has been suggested as one of the main reasons for poor bioavailability of these bioactive compounds [b66–b68].
Although the uptake of lipids and lipid digestion products has been hypothesized to be passive for many decades, scientific evidence reported that transport proteins like FATP4, FABPpm and CD36 may be involved in lipid transport across the intestinal barrier [b23]. This relatively new discovery has propagated a hypothesis that if one family of membrane transporters is common to multiple nutrients, there might be competitive inhibition of absorption of other nutrients using the same transporter. An example of this phenomenon is reported by Richelle et al. [b69], where plant sterols reduced absorption of cholesterol, carotenoids and alpha‐tocopherol in normocholesterolaemic subjects, most likely by competing with lipid membrane transporters.
Metabolism and food–drug interactions
Once the drug or bioactive food molecule has entered into an enterocyte, it may be subjected to metabolism by cytochrome 450 enzymes (CYP) (phase I) which modify the xenobiotic structure by reactions such as oxidation and reduction. In general, polyphenols are not considered as substrates for CYP enzymes, unlike drugs, but they are subjected to several phase II enzymes leading to conjugation with methyl (catechol‐O‐methyltransferases – COMT), sulfate (sulphotransferases – SULT) and glucuronyl groups (uridine‐5′‐diphosphate glucuronosyl‐transferases – UDPGT). This results in molecular forms, which are different from the original constituents of the digested food [b70]. The activity of CYP family enzymes can be inhibited or activated by co‐administered bioactive compounds or drugs. For example, inhibition of CYP enzymes can increase circulating amounts of lipid soluble vitamins, as is the case for the sesame seed lignin, sesamin, which can considerably increase γ‐tocopherol concentrations in humans [b71,b72]. Grapefruit juice has also been reported in many scientific papers in respect to food–drug interactions [b73–b75]. It has been proposed that bergamottin (a furanocoumarin) and derivatives could be responsible for the interaction [b76,b77]. Grapefruit juice can produce clinically important increases in oral drug bioavailability when co‐administered with substrates of cytochrome P450 3A4 (CYP3A4), leading to an increase in the plasma concentration of the drug [b76,b78]. This in turn should be considered as it would lead to toxic supra‐pharmacologic plasma concentrations. It has been reported in an animal study that the presence of lipids could also promote drug absorption. An example of this is the use of eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids as a vehicle for ciclosporin A, an immunosuppressive agent, for increasing its bioavailability. The suggested mechanism involved here was the inhibition of CYP3A by DHA [b79]. An opposite example, i.e. decrease in the plasma concentration of the drug, is the food–drug interaction reported in the case of St John's wort (Hypericum perforatum) and drugs such as ciclosporin and indinavir, where the interaction led to a low plasma concentration of the drug, thus compromising its therapeutic effect [b80,b81].
Improvement of bioavailability
Improving the bioavailability of bioactive food compounds is fundamental to improve their bioefficacy. Several approaches have been evaluated for the improvement of bioaccessibility and bioavailability of bioactive ingredients and drugs. These include technological and chemical modifications of the molecules to improve their solubility or the site of absorption, design of colloidal systems (micelles and vesicles), and use of nanosystems (nanoparticles), among others [b82]. Examples of materials commonly used to improve the bioavailability of drugs include chitosan, polymers, cyclodextrins and dendrimers. These materials are also applicable to many bioactive food compounds.
Nanotechnology is currently one of the major fields studied in pharmaceutical research [b83]. Although there are many cases where systemic bioavailability is improved by means of nanotechnology, the main reason for the design of such nanosystems is to improve local bioavailability, or the delivery of a certain drug to a target tissue [b83]. In principle, the use of nanosystems could facilitate the entering of the drug or bioactive compound through biological barriers, besides avoiding the metabolic modifications that could lead to low absorption. However, it is very important to understand, from an efficacy perspective, that bioavailability parameters might be influenced by the composition material and the physico‐chemical properties of the nanoparticles [b84,b85]. Therefore, human studies to identify potential risks of the use of nanoparticles should be taken into consideration. Tzeng et al. [b86] reported the use of a nanoparticle engineering process for the enhancement of antioxidant activity and solubility of kaempferol. These authors suggested that kaempferol nanoparticles could be considered as a low dose alternative in health foods and future clinical research. Curcumin, the major curcuminoid compound, is well known for many potential health benefits [b87,b88]. However, due to its insolubility in water, this compound has a very low bioavailability [b89]. A method to improve the bioavailability of curcumin in rats was achieved by introducing PLGA [poly (lactic‐co‐glycolic acid)] nanoparticles that had 5.6 fold higher bioavailability and longer half‐life when compared with free curcumin [b84]. Yu et al. [b90] developed a food grade curcuminoid organogel with high bioaccessibility and high loading of curcumin. They demonstrated that in in vitro models this preparation could be regarded as an alternative to the nanoparticle approach for improving bioavailability.
Several technologies for encapsulation of food bioactive molecules are aimed to improve bioavailability. These technologies include spray drying, coacervation, liposome entrapment, inclusion complexation, cocrystallization, nanoencapsulation, freeze drying, yeast encapsulation and emulsion [b91]. For example, Fernandez‐Garcia et al. [b92] evaluated in several in vitro digestion models an emulsifier system to improve the bioaccessibility of carotenoids. The use of microencapsulation, liposomes, gel emulsions and plant spore exines as delivery systems for PUFAs has demonstrated improved bioavailability when compared with fish oil, a typical representation of normal dietary intake [35,93–96].
Other potential ways to improve the bioavailability of bioactive compounds and drugs could be achieved through the competition and inhibition of intestinal cell transporters [b97]. The intestinal P‐glycoprotein efflux pump has been reported as one of the major contributors to the low oral bioavailability of a number of compounds reported in animal studies [b84]. In vitro experiments suggest that the bioavailability of the flavonoid hesperidin may be enhanced by inhibiting the ABC transporters by competitive exposure to other flavonoids, such as quercetin, resulting in a decrease of the efflux of hesperidin [b98]. The same approach has been suggested for (–)‐epigallocatechin 3‐gallate (EGCG), where the combination of the bioactive compound with naturally occurring inhibitors of efflux proteins resulted in increased cytosolic levels of the compound [b99].
Other approaches for improving bioavailability are structural modifications of bioactive compounds and the processing of the food matrix. For example, it has been reported in animal and human studies that quercetin, the aglycone form of rutin, is more bioavailable than the parent flavonol [b50,b100]. Carotenoids, such as lycopene and zeaxanthin, generally have a low bioavailability in humans [b101]. By processing raw tomatoes into tomato paste, the bioavailability of lycopene is increased due to release from the food matrix, but also due to isomerization of trans lycopene into the more bioavailable cis form [b102,b103]. Alternatively, entrapping lycopene with whey proteins improves its bioavailability to the same level as that of tomato paste [b104], and zeaxanthin bioavailability is improved three‐fold by homogenization with hot milk [b105]. Another example for improved bioavailability through improved bioaccessibility is phytosterols, plant sterols with chemical structures similar to that of cholesterol. When phytosterols are added to food products, changes in product texture are observed due to re‐crystallization. The insoluble crystalline phytosterols cannot be absorbed in the intestine thus resulting in a very low bioavailability. Crystallization retardation, emulsification technologies [b106] and synthesis of colloidal phytosterols leads to an enhanced solubilization of phytosterols [b107] thereby improving the bioaccessibility and consequently the bioavailability of phytosterols.
Examples on the complexicity of bioavailability of food bioactive components: (poly)phenols
Coffee
Coffee contains high levels of phenolic compounds called hydroxycinnamates, consisting principally of chlorogenic acids. They are a family of esters formed between a phenolic acid, e.g. caffeic or ferulic acid, and quinic acid. The main chlorogenic acid in coffee is 5‐caffeoylquinic acid [b108], although other caffeoylquinic, feruloylquinic and di‐caffeoylquinic acids are present in significant quantities. Phenolic metabolites of chlorogenic acids have been studied for potential bioefficacy, and controversy still remains as the results are not entirely clear [b109,b110]. In addition, only a few studies have investigated the bioavailability of coffee phenolic and chlorogenic acids.
Due to the intricate and complex metabolic pathways in humans [b111,b112], chlorogenic acids may be transformed into phenolic acids (caffeic, ferulic and isoferulic moieties), and subsequently into colonic metabolites (dihydrocaffeic and dihydroferulic acids). With extensive conjugation at the level of the intestine and the liver, many different metabolites (aglycone, sulfate, glucuronide and methyl) could then be identified from a single cup of coffee. Because of this complexity, many studies have used enzymatic cleavage to reduce the number of metabolites measured, to increase the accuracy of the quantification, thus dismissing the diversity of the coffee metabolites [b113]. Recently, conjugated metabolites were measured in great numbers [b112,b114] although validated quantification of these metabolites is yet to be performed using proper standards [b114,b115].
Research on the effect of the food matrix on the bioavailability of coffee phenolic compounds has been limited. Some reports have investigated the potential effect of proteins, especially from milk, on the bioavailability of coffee phenolics. For example, using in vitro/ex vivo modelling of the digestive process showed some binding of caffeoylquinic acid to proteins (albumin, casein), but the relevance of those findings to an in vivo setting remains to be determined. In the framework of human bioavailability studies, the main effect investigated was also the addition of milk in coffee. Renouf et al. [b116] investigated the effect of adding 10% whole milk or sugar/non‐dairy creamer to coffee on the bioavailability of phenolic acids equivalents. The milk treatment showed no significant difference in the area under the curve (AUC), maximum concentration (Cmax) or time when maximum concentration was reached (tmax) of caffeic acid, ferulic acid and isoferulic acid equivalents compared with coffee alone. With respect to the sugar/non‐dairy creamer treatment, the AUC of caffeic acid, ferulic acid and isoferulic acid equivalents were similar to coffee alone. However, Cmax of caffeic acid and isoferulic acid equivalents were significantly lower and tmax of ferulic acid and isoferulic acid equivalents were significantly longer for the sugar/non‐dairy creamer group compared with coffee. Therefore, even if the delivery of chlorogenic acid metabolites was not significantly different between treatments, the addition of sugar/non‐dairy creamer to coffee led to significant changes in plasma appearance of those metabolites (‘flatter’, but ‘longer’ curves). More recently, a report by Duarte & Farah [b117] showed that urinary excretion of chlorogenic acids and metabolites was significantly lowered when soluble coffee was reconstituted in 100% whole milk (40 ± 27% ingested dose) compared with coffee reconstituted in water (68 ± 20% ingested dose).
Tea
Tea (Camellia sinensis L.) is the second most consumed beverage worldwide after water. The different types of tea (green, oolong and black) differ depending on the extension of the oxidative processing, leading to the presence of different compounds such as flavan‐3‐ols in green tea (minimally processed), theaflavins and thearubigins in oolong (partial oxidation) and black tea (complete oxidation). Several epidemiological studies have suggested an inverse correlation between tea consumption and chronic and degenerative diseases, possibly related to their polyphenol content [b118].
As most of the studies on tea report benefits related to the consumption of green tea, the proposed beneficial effects have been attributed to the presence of flavan‐3‐ols such as (–)‐epigallocatechin 3‐gallate (EGCG). EGCG is the main flavan‐3‐ol present in green tea and it has been reported to be present in human plasma mainly in its native form [b115]. Flavan‐3‐ols are suggested to be absorbed in the small intestine and undergo extensive metabolism. Following consumption of green tea, flavan‐3‐ols appear to be rapidly absorbed having a maximum concentration (Cmax) of between 0.5 to 2 h, followed by rapid metabolism and excretion.
One limiting factor for a better absorption of flavan‐3‐ols, is the affinity of these compounds by P‐glycoprotein and multidrug resistance proteins (MRPs) leading to a significant percentage of these compounds being effluxed back into the lumen [b119]. The importance of colonic metabolism for EGCG bioavailability should not be underestimated as there is a substantial recovery of colonic metabolites in human urine [b120]. Del Rio et al. [b121] have suggested that when all the metabolites are taken into account (conjugates and microbial metabolites) the green tea flavan‐3‐ols could be considerably more bioavailable than previously reported.
Tea is usually consumed during a meal, or accompanied by creamers (dairy or non‐dairy) or sugars, and sweeteners are added in tea products such as ready‐to‐drink beverages. Many studies have reported the effect of milk addition on the bioavailability and bioaccessibility of flavan‐3‐ols from black and green teas. In general these studies have found that the addition of milk to tea had a negligible effect on bioavailability and bioaccessibility of tea flavan‐3‐ols [b122,b123].
As previously described, due to low bioaccessibility and bioavailability of most polyphenols, there have been many attempts to improve the bioavailability of these compounds. Several approaches to improve the bioavailability of EGCG have been tested, such as chemical modifications, dosing formulation and combination with other food ingredients [b82]. Another strategy to improve the absorption of flavan‐3‐ols is their administration during the fasting state; however, it is important to note that some human studies have shown that high doses of green tea preparations can be potentially hepatotoxic [b124]. Although there are many studies describing green tea bioavailability and associated health benefits, there are still many aspects that need further investigation.
Cocoa
Historically, cocoa products have been extensively used for a vast array of medicinal purposes. The long list of its medical applications includes from treatment of mental fatigue to strengthening of gums [b125]. Cocoa extract is a complex intrinsic matrix that contains many potentially bioactive compounds [b126]. These cocoa compounds include not only polyphenols but also methylxanthines such as theobromine and caffeine [b127]. Similar to tea, flavan‐3‐ols are the most important group of polyphenols in cocoa products [b128]. Specifically, (–)‐epicatechin and procyanidins (oligomers of (–)‐epicatechin compounds) are the main flavan‐3‐ols found in these foods. Contrary to some other polyphenols like quercetin and hesperetin, (–)‐epicatechin and procyanidins are not found attached to sugar moieties in plants or plant‐derived foods.
Following consumption of cocoa products, (–)‐epicatechin is absorbed within 1 to 4 h, with tmax between 1 to 2 h. Once absorbed, (–)‐epicatechin is completely glucuronidated, sulfated and/or methylated [b129,b130]. Several beneficial effects have been attributed to the consumption of (–)‐epicatechin [b128]; and the improvement of cardiovascular biomarkers is the most consistent beneficial effect demonstrated in human intervention studies [16,131–133].
According to Baba et al. [b134], (–)‐epicatechin bioaccessibility is similar when a cocoa beverage or a chocolate bar is consumed. The authors reported that 29.8 ± 5.3% and 25.3 ± 8.1% of the initial intake was absorbed when five volunteers consumed equal amounts of (–)‐epicatechin in a beverage or chocolate form, respectively.
As a beverage, cocoa is regularly consumed with milk. Many controversial scientific articles have been published about the effect of milk proteins on the bioavailability of (–)‐epicatechin. Serafini et al. [b135] reported a detrimental effect of milk over the bioavailability of (–)‐epicatechin, showing a lower (–)‐epicatechin AUC (approximately −40%) when 100 g chocolate was ingested with 200 ml of whole milk. In contrast, several other groups have demonstrated that milk has no effect on the bioavailability of cocoa polyphenols [b136–b139]. Recently, it has been proposed that milk decreases urinary excretion of cocoa flavan‐3‐ol metabolites in humans but not the plasma pharmacokinetics [b140].
In addition to the possible effect of milk, it has been demonstrated that (–)‐epicatechin bioavailability can be improved by the co‐ingestion of cocoa products with other foods. Schramm et al. [b141] showed that (–)‐epicatechin AUC and Cmax can be increased by ingestion of carbohydrates and Neilson et al. [b142] concluded that the physical form of the food and also the sucrose content may influence the tmax and Cmax of cocoa flavan‐3‐ols.
Procyanidins are also highly present in cocoa extracts. Because of their large structure and molecular weight, procyanidins are poorly absorbed [b143,b144]. Currently, only procyanidin B2 has been reported to be detected in human plasma after consumption of cocoa products [b145], suggesting that the highest molecular weight procyanidins, which are not absorbed in the small intestine, can reach the large intestine and interact with the gut microbiota. Recently, it has been suggested that procyanidins could modulate the growth of selected gut micro‐organisms with potential prebiotic benefits in humans [b146]. Consequently, further investigations of the interaction of procyanidins with the gut microbiota and especially the bioavailability of the breakdown products produced by these interactions are warranted.
Citrus fruits
Hesperidin belongs to the flavanone group of flavonoids, and it is mostly present in citrus fruits such as oranges, lemons, and grapefruits. The highest amount of hesperidin is found in the solid tissues, mainly in the pith of the fruit. Hesperidin is associated with benefits on bone health and cardiovascular health [b147,b148]. Similarly to other more complex flavonoid glycosides, hesperidin is absorbed in the large intestine where it is hydrolyzed by the gut microbiota and the aglycone is then absorbed by passive transport [b6,b149]. Several bioavailability studies on hesperidin have shown a late tmax value (4.4 h–7.0 h) suggesting that absorption takes place in the colon [150–154]. However, modification of hesperidin to hesperetin‐7‐glucoside, by enzymaticly cleaving the rhamnose moiety with a α‐L‐rhamnose, has been known, not only to increase the bioavailability of this flavanone [b155,b156], but also to alter the absorption kinetics by having a much earlier tmax (0.6 h vs. 7 h) [b154] suggesting that the site of absorption has changed.
Dosage also affects the bioavailability of hesperidin. It has been shown that the relative absorption is increased with higher doses of hesperidin. This is the case also for the relative urinary excretion of the flavanone [b152,b154].
The food matrix has not been shown to have an impact on hesperidin bioavailability. Consumption of orange juice with full‐fat yogurt did not significantly affect the Cmax or tmax values [b153], nor did food processing have an effect on hesperidin bioavailability when comparing the consumption of orange fruits with the consumption of orange juice [b150].
Lipids
PUFAs
Bioactive PUFA, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are lipids that may exert beneficial biological functions in the human body such as prevention of heart disorders and maintenance of cognitive function [b157–b160]. Dietary sources of PUFA are usually limited to single cell oils, marine derived fatty fishes, fish oil and related fractions, and are incorporated into a variety of food products [b157]. The underlying mechanism of apical uptake of lipid digestion products still remains to be fully elucidated. However both active (apical membrane proteins: fatty acid binding protein and fatty acid translocase) and passive transporters have been proposed [b161]. Intestinal cell models have suggested saturable transport for long chain fatty acids but not for short chain fatty acids [b162].
Hypothetically, the bioavailability of matrix embedded PUFA may differ from its supplemental counterpart. Many studies have focused on the bioavailability of EPA and DHA but the mechanism of their uptake into absorptive epithelial cells and intracellular events leading to basolateral secretion is still derived from knowledge obtained for long chain fatty acids. In some pathological states, such as malabsorptive conditions, absorption of PUFA is reduced. Similarly, Orlistat™, a lipase inhibitor, may also lead to a reduction in the absorption of total lipids by as much as 30% [b163]. Therefore, in order to modulate bioavailability of these bioactive PUFA a clear understanding of physiologically orchestrated steps of pre‐absorptive and absorptive events is essential.
Conclusions
Identifying the bioavailability of bioactive food compounds and pharmaceutical drugs is essential when evaluating their potential health benefits as well as their toxicity. The bioavailability of orally ingested drugs includes the same steps as in bioactive food compounds (LADME) and faces some of the same challenges including transporters, molecular structures, and enzymes. However, unravelling the bioavailability of bioactive food compounds is even more challenging than with drugs since many other factors such as bioaccessibility, food matrix effect and the gut microbiota can affect bioactive food compounds during digestion.
Bioactive food molecules are a diverse group of compounds. They include lipophilic and hydrophilic molecules with complex chemical structures resulting in a number of different absorption mechanisms. For polyphenols, even within the same subclass of compounds, there are significant differences in absorption. For PUFAs, their bioaccessibility is a challenge since these lipids can be represented in different dietary forms and structures, i.e. triacylglycerides or phospholipids. Liberation of PUFA from these constituents varies, affecting their extent of bioavailability. Although there are many studies reporting the bioavailability and the bioefficacy of these bioactive food components, understanding their interactions, metabolism and mechanism of action warrants further work. In addition, it should be considered that current analytical methods for estimating bioavailability in humans through identification of main metabolites have limitations. For example, if we only look at urinary excretion data we underestimate the actual bioavailability, as for most of the bioactive food compounds their metabolism has not been fully characterized.
The next important step in understanding the bioavailability of bioactive food compounds is to identify properly the circulating metabolites in order to have a better understanding of the fate of the parent compounds. Only when the circulating forms of a bioactive food molecule or a drug are known, a more complete picture related to bioavailability and possible correlation to bioefficacy can be obtained. For drugs, this is a requirement when performing bioavailability studies and this type of approach can be translated to nutrition research too.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare MJR, MR, CC‐H, L A‐G, SKT and MSP had support from Nestle Research Center, Nestec Ltd. for the submitted work, MJR, MR, CC‐H, L A‐G, and SKT are and have been employees with Nestle Research Center, Nestec Ltd. in the previous 3 years, MSP is and has been an employee with Nestle Research Center, Nestec Ltd. in the previous 1 year and declares no financial relationships with any other organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work
References
- 1.Kussmann M, Affolter M, Nagy K, Holst B, Fay LB. Mass spectrometry in nutrition: understanding dietary health effects at the molecular level. Mass Spectrom Rev. 2007;26:727–750. doi: 10.1002/mas.20147. [DOI] [PubMed] [Google Scholar]
- 2.Espìn JC, Garcìa‐Conesa MT, Tomàs‐Barberàn FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(1 Suppl.):317S–325. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 4.Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 5.Patil BS, Jayaprakasha GK, Chidambara Murthy KN, Vikram A. Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem. 2009;57:8142–8160. doi: 10.1021/jf9000132. [DOI] [PubMed] [Google Scholar]
- 6.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl.):230S–242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 7.Williamson G, Clifford MN. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br J Nutr. 2010;104:S48–S66. doi: 10.1017/S0007114510003946. [DOI] [PubMed] [Google Scholar]
- 8.U.S.Department of Health and Human Services. Rockville, MD: Center for Drug Evaluation and Research (CDER); 2003. Food and Drug Administration (FaDA). Guidance for industry: bioavailability and bioequivalence studies for orally administrated drug products general considerations. [Google Scholar]
- 9.Benito P, Miller D. Iron absorption and bioavailability: an updated review. Nutr Res. 1998;18:581–603. [Google Scholar]
- 10.Fernàndez‐Garcìa E, Carvajal‐Lérida I, Pérez‐Gàlvez A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr Res. 2009;29:751–760. doi: 10.1016/j.nutres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 12.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130(8S Suppl.):2073S–2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 13.De Roos B, Mavrommatis Y, Brouwer IA. Long‐chain n‐3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158:413–428. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid‐rich foods, and cardiovascular risk: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Kris‐Etherton PM, Hecker KD, Binkoski AE. Polyunsaturated fatty acids and cardiovascular health. Nutr Rev. 2004;62:414–426. doi: 10.1111/j.1753-4887.2004.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 16.Trikalinos TA, Lee J, Moorthy D, Yu WW, Lau J, Lichtenstein AH, Chung M. Effects of eicosapentanoic acid and docosahexanoic acid on mortality across diverse settings: systematic review and meta‐analysis of randomized trials and prospective cohorts. AHRQ Tech Rev. 2012;17:4. [PubMed] [Google Scholar]
- 17.Vauzour D, Rodriguez‐Mateos A, Corona G, Oruna‐Concha MJ, Spencer JPE. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saura‐Calixto F, Serrano J, Goñi I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007;101:492–501. [Google Scholar]
- 19.Gropper SS, Smith JL. 5 edn. Belmont: Wadsworth; 2009. The digestive system: mechanism for nurishing the body; pp. 33–62. (eds). In: Advanced Nutrition and Human Metabolism. [Google Scholar]
- 20.Neilson AP, Ferruzzi MG. Influence of formulation and processing on absorption and metabolism of flavan‐3‐ols from tea and cocoa. Annu Rev Food Sci Technol. 2011;2:125–151. doi: 10.1146/annurev-food-022510-133725. [DOI] [PubMed] [Google Scholar]
- 21.Garrett DA, Failla ML, Sarama RJ. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J Agric Food Chem. 1999;47:4301–4309. doi: 10.1021/jf9903298. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niot I, Poirier H, Tran TTT, Besnard P. Intestinal absorption of long‐chain fatty acids: evidence and uncertainties. Prog Lipid Res. 2009;48:101–115. doi: 10.1016/j.plipres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Tang G, Serfaty‐Lacrosniere C, Camilo ME, Russell RM. Gastric acidity influences the blood response to a beta‐carotene dose in humans. Am J Clin Nutr. 1996;64:622–626. doi: 10.1093/ajcn/64.4.622. [DOI] [PubMed] [Google Scholar]
- 25.Mullen W, Edwards CA, Serafini M, Crozier A. Bioavailability of pelargonidin‐3‐O‐glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J Agric Food Chem. 2008;56:713–719. doi: 10.1021/jf072000p. [DOI] [PubMed] [Google Scholar]
- 26.Welch IM, Davison PA, Worlding J, Read NW. Effect of ileal infusion of lipid on jejunal motor patterns after a nutrient and nonnutrient meal. Am J Physiol. 1988;255:G800–G806. doi: 10.1152/ajpgi.1988.255.6.G800. [DOI] [PubMed] [Google Scholar]
- 27.Welch IM, Cunningham KM, Read NW. Regulation of gastric emptying by ileal nutrients in humans. Gastroenterology. 1988;94:401–404. doi: 10.1016/0016-5085(88)90428-3. [DOI] [PubMed] [Google Scholar]
- 28.Walsh KR, Zhang YC, Vodovotz Y, Schwartz SJ, Failla ML. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. J Agric Food Chem. 2003;51:4603–4609. doi: 10.1021/jf0342627. [DOI] [PubMed] [Google Scholar]
- 29.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full‐fat than with fat‐reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- 30.Berry SE, Tydeman EA, Lewis HB, Phalora R, Rosborough J, Picout DR, Ellis PR. Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects. Am J Clin Nutr. 2008;88:922–929. doi: 10.1093/ajcn/88.4.922. [DOI] [PubMed] [Google Scholar]
- 31.Mateo Anson N, van den Berg R, Havenaar R, Bast A, Haenen GRMM. Bioavailability of ferulic acid is determined by its bioaccessibility. J Cereal Sci. 2009;49:296–300. [Google Scholar]
- 32.Anson NM, Aura AM, Selinheimo E, Mattila I, van den Poutanen K, Berg R, Havenaar R, Bast A, Haenen GRMM. Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo 1‐3. J Nutr. 2011;141:137–143. doi: 10.3945/jn.110.127720. [DOI] [PubMed] [Google Scholar]
- 33.Richelle M, Sabatier M, Steiling H, Williamson G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227–238. doi: 10.1079/bjn20061817. [DOI] [PubMed] [Google Scholar]
- 34.Singh H, Ye A, Horne D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog Lipid Res. 2009;48:92–100. doi: 10.1016/j.plipres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Nawar WW. Lipids. In: Fennema OR, editor. 3 edn. New York, NY: Marcel Dekker; 1996. pp. 225–319. . In: Food Chemistry. ed. [Google Scholar]
- 36.Fernández‐García E, Carvajal‐Lérida I, Jarén‐Galán M, Garrido‐Fernández J, Pérez‐Gálvez A, Hornero‐Méndez D. Carotenoid bioavailability from foods: from plant pigments to efficient biological activities. Food Res Int. 2012;46:438–450. [Google Scholar]
- 37.Cansell M, Nacja F, Combe N. Marine lipid‐based liposomes increase in vivo FA bioavailability. Lipids. 2003;38:551–559. doi: 10.1007/s11745-003-1341-0. [DOI] [PubMed] [Google Scholar]
- 38.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 39.Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr. 2010;104:S67–90. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
- 40.Viskupicova J, Ondrejovic M, Sturdík E. Bioavailability and metabolism of flavonoids. J Food Nutr Res. 2008;47:151–162. [Google Scholar]
- 41.Williamson G. The use of flavonoid aglycones in in vitro systems to test biological activities: based on bioavailability data, is this the valid approach? Phytochem Rev. 2002;1:215–222. [Google Scholar]
- 42.Lolito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti‐inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51:454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer JPE, Abd El Mohsen MM, Rice‐Evans C. Cellular uptake and metabolism of flavonoids and their metabolites: implications for their bioactivity. Arch Biochem Biophys. 2004;423:148–161. doi: 10.1016/j.abb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Scholz S, Williamson G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int J Vitam Nutr Res. 2007;77:224–235. doi: 10.1024/0300-9831.77.3.224. [DOI] [PubMed] [Google Scholar]
- 45.Appeldoorn MM, Vincken JP, Aura AM, Hollman PCH, Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2‐(3,4‐dihydroxyphenyl)acetic acid and 5‐(3,4‐dihydroxyphenyl)‐γ‐ valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–1092. doi: 10.1021/jf803059z. [DOI] [PubMed] [Google Scholar]
- 46.Hollman PC, van Trijp JM, van der Buysman MN, Gaag MS, de Mengelers MJ, Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 47.Hollman PC, van Bijsman MN, Gameren Y, de Cnossen EP, Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic Res. 1999;31:569–573. doi: 10.1080/10715769900301141. [DOI] [PubMed] [Google Scholar]
- 48.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 49.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 50.Erlund I, Kosonen T, Alfthan G, Maenpaa J, Perttunen K, Kenraali J, Parantainen J, Aro A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol. 2000;56:545–553. doi: 10.1007/s002280000197. [DOI] [PubMed] [Google Scholar]
- 51.Hollman PCH, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 52.Ottaviani JI, Momma TY, Heiss C, Kwik‐Uribe C, Schroeter H, Keen CL. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic Biol Med. 2011;50:237–244. doi: 10.1016/j.freeradbiomed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Rusznyàk S, Szent‐Györgyi A. Vitamin P: flavonols as vitamins [5] Nature. 1936;138:27. [Google Scholar]
- 54.Boileau TWM, Boileau AC, Erdman J. Bioavailability of all‐trans and cis‐isomers of lycopene. Exp Biol Med. 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 55.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R‐ and S‐equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 56.Brand W, Shao J, Hoek‐Van Den Hil EF, Van Elk KN, Spenkelink B, De Haan LHJ, Rein MJ, Dionisi F, Williamson G, Van Bladeren PJ, Rietjens IMCM. Stereoselective conjugation, transport and bioactivity of S‐ and R‐hesperetin enantiomers in vitro. J Agric Food Chem. 2010;58:6119–6125. doi: 10.1021/jf1008617. [DOI] [PubMed] [Google Scholar]
- 57.Lévèques A, Actis‐Goretta L, Rein MJ, Williamson G, Dionisi F, Giuffrida F. UPLC‐MS/MS quantification of total hesperetin and hesperetin enantiomers in biological matrices. J Pharm Biomed Anal. 2012;57:1–6. doi: 10.1016/j.jpba.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 58.Richelle M, Lambelet P, Rytz A, Tavazzi I, Mermoud AF, Juhel C, Borel P, Bortlik K. The proportion of lycopene isomers in human plasma is modulated by lycopene isomer profile in the meal but not by lycopene preparation. Br J Nutr. 2012;107:1482–1488. doi: 10.1017/S0007114511004569. [DOI] [PubMed] [Google Scholar]
- 59.Ross AB, Vuong LT, Ruckle J, Synal HA, Schulze‐König T, Wertz K, Rümbeli R, Liberman RG, Skipper PL, Tannenbaum SR, Bourgeois A, Guy PA, Enslen M, Nielsen ILF, Kochhar S, Richelle M, Fay LB, Williamson G. Lycopene bioavailability and metabolism in humans: an accelerator mass spectrometry study. Am J Clin Nutr. 2011;93:1263–1273. doi: 10.3945/ajcn.110.008375. [DOI] [PubMed] [Google Scholar]
- 60.Brand W, Schutte ME, van Williamson G, Zanden JJ, Cnubben NHP, Groten JP, Van Bladeren PJ, Rietjens IMCM. Flavonoid‐mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food‐borne toxic compounds and bioactive ingredients. Biomed Pharmacother. 2006;60:508–519. doi: 10.1016/j.biopha.2006.07.081. [DOI] [PubMed] [Google Scholar]
- 61.Kerns EH, Di L. 1st edn. London: Elsevier; 2008. pp. 103–122. Transporters. Drug‐like Properties: Concepts, Structure Design and Methods, from ADME to Toxicity Optimization. [Google Scholar]
- 62.Chow S‐C, Liu J‐P. Introduction. In: Chow S‐C, Liu J‐P, editors. 3rd edn. Boca Raton: Chapman & Hall/CRC; 2009. pp. 3–11. . In: Design and Analysis of Bioavailability and Bioequivalence Studies. eds. [Google Scholar]
- 63.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- 64.Whitley AC, Sweet DH, Walle T. The dietary polyphenol ellagic acid is a potent inhibitor of hOAT1. Drug Metab Dispos. 2005;33:1097–1100. doi: 10.1124/dmd.105.004275. [DOI] [PubMed] [Google Scholar]
- 65.Itagaki S, Kobayashi Y, Otsuka Y, Kubo S, Kobayashi M, Hirano T, Iseki K. Food‐drug interaction between ferrulic acid and nateglinide involving the fluorescein/H+ cotransport system. J Agric Food Chem. 2005;53:2499–2502. doi: 10.1021/jf047990i. [DOI] [PubMed] [Google Scholar]
- 66.Morris ME, Zhang S. Flavonoid‐drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Sesink ALA, Arts ICW, De Boer VCJ, Breedveld P, Schellens JHM, Hollman PCH, Russel FGM. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol Pharmacol. 2005;67:1999–2006. doi: 10.1124/mol.104.009753. [DOI] [PubMed] [Google Scholar]
- 68.Williamson G, Aeberli I, Miguet L, Zhang Z, Sanchez MB, Crespy V, Barron D, Needs P, Kroon PA, Glavinas H, Krajcsi P, Grigorov M. Interaction of positional isomers of quercetin glucuronides with the transporter ABCC2 (cMOAT, MRP2) Drug Metab Dispos. 2007;35:1262–1268. doi: 10.1124/dmd.106.014241. [DOI] [PubMed] [Google Scholar]
- 69.Richelle M, Enslen M, Hager C, Groux M, Tavazzi I, Godin JP, Berger A, Métairon S, Quaile S, Piguet‐Welsch C, Sagalowicz L, Green H, Fay LB. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta‐carotene and alfa‐tocopherol in normocholesterolemic humans. Am J Clin Nutr. 2004;80:171–177. doi: 10.1093/ajcn/80.1.171. [DOI] [PubMed] [Google Scholar]
- 70.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81(1 Suppl.):243S–255. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 71.Averina ES, Kutyrev IA. Perspectives on the use of marine and freshwater hydrobiont oils for development of drug delivery systems. Biotechnol Adv. 2011;29:548–557. doi: 10.1016/j.biotechadv.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Frank J, Lee S, Leonard SW, Atkinson JK, Kamal‐Eldin A, Traber MG. Sex differences in the inhibition of α‐tocopherol metabolism by a single dose of dietary sesame oil in healthy subjects. Am J Clin Nutr. 2008;87:1723–1729. doi: 10.1093/ajcn/87.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becquemont L, Verstuyft C, Kerb R, Brinkmann U, Lebot M, Jaillon P, Funck‐Brentano C. Effect of grapefruit juice on digoxin pharmacokinetics in humans. Clin Pharmacol Ther. 2001;70:311–316. [PubMed] [Google Scholar]
- 74.Dresser GK, Bailey DG. The effects of fruit juices on drug disposition: a new model for drug interactions. Eur J Clin Invest. 2003;33:10–16. doi: 10.1046/j.1365-2362.33.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 75.Fuhr U. Drug interactions with grapefruit juice. Extent, probable mechanism and clinical relevance. Drug Saf. 1998;18:251–272. doi: 10.2165/00002018-199818040-00002. [DOI] [PubMed] [Google Scholar]
- 76.Edwards DJ, Bellevue FH, III, Woster PM. Identification of 6′,7′‐dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996;24:1287–1290. [PubMed] [Google Scholar]
- 77.He K, Iyer KR, Hayes RN, Sinz MW, Woolf TF, Hollenberg PF. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;11:252–259. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- 78.Hanley MJ, Cancalon P, Widmer WW, Greenblatt DJ. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7:267–286. doi: 10.1517/17425255.2011.553189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirunpanich V, Katagi J, Sethabouppha B, Sato H. Demonstration of docosahexaenoic acid as a bioavailability enhancer for CYP3A substrates: in vitro and in vivo evidence using cyclosporin in rats. Drug Metab Dispos. 2006;34:305–310. doi: 10.1124/dmd.105.007088. [DOI] [PubMed] [Google Scholar]
- 80.Di YM, Li CG, Xue CC, Zhou SF. Clinical drugs that interact with St John's wort and implication in drug development. Curr Pharm Des. 2008;14:1723–1742. doi: 10.2174/138161208784746798. [DOI] [PubMed] [Google Scholar]
- 81.Mannel M. Drug interactions with St John's wort : mechanisms and clinical implications. Drug Saf. 2004;27:773–797. doi: 10.2165/00002018-200427110-00003. [DOI] [PubMed] [Google Scholar]
- 82.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52:S139–S151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 83.Yliperttula M, Urtti A. Nanotechnology for improved drug bioavailability. In: Van de Waterbeemd H, Testa B, editors. 2nd edn. Weinheim: Wiley‐VCH; 2009. pp. 597–604. . In: Drug Bioavailability: Estimation of Solubility, Permeability, Absorption and Bioavailability. eds. [Google Scholar]
- 84.European Food Safety Authoriy. 2011. Scientific opinion: guidence to the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain.
- 85.Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, Wang H, Zhou Q, Yu S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59:9280–9289. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 86.Tzeng CW, Yen FL, Wu TH, Ko HH, Lee CW, Tzeng WS, Lin CC. Enhancement of dissolution and antioxidant activity of kaempferol using a nanoparticle engineering process. J Agric Food Chem. 2011;59:5073–5080. doi: 10.1021/jf200354y. [DOI] [PubMed] [Google Scholar]
- 87.Menon VP, Sudheer AR. Antioxidant and anti‐inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 90.Yu H, Shi K, Liu D, Huang Q. Development of a food‐grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012;131:48–54. [Google Scholar]
- 91.Fang Z, Bhandari B. Encapsulation of polyphenols – A review. Trends Food Sci Tech. 2010;21:510–523. [Google Scholar]
- 92.Fernandez‐Garcia E, Rincon F, Perez‐Galvez A. Developing an emulsifier system to improve the bioaccessibility of carotenoids. J Agric Food Chem. 2008;56:10384–10390. doi: 10.1021/jf801910y. [DOI] [PubMed] [Google Scholar]
- 93.Haug IJ, Sagmo LB, Zeiss D, Olsen IC, Draget KI, Seternes T. Bioavailability of EPA and DHA delivered by gelled emulsions and soft gel capsules. Eur J Lipid Sci Tech. 2011;113:137–145. [Google Scholar]
- 94.Higgins S, Carroll YL, O'Brien NM, Morrissey PA. Use of microencapsulated fish oil as a means of increasing n‐3 polyunsaturated fatty acid intake. J Hum Nutr Diet. 1999;12:265–271. [Google Scholar]
- 95.Wakil A, MacKenzie G, Diego‐Taboada A, Bell JG, Atkin SL. Enhanced bioavailability of eicosapentaenoic acid from fish oil after encapsulation within plant spore exines as microcapsules. Lipids. 2010;45:645–649. doi: 10.1007/s11745-010-3427-y. [DOI] [PubMed] [Google Scholar]
- 96.Wallace JMW, McCabe AJ, Robson PJ, Keogh MK, Murray CA, Kelly PM, Márquez‐Ruiz G, McGlynn H, Gilmore WS, Strain JJ. Bioavailability of n‐3 polyunsaturated fatty acids (PUFA) in foods enriched with microencapsulated fish oil. Ann Nutr Metab. 2000;44:157–162. doi: 10.1159/000012839. [DOI] [PubMed] [Google Scholar]
- 97.Scheepens A, Tan K, Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010;5:75–87. doi: 10.1007/s12263-009-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brand W, Van Der Wel PAI, Rein MJ, Barron D, Williamson G, Van Bladeren PJ, Rietjens IMCM. Metabolism and transport of the citrus flavonoid hesperetin in Caco‐2 cell monolayers. Drug Metab Dispos. 2008;36:1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 99.Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance‐associated proteins in regulating cellular levels of (‐)‐epigallocatechin‐3‐gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 100.Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J Agric Food Chem. 2002;50:618–621. doi: 10.1021/jf010919h. [DOI] [PubMed] [Google Scholar]
- 101.Biehler E, Bohn T. Methods for assessing aspects of carotenoid bioavailability. Curr Nutr Food Sci. 2010;6:44–69. [Google Scholar]
- 102.Gärtner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr. 1997;66:116–122. doi: 10.1093/ajcn/66.1.116. [DOI] [PubMed] [Google Scholar]
- 103.Porrini M, Riso P, Testolin G. Absorption of lycopene from single or daily portions of raw and processed tomato. Br J Nutr. 1998;80:353–361. doi: 10.1079/096582198388300. [DOI] [PubMed] [Google Scholar]
- 104.Richelle M, Bortlik K, Liardet S, Hager C, Lambelet P, Baur M, Applegate LA, Offord EA. A food‐based formulation provides lycopene with the same bioavailability to humans as that from tomato paste. J Nutr. 2002;132:404–408. doi: 10.1093/jn/132.3.404. [DOI] [PubMed] [Google Scholar]
- 105.Benzie IFF, Chung WY, Wang J, Richelle M, Bucheli P. Enhanced bioavailability of zeaxanthin in a milk‐based formulation of wolfberry (Gou Qi Zi; Fructus barbarum L.) Br J Nutr. 2006;96:154–160. doi: 10.1079/bjn20061796. [DOI] [PubMed] [Google Scholar]
- 106.Pouteau EB, Monnard IE, Piguet‐Welsch C, Groux MJA, Sagalowicz L, Berger A. Non‐esterified plant sterols solubilized in low fat milks inhibit cholesterol absorption: a stable isotope double‐blind crossover study. Eur J Nutr. 2003;42:154–164. doi: 10.1007/s00394-003-0406-6. [DOI] [PubMed] [Google Scholar]
- 107.Rossi L, Seijen Ten Hoorn JWM, Melnikov SM, Velikov KP. Colloidal phytosterols: synthesis, characterization and bioaccessibility. Soft Matter. 2010;6:928–936. [Google Scholar]
- 108.Clifford MN. Chlorogenic acids and other cinnamates – Nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–1043. [Google Scholar]
- 109.George SE, Ramalakshmi K, Rao LJM. A perception on health benefits of coffee. Crit Rev Food Sci Nutr. 2008;48:464–486. doi: 10.1080/10408390701522445. [DOI] [PubMed] [Google Scholar]
- 110.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 111.Ferruzzi MG. The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiol Behav. 2010;100:33–41. doi: 10.1016/j.physbeh.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 112.Stalmach A, Mullen W, Barron D, Uchida K, Yokota T, Cavin C, Steiling H, Williamson G, Crozier A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab Dispos. 2009;37:1749–1758. doi: 10.1124/dmd.109.028019. [DOI] [PubMed] [Google Scholar]
- 113.Renouf M, Guy PA, Marmet C, Fraering AL, Longet K, Moulin J, Enslen M, Barron D, Dionisi F, Cavin C, Williamson G, Steiling H. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: small intestine and colon are key sites for coffee metabolism. Mol Nutr Food Res. 2010;54:760–766. doi: 10.1002/mnfr.200900056. [DOI] [PubMed] [Google Scholar]
- 114.Redeuil K, Smarrito‐Menozzi C, Guy P, Rezzi S, Dionisi F, Williamson G, Nagy K, Renouf M. Identification of novel circulating coffee metabolites in human plasma by liquid chromatography‐mass spectrometry. J Chromatogr A. 2011;1218:4678–4688. doi: 10.1016/j.chroma.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 115.Williamson G, Dionisi F, Renouf M. Flavanols from green tea and phenolic acids from coffee: critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Mol Nutr Food Res. 2011;55:864–873. doi: 10.1002/mnfr.201000631. [DOI] [PubMed] [Google Scholar]
- 116.Renouf M, Marmet C, Guy P, Fraering AL, Longet K, Moulin J, Enslen M, Barron D, Cavin C, Dionisi F, Rezzi S, Kochhar S, Steiling H, Williamson G. Nondairy creamer, but not milk, delays the appearance of coffee phenolic acid equivalents in human plasma. J Nutr. 2010;140:259–263. doi: 10.3945/jn.109.113027. [DOI] [PubMed] [Google Scholar]
- 117.Duarte GS, Farah A. Effect of simultaneous consumption of milk and coffee on chlorogenic acids' bioavailability in humans. J Agric Food Chem. 2011;59:7925–7931. doi: 10.1021/jf201906p. [DOI] [PubMed] [Google Scholar]
- 118.Gupta J, Siddique YH, Beg T, Ara G, Afzal M. A review on the beneficial effects of tea polyphenols on human health. Int J Pharm. 2008;4:314–338. [Google Scholar]
- 119.Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006;7:755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 120.Crozier A, Del RD, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010;31:446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 121.Del Rio D, Calani L, Cordero C, Salvatore S, Pellegrini N, Brighenti F. Bioavailability and catabolism of green tea flavan‐3‐ols in humans. Nutrition. 2010;26:1110–1116. doi: 10.1016/j.nut.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 122.van der Burg‐Koorevaar MC, Miret S, Duchateau GS. Effect of milk and brewing method on black tea catechin bioaccessibility. J Agric Food Chem. 2011;59:7752–7758. doi: 10.1021/jf2015232. [DOI] [PubMed] [Google Scholar]
- 123.van het Hof KH, Kivits GA, Weststrate JA, Tijburg LB. Bioavailability of catechins from tea: the effect of milk. Eur J Clin Nutr. 1998;52:356–359. doi: 10.1038/sj.ejcn.1600568. [DOI] [PubMed] [Google Scholar]
- 124.Mazzanti G, Menniti‐Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 125.Dillinger TL, Barriga P, Escárcega S, Jimenez M, Lowe DS, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr. 2000;130(8 Suppl.):2057S–2072. doi: 10.1093/jn/130.8.2057S. [DOI] [PubMed] [Google Scholar]
- 126.Bruinsma K, Taren DL. Chocolate: food or drug? J Am Diet Assoc. 1999;99:1249–1256. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]
- 127.Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–26. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- 128.Aron PM, Kennedy JA. Flavan‐3‐ols: nature, occurrence and biological activity. Mol Nutr Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 129.Kuhnle G, Spencer JPE, Schroeter H, Shenoy B, Debnam ES, Srai SKS, Rice‐Evans C, Hahn U. Epicatechin and catechin are O‐methylated and glucuronidated in the small intestine. Biochem Biophys Res Commun. 2000;277:507–512. doi: 10.1006/bbrc.2000.3701. [DOI] [PubMed] [Google Scholar]
- 130.Shali NA, Curtis CG, Powell GM, Roy AB. Sulphation of the flavonoids quercetin and catechin by rat liver. Xenobiotica. 1991;21:881–893. doi: 10.3109/00498259109039528. [DOI] [PubMed] [Google Scholar]
- 131.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 132.Hodgson JM, Croft KD. Tea flavonoids and cardiovascular health. Mol Aspects Med. 2010;31:495–502. doi: 10.1016/j.mam.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 133.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik‐Uribe C, Schmitz HH, Kelm M. (‐)‐Epicatechin mediates beneficial effects of flavanol‐rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baba S, Osakabe N, Yasuda A, Natsume M, Takizawa T, Nakamura T, Terao J. Bioavailability of (–)‐epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic Res. 2000;33:635–641. doi: 10.1080/10715760000301151. [DOI] [PubMed] [Google Scholar]
- 135.Serafini M, Bugianesi R, Maiani G, Valtuena S, De SS, Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- 136.Keogh JB, McInerney J, Clifton PM. The effect of milk protein on the bioavailability of cocoa polyphenols. J Food Sci. 2007;72:S230–S233. doi: 10.1111/j.1750-3841.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 137.Roura E, Andres‐Lacueva C, Estruch R, Mata‐Bilbao ML, Izquierdo‐Pulido M, Waterhouse AL, Lamuela‐Raventos RM. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann Nutr Metab. 2007;51:493–498. doi: 10.1159/000111473. [DOI] [PubMed] [Google Scholar]
- 138.Roura E, Andres‐Lacueva C, Estruch R, Lourdes Mata BM, Izquierdo‐Pulido M, Lamuela‐Raventos RM. The effects of milk as a food matrix for polyphenols on the excretion profile of cocoa (‐)‐epicatechin metabolites in healthy human subjects. Br J Nutr. 2008;100:846–851. doi: 10.1017/S0007114508922534. [DOI] [PubMed] [Google Scholar]
- 139.Schroeter H, Holt RR, Orozco TJ, Schmitz HH, Keen CL. Nutrition: milk and absorption of dietary flavanols. Nature. 2003;426:787–788. doi: 10.1038/426787b. [DOI] [PubMed] [Google Scholar]
- 140.Mullen W, Borges G, Donovan JL, Edwards CA, Serafini M, Lean ME, Crozier A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan‐3‐ol metabolites in humans. Am J Clin Nutr. 2009;89:1784–1791. doi: 10.3945/ajcn.2008.27339. [DOI] [PubMed] [Google Scholar]
- 141.Schramm DD, Karim M, Schrader HR, Holt RR, Kirkpatrick NJ, Polagruto JA, Ensunsa JL, Schmitz HH, Keen CL. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003;73:857–869. doi: 10.1016/s0024-3205(03)00373-4. [DOI] [PubMed] [Google Scholar]
- 142.Neilson AP, George JC, Janle EM, Mattes RD, Ralf R, Matusheski NV, Ferruzzi MG. Influence of chocolate matrix composition on cocoa flavan‐3‐ol bioaccessibility in vitro and bioavailability in humans. J Agric Food Chem. 2009;57:9418–9426. doi: 10.1021/jf902919k. [DOI] [PubMed] [Google Scholar]
- 143.Donovan JL, Manach C, Rios L, Morand C, Scalbert A, Rémésy C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br J Nutr. 2002;87:299–306. doi: 10.1079/bjnbjn2001517. [DOI] [PubMed] [Google Scholar]
- 144.Gonthier MP, Donovan JL, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 145.Holt RR, Lazarus SA, Cameron Sullards M, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. Procyanidin dimer B2 [epicatechin‐(4β‐8)‐epicatechin] in human plasma after the consumption of a flavanol‐rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 146.Tzounis X, Rodriguez‐Mateos A, Vulevic J, Gibson GR, Kwik‐Uribe C, Spencer JPE. Prebiotic evaluation of cocoa‐derived flavanols in healthy humans by using a randomized, controlled, double‐blind, crossover intervention study. Am J Clin Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 147.Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil‐Izquierdo A, Mardon J, Lebecque P, Davicco MJ, Chee WSS, Coxam V, Offord E. Hesperidin inhibits ovariectomized‐induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol. 2008;104:648–654. doi: 10.1152/japplphysiol.00441.2007. [DOI] [PubMed] [Google Scholar]
- 148.Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, Senese N, Tesauro M, Lauro D, Cardillo C, Quon MJ. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E782–E792. doi: 10.1210/jc.2010-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Manach C, Morand C, Demigne C, Texier O, Regerat F, Remesy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409:12–16. doi: 10.1016/s0014-5793(97)00467-5. [DOI] [PubMed] [Google Scholar]
- 150.Brett GM, Hollands W, Needs PW, Teucher B, Dainty JR, Davis BD, Brodbelt JS, Kroon PA. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br J Nutr. 2009;101:664–675. doi: 10.1017/S000711450803081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Erlund I, Silaste ML, Alfthan G, Rantala M, Kesaniemi YA, Aro A. Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur J Clin Nutr. 2002;56:891–898. doi: 10.1038/sj.ejcn.1601409. [DOI] [PubMed] [Google Scholar]
- 152.Manach C, Morand C, Gil‐Izquierdo A, Bouteloup‐Demange C, Remesy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003;57:235–242. doi: 10.1038/sj.ejcn.1601547. [DOI] [PubMed] [Google Scholar]
- 153.Mullen W, Archeveque MA, Edwards CA, Matsumoto H, Crozier A. Bioavailability and metabolism of orange juice flavanones in humans: impact of a full‐fat yogurt. J Agric Food Chem. 2008;56:11157–11164. doi: 10.1021/jf801974v. [DOI] [PubMed] [Google Scholar]
- 154.Nielsen IL, Chee WS, Poulsen L, Offord‐Cavin E, Rasmussen SE, Frederiksen H, Enslen M, Barron D, Horcajada MN, Williamson G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double‐blind, crossover trial. J Nutr. 2006;136:404–408. doi: 10.1093/jn/136.2.404. [DOI] [PubMed] [Google Scholar]
- 155.De Goede J, Geleijnse JM, Boer JMA, Kromhout D, Verschuren WMM. Marine (n‐3) fatty acids, fish consumption, and the 10‐year risk of fatal and nonfatal coronary heart disease in a large population of Dutch adults with low fish intake. J Nutr. 2010;140:1023–1028. doi: 10.3945/jn.109.119271. [DOI] [PubMed] [Google Scholar]
- 156.Gonzàlez‐Barrio R, Trindade LM, de Manzanares P, Graaff LH, Tomàs‐Barberàn FA, Espìn JC. Production of bioavailable flavonoid glucosides in fruit juices and green tea by use of fungal alpha‐L‐rhamnosidases. J Agric Food Chem. 2004;52:6136–6142. doi: 10.1021/jf0490807. [DOI] [PubMed] [Google Scholar]
- 157.Innis SM. Dietary (n‐3) fatty acids and brain development. J Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 158.Quinn JF, Raman R, Thomas RG, Yurko‐Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, Shinto L, Aisen PS. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vedtofte MS, Jakobsen MU, Lauritzen L, Heitmann BL. Dietary α‐linolenic acid, linoleic acid, and n‐3 long‐chain PUFA and risk of ischemic heart disease. Am J Clin Nutr. 2011;94:1097–1103. doi: 10.3945/ajcn.111.018762. [DOI] [PubMed] [Google Scholar]
- 160.Wang W, Shinto L, Connor WE, Quinn JF. Nutritional biomarkers in Alzheimer's disease: the association between carotenoids, n‐3 fatty acids, and dementia severity. J Alzheimers Dis. 2008;13:31–38. doi: 10.3233/jad-2008-13103. [DOI] [PubMed] [Google Scholar]
- 161.Minich DM, Vonk RJ, Verkade HJ. Intestinal absorption of essential fatty acids under physiological and essential fatty acid‐deficient conditions. J Lipid Res. 1997;38:1709–1721. [PubMed] [Google Scholar]
- 162.Trotter PJ, Ho SY, Storch J. Fatty acid uptake by Caco‐2 human intestinal cells. J Lipid Res. 1996;37:336–346. [PubMed] [Google Scholar]
- 163.Zhi J, Melia AT, Eggers H, Joly R, Patel IH. Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers. J Clin Pharmacol. 1995;35:1103–1108. doi: 10.1002/j.1552-4604.1995.tb04034.x. [DOI] [PubMed] [Google Scholar]


