Abstract
OBJECTIVES The aim of this overview of systematic reviews (SRs) is to evaluate critically the evidence regarding interactions between herbal medicinal products (HMPs) and synthetic drugs.
METHODS Four electronic databases were searched to identify relevant SRs.
RESULTS Forty‐six SRs of 46 different HMPs met our inclusion criteria. The vast majority of SRs were of poor methodological quality. The majority of these HMPs were not associated with severe herb–drug interactions. Serious herb–drug interactions were noted for Hypericum perforatum and Viscum album. The most severe interactions resulted in transplant rejection, delayed emergence from anaesthesia, cardiovascular collapse, renal and liver toxicity, cardiotoxicity, bradycardia, hypovolaemic shock, inflammatory reactions with organ fibrosis and death. Moderately severe interactions were noted for Ginkgo biloba, Panax ginseng, Piper methysticum, Serenoa repens and Camellia sinensis. The most commonly interacting drugs were antiplatelet agents and anticoagulants.
CONCLUSION The majority of the HMPs evaluated in SRs were not associated with drug interactions with serious consequences. However, the poor quality and the scarcity of the primary data prevent firm conclusions.
Keywords: drug interactions, herbal medicine, safety, systematic reviews
Introduction
The prevalence of use of herbal medicinal products (HMPs) is high and continues to increase. This applies to the UK [1] as well as other parts of the world [2]. It is therefore important to be aware of the safety issues associated with the administration of HMPs [3–5]. HMPs contain pharmacologically active ingredients, some of which might interact with synthetic drugs [4] which, in turn, could endanger the health of patients [5–7].
The aim of this article is to provide an overview and critical evaluation of the evidence from systematic reviews (SRs) of herb–drug interactions.
Methods
Electronic literature searches were conducted in January 2012 to identify SRs of herb–drug interactions. The following electronic databases were used: MEDLINE and EMBASE (via OVID), AMED and CINHAL (via EBSCO) and Cochrane Database. Search terms were constructed using ‘herbal medicine’ and ‘adverse events’ terms and their derivatives and MeSH terms, and ‘review’ in the title of the article (details of the search strategy are presented in the appendix). Our own extensive departmental files were hand‐searched.
No restrictions of language or time of publication were imposed. Abstracts of reviews thus located were inspected and those appearing to meet the inclusion criteria were retrieved for further evaluation by both authors. Systematic reviews were defined as articles that included an explicit and repeatable methodology. To get included, SRs had to focus on herb–drug interactions. If, for one specific HMP, multiply SRs were found, the most up‐to‐date, methodologically sound and independent one was included. Reviews of mixtures of more than one HMP and SRs of polyherbals were excluded. Non‐systematic reviews and/or reviews pertaining to the effectiveness of HMPs were also excluded. The methodological quality of all SRs was assessed using the modified Oxman score [8]. This is a validated tool that applies the following criteria for assessing the methodological quality of review articles: reporting of search methods and their comprehensiveness, repeatability of eligibility criteria, avoidance of selection bias and reliability of conclusions. These domains were scored as follows: 1 (fulfilled), 0 (partially fulfilled) or −1 (not fulfilled). A final result of 0 or below means the review has major flaws, 1–2 minor flaws and 3–5 minimal or no flaws.
Results
Our searches generated 4366 articles, of which 4320 had to be excluded (Figure 1). Thus 46 SRs met our inclusion criteria (Table 1) [9–54]. The following herbs were considered to interact with synthetic drugs: Aloe vera, Boswellia serrata, Calendula officinalis, Camellia sinensis, Cassia senna, Caulophyllum thalictroides, Cinnamomum spp., Cimicifuga racemosa, Cnicus benedictus, Commifora mukul, Crataegus spp., Crocus sativus, Curcuma longa, Echinacea spp., Ganoderma lucidum, Ginkgo biloba, Grifola frondosa, Gymnema sylvestre, Harpagophytum procumbens, Herbae pulvis standardisatus, Hippocastanaceae, Hypericum perforatum, Lagerstroemia speciosa, Larrea tridentate, Lavandula angustifolia miller, Medicago sativa, Melissa officinalis, Mentha piperita, Mentha spicata/Mentha viridis, Momordica charantia, Morinda citrifolia, Panax ginseng, Piper methysticum, Pelargonium sidoides, Perna canaliculus, Petasites hybridus, Rosmarinus officinalis, Serenoa repens, Salvia hispanica, Stevia rebaudiana, Taraxacum officinale, Thymus vulgaris, Trifolium pretense, Trigonella foenum‐graecum, Viscum album and Vitex agnus‐castus.
Figure 1.
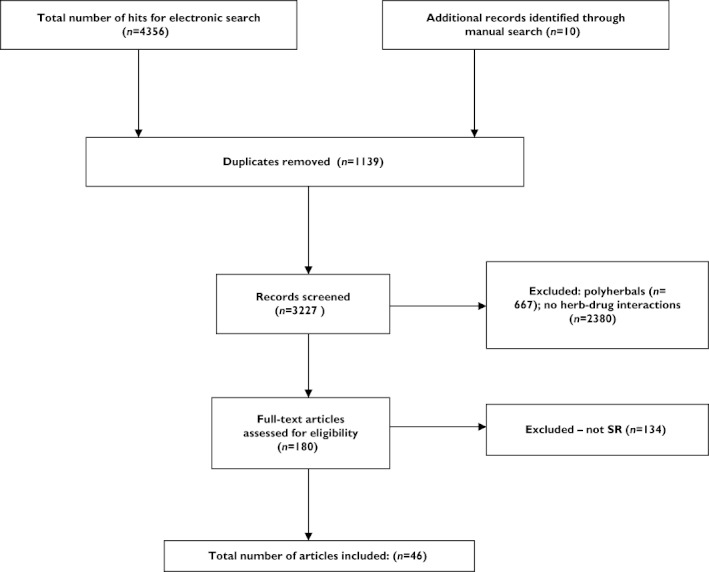
Flow diagram
Table 1.
Key data from the included SRs
| First author (year) Country [Ref] | Type of primary data | *(n) | HMPs evaluated | Drugs that interact | Type of interactions | Clinical outcomes | Mechanism of action | Overall judgment | Quality of SR | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Armbruer (2012) USA [40] | RCTs, NRCT, CCT, SRs, MAs, CR, CS, AS, in vitro | <100 | Cinnamon (Cinnamomum spp.) | 1. Antibiotics | Synergism with 1†, 2†, 3†and‡, 4, 5‡, 6†and‡,7, 9‡, 10†, 11‡, 12†and‡, 13‡, | Increased risk of hypoglycaemia, bleeding (theoretical) | Inhibited arachidonic acid release and thromboxane B2 formation; inhibition of HMG‐CoA activity, inhibition of aminopyrine N‐demethylation | Only minor concerns | −4 | Most interactions were theoretical, limited evidence in humans. Caution for patients with diabetes, autoimmune diseases, liver damage, and for patients using antiarrhythmic agents, antilipemics and anticoagulant or antiplatelet agents. |
| 2. Anticoagulants or antiplatelets | ||||||||||
| 3. Antidiabetics | ||||||||||
| 4. Antifungals | ||||||||||
| 5. Antilipaemics | ||||||||||
| 6. Antineoplastic agents | ||||||||||
| 7. Antiretroviral agents | ||||||||||
| 8. Cytochrome P450 metabolized agents | ||||||||||
| 9. Anxiolytics | ||||||||||
| 10. Oestrogens | ||||||||||
| 11. Hepatotoxic agents | ||||||||||
| 12. Immunosuppressants | ||||||||||
| 13. Sympathomimetics | ||||||||||
| Barrette (2012) USA [41] | RCTs, CCT, SRs, CR, CS, AS, in vitro | <100 | Black cohosh (Cimicifuga racemosa) | 1. Antihistamines | Inhibition of 1†, 8, synergism with 2, 3†, 4, | GI upset | It is not clear how (or if) black cohosh interacts with estrogens/estrogen receptors and/or progestins | Only minor concerns | −4 | Interaction data in this area were lacking. Caution for patients with known estrogen sensitive conditions, such as breast cancer, uterine cancer or endometriosis; in patients on hormone replacement therapy, including tamoxifen or raloxifene; in epileptic patients; in patients on antihypertensive medications; and in patients with liver disease. |
| 2. Antihypertensives | ||||||||||
| 3. Antilipemic agents | ||||||||||
| 4. Antineoplastic agents | ||||||||||
| 5. Antiseizures | ||||||||||
| 6. OEstrogens | ||||||||||
| 7. Hepatotoxic agents | ||||||||||
| 8. Oral agents | ||||||||||
| 9. Tamoxifen, raloxifene | ||||||||||
| Basch (2003) USA [9] | CCTs, CRs | <10 | Bitter melon (Momordica charantia) | 1. Hypoglycaemics | Synergism with 1 | Lowered blood glucose concentrations | Decreased hepatic gluconeogenesis, increased hepatic glycogen synthesis; increased pancreatic insulin secretion. | Only minor concerns | −4 | Low quality and quantity of the available evidence regarding interactions. Caution for patients with diabetes |
| Basch (2003) USA [10] | CS, CR | <10 | Alfalfa (Medicago sativa) | 1. Hypoglycaemics | Synergism with 1 and 2 | Increased drug‐induced photosensitivity, lowered blood glucose, total cholesterol or LDL | Saponins may reduce cholesterol absorption | Only minor concerns | 2 | Chlorpromazine was reported to increase drug‐induced photosensitivity when taken in combination with alfalfa |
| 2. Cholesterol lowering agents | ||||||||||
| 3. Chlorpromazine | ||||||||||
| Basch (2004) USA [11] | RCT, SRS | <10 | Thyme (Thymus vulgaris) | 1. 5‐fluorouracil | Synergism with 1 | n.k. | Thymol increases the stratum corneum lipids fluidity and perturbing the barrier integrity of the epidermis | Only minor concerns | −4 | Thyme may decrease concentrations of thyroid hormone; caution for patients taking hepatotoxic agents |
| Basch (2005) USA [42] | RCTs, NRCT, CCT, SRs, MAs, OS, CR, CS, AS, in vitro | <100 | Echinacea spp. | 1. Amoxicillin | Synergism with 2†, 3, 4, 5‡ and inhibition of 3 | Rhabdomyolysis, shock, and death (causality questioned) | Selective modulation of the catalytic activity of CYP3A at hepatic and intestinal sites | Only minor concerns | −4 | Use cautiously in patients using cytochrome P450‐metabolized agents or hepatotoxic drugs |
| 2. Antineoplastic agents | ||||||||||
| 3. Cytochrome P450‐metabolized agents | ||||||||||
| 4. Hepatotoxic agents | ||||||||||
| 5. Hydrophilic agents | ||||||||||
| Basch (2006) USA [12] | RCTs, CS, comparison study, AS | <10 | Calendula (Calendula officinalis) | 1. Sedatives | Synergism with 1 and 2‡ | n.k. | Insufficient evidence to determine pharmacodynamics/kinetics | Only minor concerns | 0 | Systemic effects in humans were not clear; caution for patients taking sedatives |
| 2. Antihypertensives | ||||||||||
| Basch (2004) USA [13] | RCTs, NRCT,CS, AS | <10 | Lavender (Lavandula angustifolia Miller) | 1. Sedatives, | Synergism with 1, 2, 3 and 4‡ | n.k. | Linalool binds to glutamate and increases GABA concentrations‡ | Only minor concerns | −4 | Use cautiously in patients taking sedatives, anticoagulants, antiplatelet agents and anti‐epileptic drugs |
| 2. Anticoagulants, NSAIDs, anti‐platelet agents, | ||||||||||
| 3. Anti‐seizures | ||||||||||
| 4. Cholesterol lowering agents‡ | ||||||||||
| Basch (2004) USA [14] | RCTs, NRCTs, SR, comparison study, in vitro | <100 | Boswellia (Boswellia serrata) | 1. Leukotriene inhibitors | Inhibition of 1 and synergism with 2† | n.k. | Inhibition of lipoxygenase to produce 5‐HETE, LTB4 and HLE† | Only minor concerns | −4 | Use cautiously in patients taking leukotriene inhibitors |
| 2. Anti‐neoplastic agents† | ||||||||||
| Basch (2012) USA [43] | RCTs, NRCT, CCT, SRs, OS, cohort study, CR, CS, CCS, AS, in vitro | <100 | Mistletoe (Viscum album) | 1. Antidiabetic agents | Synergism with 1†, 2, 3†and‡, 4, 5, 6, inhibition of 7† | Organ fibrosis and death, cardiotoxicity, bradycardia, hypovolaemic shock and CVD collapse, inflammatory reaction | Mistletoe lectins agglutinate human erythrocytes and react with immunoglobulins | Serious concerns | −4 | Use cautiously in patients with cardiovascular disease, uncontrolled hyperthyroidism, seizures, glaucoma and diabetics. |
| 2. Antihypertensives | ||||||||||
| 3. Antineoplastic agents | ||||||||||
| 4. Cholinergic agents | ||||||||||
| 5. CNS depressants | ||||||||||
| 6. Diuretics | ||||||||||
| 7. Immunosuppressants | ||||||||||
| 8. Thyroid hormones | ||||||||||
| Basch (2012) USA [44] | RCTs, NRCT, CCT, SRs, MA, CR, AS, in vitro | <100 | Hawthorn (Crataegus spp.) | 1. Alpha agonists | Inhibition of 1†and‡, 2†, synergism with 3†and‡, 4†and‡, 5‡, 6‡, 7‡, 8‡ | Increased risk of bleeding† | Inhibition of thromboxane A2 biosynthesis† | Only minor concerns | −4 | Although possible safe co‐administration of hawthorn and cardiac glycosides has been suggested, close monitoring during dose titration is warranted. |
| 2. Anticoagulants and antiplatelets | ||||||||||
| 3. Antihypertensives | ||||||||||
| 4. Antilipaemic agents | ||||||||||
| 5. β‐adrenoceptor blockers | ||||||||||
| 6. Digoxin, digitoxin | ||||||||||
| 7. Phosphodiesterase inhibitors | ||||||||||
| 8. Vasodilators | ||||||||||
| Basch (2012) USA [45] | RCTs, CCT, SRs, MAs, CR, AS, in vitro | <100 | Green tea (Camellia sinensis) | 1. Analgesics | Synergism with 1, 4† and ‡, 6, 7, 9, 15, 16 inhibition of 2, 8, 10, 13, 14†, | Increased risk of toxic effects, hypertensive crisis;, impaired iron metabolism and microcytic anaemia, increased blood pressure; ischaemic stroke | EGCG inhibits the IL‐1 beta‐induced activity and expression of cyclooxygenase‐2 and nitric oxide synthase‐2; caffeine acts via blockade of adenosine receptors, and theoretically, may antagonize the effects of adenosine | Some concerns | −4 | Use cautiously in patients taking analgesics, antilipaemics, antiseizures, antivirals, β‐adrenoceptor blockers, cytochrome P450‐metabolized agents, hepatotoxic agents, hormonal agents and sedatives. |
| 2. Antiandrogens | ||||||||||
| 3. Antiarthritics | ||||||||||
| 4. Anticoagulants and antiplatelets | ||||||||||
| 5. Antidepressants | ||||||||||
| 6. Antilipaemics | ||||||||||
| 7. Antivirals | ||||||||||
| 8. Cytochrome P450metabolized agents | ||||||||||
| 9. Hepatotoxic agents | ||||||||||
| 10. Oestrogen | ||||||||||
| 11. Hypertensives | ||||||||||
| 12. Hypoglycaemics | ||||||||||
| 13. Sedatives | ||||||||||
| 14. P‐glycoprotein modulators | ||||||||||
| 15. Antiseizures | ||||||||||
| 16. β−adrenoceptor agonists | ||||||||||
| Brendler (2006) Germany [15] | RCTs, CS, AS | <100 | Devil's claw (Harpagophytum procumbens) | 1. Anti‐arrhythmic agents | Synergism with 1‡, inhibition of 2‡ | Decreased HR ‡ | Release of inflammatory mediators†; inhibition of arachidonic acid metabolism pathways† | Only minor concerns | 0 | Use with anticoagulant and antiplatelet agents should be approached with caution |
| 2. Inotropic agents | ||||||||||
| 3. Anticoagulant/antiplatelet agents | ||||||||||
| Brendler (2012) Germany [46] | RCTs, AS, in vitro | <100 | Noni (Morinda citrifolia) | 1. Anti‐angiogenic drugs | Synergism with 1†, 2†, 3‡, 5‡, 6, inhibition of 3, 7‡ | Decreased gastric transit time‡, increased risk of hyperkalemia, | Inhibition of copper‐induced LDL oxidation; of Ras oncogene function, cell transformation; of the tumour‐promoting effect of TNF‐a, and activator protein‐1 transactivation. | Only minor concerns | −4 | Use cautiously in patients using warfarin or other anticoagulants, antihypertensives or ACE inhibitors. |
| 2. Antibiotics | ||||||||||
| 3. Anticoagulants | ||||||||||
| 4. Antihypertensives | ||||||||||
| 5. Anti‐inflammatory agents | ||||||||||
| 6. Hepatotoxic agents | ||||||||||
| 7. Immunosuppressants | ||||||||||
| Ceurvels, (2012) USA [47] | CR, AS | <10 | Blue cohosh (Caulophyllum thalictroides) | 1. Antidiabetic agents | Synergism with 3 and 5 | Coronary vasoconstriction, tachycardia, increase in blood pressure, diaphoresis, abdominal pain, vomiting and muscle weakness | caulosaponin and caulophyllosaponin, have been shown to have labour induction properties | Only minor concerns | −4 | Use cautiously in patients who are pregnant or breast‐feeding; in patients who smoke or are quitting smoking due to possible nicotine toxicity; and in patients with diabetes |
| 2. Cardiovascular drugs | ||||||||||
| 3. Nicotine | ||||||||||
| 4. Oxytocin | ||||||||||
| 5. Cocaine | ||||||||||
| Ernst (2005) UK [16] | CRs | <100 | Ginkgo biloba | 1. Paracetamol (acetaminophen) | Inhibition of 1, 3, 4, 6 | Haemorrhage, bleeding, apraxia, death, haematoma, hyphaema, permanent neurological deficit | Inhibition of platelet aggregation | Some concerns | 5 | Use cautiously in patients taking anaesthetics, analgesics, anticoagulants and antiplatelet agents |
| 2. lisinopril | ||||||||||
| 3. anaesthetics | ||||||||||
| 4. aspirin | ||||||||||
| 5. warfarin | ||||||||||
| 6. ibuprofen | ||||||||||
| Giles (2005) USA [17] | RCT, OLS, SR | <100 | Butterbur (Petasites hybridus) | 1. Anticholinergics | Synergism with 1 | Increased liver enzyme levels | Reduction of smooth muscle spasm; inhibition of lipoxygenase activity and down‐regulation of leukotriene synthesis‡ | Only minor concerns | 1 | Administration of butterbur with anticholinergics may not be advisable |
| Keifer (2007) USA [48] | RCTs, AS, in vitro | <10 | Peppermint (Mentha piperita) | 1. Antibiotics | Synergism with 1,†3,† 6,† and inhibition of 2,† 4,‡ 5†and ‡ | n.k. | Menthol and menthyl acetate may inhibit CYP3A4‐mediated nifedipine metabolism and increase felodopine concentrations | Only minor concerns | 2 | No documented interactions in humans. |
| 2. Benzoic acid | ||||||||||
| 3. Calcium channel blockers | ||||||||||
| 4. Ciclosporin | ||||||||||
| 5. Cytochrome P450 metabolized agents | ||||||||||
| 6. Oxytetracycline | ||||||||||
| Nelsen (2002) USA [18] | RCTs, CS, in vitro | <100 | Red clover (Trifolium pratense) | 1. Cytochrome P450‐metabolized agents | Inhibition of 1†, 2 and synergism with 2 | Alleviated GrRH, FSH and LH concentrations | Binding to estradiol receptors (estradiol‐α and estradiol‐β) | Only minor concerns | 1 | Red clover may have synergistic effects with anticoagulants or antiplatelet agents; use cautiously in patients taking hormonal agents |
| 2. HRT and OCPs | ||||||||||
| Sweeney (2005) USA [19] | Case series, in vitro, AS | <10 | Dandelion (Taraxacum officinale) | 1. Ciprofloxacin | Inhibition of 1‡, synergism with 3†, 4‡ | Inhibition of platelet aggregation† | Sesquiterpenes lactones may act as anti‐inflammatory agents; lactones may increase gastric acid secretion; increased bile production and release; inulin may act to buffer blood glucose concentrations | Only minor concerns | −4 | Patients using antihypertensive and/or antidiabetic agents or insulin should be monitored closely while using dandelion. |
| 2. Hypoglycaemic drugs | ||||||||||
| 3. Anticoagulants | ||||||||||
| 4. Cytochrome P450 1A2 and 2E metabolized agents | ||||||||||
| Tiffany (2002) USA [20] | RCTs, SR, OS, AS | <100 | Horse chestnut seed extract (Hippocastanaceae) | 1. Hypoglycaemic agents | Synergism with 1‡ | n.k. | Inhibition of the normal increase of serum glucose concentrations | Only minor concerns | 1 | No documented interactions in humans. |
| Ulbricht (2003) USA [21] | CR, AS | <100 | Chaparral (Larrea tridentate) | 1. Cytochrome P450 metabolized agents | Inhibition of 1‡ | Increased renal and liver toxicity (theoretically) | Diminished platelet aggregation; decrease plasma glucose concentrations; blocked cellular respiration and exerted antioxidant effects; inhibited induction of ornithine decarboxylase† and ‡ | Only minor concerns | 2 | Chaparral should be avoided in combination with potentially hepatotoxic agents |
| Ulbricht (2004) USA [22][22] | RCTs, NRCT, OS, AS | <100 | Belladonna (Herbae pulvis standardisatus) | 1. Cisapride | Inhibition of 1, 2‡ | Delayed gastrointestinal transit time | Inhibition of the muscarinic actions of acetylcholine | Only minor concerns | −4 | Avoid concomitant use with alcohol, anti‐arrhythmics,antidepressants, anticholinergic and drugs that interact with atropine |
| 2. Tacrine | ||||||||||
| Ulbricht (2005) USA [23] | RCT, CS, AS | <100 | Lemon balm (Melissa officinalis) | 1. Barbiturates | Synergism with 1‡, 2, inhibition of 4† | Hypnosis‡, sedation | Reduced pituitary and serum TSH concentrations‡ | Only minor concerns | 0 | Use cautiously in patients taking hormonal agents and sedatives |
| 2. Sedatives | ||||||||||
| 3. Nicotine and scopolamine | ||||||||||
| 4. SSRIs | ||||||||||
| Ulbricht (2005) USA [24] | RCTs, CS, AS | <100 | Guggul (Commifora mukul) | 1. Propranolol | Inhibition of 1, 2, synergism with 3‡, 5 | Increased risk of bleeding (theoretical) | Guggulsterones have been reported to function as antagonists of the farsenoid X receptor | Only minor concerns | 1 | Guggulipid should be used with caution in patients taking thyroid drugs. |
| 2. Diltiazem | ||||||||||
| 3. Thyroid agents | ||||||||||
| 4. Lipid‐lowering agents | ||||||||||
| 5. Anticoagulants, antiplatelet agents | ||||||||||
| Ulbricht (2005) USA [25] | RCTs CRs, in vitro | <100 | Kava (Piper methysticum) | 1. Cytochrome P450 substrates | Inhibition of 1†, 2, 3†, and synergism with 5 | Coma, sedation, lethargy, drowsiness | Kavalactones or kavapyrones may alter central GABA transmission, blocking ion channels; inhibition of thromboxane synthesis and cyclooxygenase. | Some concerns | 3 | Anesthesiologists recommend stop taking kava 2–3 weeks prior to surgery; patients with Parkinson's disease should avoid kava. Avoid combining kava with hepatotoxic agents. |
| 2. Dopamine agonists and antagonists | ||||||||||
| 3. Monoamine oxidase inhibitors | ||||||||||
| 4. Antiplatelet agents | ||||||||||
| 5. Sedatives/CNS depressants | ||||||||||
| Ulbricht (2006) USA [49] | RCTs, SRs, CR, CS, AS, in vitro | <100 | Saw palmetto (Serenoa repens) | 1. Androgenic drugs | Inhibition of 1, synergism with 2, 3, 4, 6 and 10 | Severe intra‐operative and cerebral haemorrhage, hypertension, nausea or vomiting | Inhibition of lipooxygenase and cyclooxygenase; exerted activity on estrogen receptors; stimulation of macrophage phagocytosis and NK cell synthesis of interferon‐gamma† | Some concerns | −4 | Use cautiously in patients with hypertension, hormone‐sensitive conditions and bleeding disorders |
| 2. Anti‐androgenic drugs | ||||||||||
| 3. Anticoagulants and antiplatelets | ||||||||||
| 4. Antibiotics | ||||||||||
| 5. Antihypertensives | ||||||||||
| 6. Anti inflammatory agents | ||||||||||
| 7. Cytochrome P450 metabolized agents | ||||||||||
| 8. Metronidazole or disulfiram | ||||||||||
| 9. Hormonal agents | ||||||||||
| 10. Immunomodulators | ||||||||||
| Ulbricht (2007) USA [27] | RCTs, NRCT, CCT, SRs, MA, CRs, CS, AS | <10 | Fenugreek (Trigonella foenum‐graecum) | 1. Anti‐arrhythmic agents/cardiac glicosides/potassium depletings | Synergism with 2, 5 inhibition of 3‡ | Increased INR, reduced potassium levels, lowered LDL, TG, and total cholesterol ‡ and †, improved insulin resistance‡ | Modulation of beta‐glucuronidase and mucinase activities, DNA fragmentation by protodiosgenin; phosphorylation of insulin receptor, or activation of insulin signalling pathway | Only minor concerns | 2 | Use cautiously in patients taking antidiabetics and antilipemics |
| 2. Antidiabetic agents | ||||||||||
| 3. Anticoagulants and antiplatelets | ||||||||||
| 4. Antilipaemic agents | ||||||||||
| 5. Laxatives | ||||||||||
| Ulbricht (2007) USA [28] | RCT, AS | <10 | Banaba (Lagerstroemia speciosa) | 1. Hypoglycaemic agents | Synergism with 1‡ | n.k. | Increase the rate of glucose uptake and decrease the isoprenaline‐induced glycerol release | Only minor concerns | 1 | Lagerstroemin may activate insulin receptors, use cautiously in diabetic patients |
| Ulbricht (2007) USA [50] | RCTs, CR, AS, in vitro | <100 | Aloe vera | 1. Insulin | Synergism with 1, 6 and 7 | Potassium depletion, hypokalaemia, increased hypoglycaemic effect | Anthraquinone glycosides act as laxatives; stimulation of β cells | Only minor concerns | 2 | Use cautiously in patients with diabetes or glucose intolerance. |
| 2. Oral hypoglycemic agents | Avoid oral aloe latex in patients with renal insufficiency, cardiac disease, or electrolyte abnormalities | |||||||||
| 3. Laxatives | ||||||||||
| 4. Sevoflurane | ||||||||||
| 5. Thyroid hormones | ||||||||||
| 6. Topical hydrocortisone | ||||||||||
| 7. Zidovudine | ||||||||||
| Ulbricht (2008) USA [39] | AS, in vitro | <100 | Blessed thistle (Cnicus benedictus) | 1. Antibiotics | Synergism with 1†, 2†, 3† and ‡ | Increasing bleeding risk (theoretical) | Cnicin and arctigenin have exhibited cytotoxic activity against some tumor cells via inhibition of cellular DNA, RNA or protein synthesis† | Only minor concerns | −4 | Limited evidence in humans |
| 2. Anticoagulant and antiplatelet agents | ||||||||||
| 3. Antineoplastic agents | ||||||||||
| Ulbricht (2009) USA [29] | RCTs, AS | <10 | Chia (Salvia hispanica) | 1. Anticoagulants and antiplatelets | Synergism with 1‡, 2,3 | Lowered blood pressure | Increased levels of alphalinolenic acid, fibre, protein and magnesium | Only minor concerns | 0 | Caution is advised as high doses of omega‐3 fatty acids in Chia are known to increase the risk of bleeding, use cautiously in patients taking antioxidants |
| 2. Antihypertensives | ||||||||||
| 3. Antioxidants | ||||||||||
| 4. Cytochrome P450‐metabolized agents | ||||||||||
| Ulbricht (2009) USA [26] | RCTs, CR | >100 | Ginseng (Panax ginseng) | 1. DHT | Synergism with 1, 4, 5,7 inhibition of 2, 7, 8 | Mania, headache, tremor, and insomnia, reduced blood glucose, HbA1c, plasma cholesterol, triglyceride, LDL, and NEFA; elevated HDL, LH, FSH; altered BP | Inhibition of platelet aggregation and CYP2D6 | Some concerns | −4 | Caution is advised about concomitant use with warfarin, oral hypoglycaemic agents, insulin, antilipaemics anti‐arrhythmias hormonal agents diuretics |
| 2. anticoagulant | ||||||||||
| 3. antidepressants | ||||||||||
| 4. antidiabetics | ||||||||||
| 5. antilipaemics | ||||||||||
| 6. calcium channel blockers | ||||||||||
| 7. digoxin | ||||||||||
| 8. diuretics | ||||||||||
| Ulbricht (2009) USA [35] | RCTs, SR, CRs, AS, in vitro, | <100 | Green‐lipped mussel (Perna canaliculus) | 1. Anti‐inflammatory agents and corticosteroids | Synergism with 1† and ‡, 2‡ | n.k. | Inhibition of lipoxygenase | Only minor concerns | −4 | Limited evidence in humans |
| 2. Leukotriene receptor antagonists | ||||||||||
| Ulbricht (2009) USA [51] | Review, NRCT, CS, AS, in vitro | <100 | Maitake mushroom (Grifola frondosa) | 1. Antidiabetic agents | Synergism with 2†and‡ 3 | n.k. | Beta‐glucans are distributed to the liver and spleen with a prolonged half‐life | Only minor concerns | −4 | Use cautiously in patients using antihypertensives, antidiabetic agents and immunomodulators. |
| 2. Antineoplastic agents | ||||||||||
| 3. Antiviral agents | ||||||||||
| 4. Immunosuppressants | ||||||||||
| Ulbricht (2010) USA [52] | RCTs, CCT, CS, AS, in vitro | <100 | Reishi mushroom (Ganoderma lucidum) | 1. Antibiotics | Synergism with 1†, 2, 3, 5†and‡, 6† and inhibition of 8 | Increased risk of bleeding (theoretical) | Inhibitory activity on angiotensin converting enzyme† | Only minor concerns | −4 | Use cautiously in patients using anti‐inflamatory agents anticoagulants and antiplatelet agents |
| 2. Anticoagulant and antiplatelets | ||||||||||
| 3. NSAIDs | ||||||||||
| 4. Antidiabetics | ||||||||||
| 5. Antineoplastic agents | ||||||||||
| 6. Antiviral agents | ||||||||||
| 7. Cardiovascular agents | ||||||||||
| 8. Neurologic agents | ||||||||||
| Ulbricht (2010) USA [30] | RCTs, CCTs, AS | <10 | Stevia (Stevia rebaudiana) | 1. Sodium monoketocholate | Synergism with 1, 2, 3, 4‡ | Decreased glucose levels and blood pressure, inhibition of rotavirus | Inhibition of oxidative phosphorylation, ATPase activity, NADH‐oxidase activity, succinate‐oxidase activity, succinate dehydrogenase, and L‐glutamate dehydrogenase†; inhibition of ketogenesis and [14C] CO2 production from [1‐14C] palmitate‡ | Only minor concerns | 0 | Use cautiously in patients using diuretics and antihypertensives |
| 2. Vasodilators | ||||||||||
| 3. Diuretics | ||||||||||
| 4. Calcium channel blockers | ||||||||||
| Ulbricht (2010)USA [37] | RCTs, OS, CRs, in vitro | <100 | Umckaloabo (Pelargonium sidoides) | 1. Anticoagulant and antiplatelet agents | Synergism with 1†, 3, 4; inhibition of 5† | Cardiovascular complications, hepatotoxicity, increased risk of bleeding (theoretical), laxative effect | Gallic acid may stimulate a release of TNF, stimulate interferon activity and increase NK activity | Only minor concerns | −4 | Use cautiously in patients using anticoagulants or antiplatelet agents |
| 2. Cardiovascular agents | ||||||||||
| 3. Hepatotoxic agents | ||||||||||
| 4. Laxatives | ||||||||||
| 5. Immunosuppressants | ||||||||||
| Ulbricht (2010)USA [34] | RCTs, CRs, AS, in vitro | <100 | Rosemary (Rosmarinus officinalis) | 1. Immunosupressants | Synergism with 3, 4, 5† and ‡ | Increased risk of bleeding, hypotension | Inhibition of ACE, and platelets aggregation‡, decreased fibronectin and fibrin‡ | Only minor concerns | −4 | Use cautiously in patients using salicylates, cytochrome P450 metabolized drugs and anti‐diabetic agents |
| 2. Cytochrome P450‐metabolized agents | ||||||||||
| 3. Anxiolytics | ||||||||||
| 4. Antibiotics | ||||||||||
| 5. Anticoagulants or antiplatelets | ||||||||||
| Ulbricht (2010) USA [32] | RCTs, NRCTs, in vitro, AS | <100 | Spearmint (Mentha spicata, Mentha viridis) | 1. Nephrotoxic agents | Synergism with 1‡, 2‡, inhibition of 3‡ | n.k. | Decreased expression of cytochrome P450scc and cytochrome P450C17 enzymes | Only minor concerns | −4 | Interactions in humans are hypothetical |
| 2. Hepatotoxic agents | ||||||||||
| 3. Cytochrome P450‐metabolized agents | ||||||||||
| Ulbricht (2011) USA [33] | RCTs, SR, ET, OLS, CS | <100 | Saffron (Crocus sativus) | 1. SSRIs | Synergism with 1, 2, 3, 4, 5 and inhibition of 6† and ‡ | n.k. | Trans‐crocin‐4 may inhibit Abeta fibrillogenesis and platelet aggregation‡ | Only minor concerns | −4 | Use cautiously in patients using anticoagulants or antiplatelet agents, hormonal agents, antidepressants and antihypertensives |
| 2. MAOIs | ||||||||||
| 3. Fertility agents | ||||||||||
| 4. Alzheimer's agents | ||||||||||
| 5. Anti hypertensives | ||||||||||
| 6. Anticoagulants or antiplatelets | ||||||||||
| Ulbricht (2011) USA [36] | RCTs, SRs, CCTs, | <100 | Senna (Cassia senna) | 1. Digoxin | Synergism with 1†, 2, 3†, 4† | Lowered serum estrogen concentrations and potassium levels; increased risk of excessive bleeding and gallstones | Decreased deoxycholic acid and biliary cholesterol saturation | Only minor concerns | −4 | Use cautiously in patients using anticoagulant and antiplatelet agents |
| 2. Anticoagulant and antiplatelet agents | ||||||||||
| 3. Antibiotics | ||||||||||
| 4. Antineoplastics | ||||||||||
| Ulbricht (2011) USA [38] | RCT, CCTs, CS, AS | <100 | Gymnema (Gymnema sylvestre) | 1. Antidiabetic agents | Synergism with 1, 2 | Hypoglycemia | Reductions of serum TG, total cholesterol, VLDL and LDL | Only minor concerns | −4 | Supervision is needed in diabetic patients |
| 2. antilipaemic agents | ||||||||||
| Ulbricht (2011) USA [53] | RCTs,SRs, CCT, CS, CR | <100 | Turmeric (Curcuma longa) | 1. Paracetamol (acetaminophen) | Inhibition of 1†, 12†and‡, 15†, 19, 21 synergism with 2‡, 3†, 4‡, 5† and‡, 6, 8†and‡, 9‡, 10†and‡, 11†and‡, 13†, 14‡, 15‡, 17‡, 18†, 19, 20†, 21†, 23 | Increased risk of bleeding, transient hypotension, bradycardia, and vasodilation | Diferuloylmethane is believed to be the principal pharmacological agent responsible for all interactions | Only minor concerns | −4 | Use cautiously in patients using beta blockers or those with metabolic syndrome or increased risk of bleeding. |
| 2. acetylcholinesterase inhibitors | ||||||||||
| 3. amiloride | ||||||||||
| 4. antibiotics | ||||||||||
| 5. anticoagulants and antiplatelets | ||||||||||
| 6. antidiabetic agents | ||||||||||
| 7. antihypertensives | ||||||||||
| 8. Anti‐inflammatory agents | ||||||||||
| 9. Antilipemic agents | ||||||||||
| 10. Antineoplastic agents | ||||||||||
| 11. Celecoxib | ||||||||||
| 12. Cytochrome P450‐metabolized agents | ||||||||||
| 13. Erythromycin | ||||||||||
| 14. Erythropoietin | ||||||||||
| 15. Hormonal agents | ||||||||||
| 16. NSAIDs | ||||||||||
| 17. Norfloxacin | ||||||||||
| 18. Oxaliplatin | ||||||||||
| 19. P‐glycoprotein‐regulated drugs | ||||||||||
| 20. Retinol | ||||||||||
| 21. Talinolol | ||||||||||
| 22. Taxol | ||||||||||
| 23. Warfarin | ||||||||||
| Vora (2012) USA [54] | RCT | <10 | Chasteberry (Vitex agnus‐castus) | 1. Prolactin | Synergism with 1 (low doses) | n.k. | Constituents of chasteberry bind to dopamine‐2 receptors in the pituitary, thereby inhibiting prolactin secretion† | Only minor concerns | −4 | Use cautiously in patients taking oral contraceptives or HRT or in patients taking dopamine agonists or antagonists. Avoid using in patients with hormone sensitive cancers or conditions, in patients who are pregnant or breastfeeding or in women undergoing in vitro fertilization. |
| Whitten (2006) Australia [31] | RCTs | <100 | St John's wort (Hypericum perforatum) | 1. Immunosupressants | Inhibition of 1, 2, 4, 5, 6 and 7 | Transplant rejection, unwanted pregnancy, mania, orofacial dystonia, delayed emergence from anaesthesia, CVD collapse | Induction of CYP3A enzymes and/or intestinal P‐glycoprotein | Serious concerns | 5 | High doses of this HMP cause significant changes in pharmacokinetic measurements consistent with CYP3A induction. Avoid in transplant patients or those requiring anaesthesia. |
| 2. Antiretrovirals | ||||||||||
| 3. Hormonal therapy | ||||||||||
| 4. CVD drugs | ||||||||||
| 5. Anicancer drugs | ||||||||||
| 6. CNS drugs | ||||||||||
| 7. Antimicrobals |
Range of primary data.
Based on in vitro studies.
Based on animal studies.
ACE, angiotensin‐converting enzyme; AS, animal study; ATP, Adenosine triphosphate; BP, blood pressure; CAM, complementary and alternative medicine; CCS, case control study; CCT, controlled clinical trial; CNS, central nervous system; CR, case report; CS, case series; CVD, cardiovascular; CYP2D6, cytochrome CYP 2D6; DHT, dihydrotestosterone; EGSG, epigallocatechin gallate; ET, equivalence trial; FSH, follicle stimulating hormone; GABA, gamma‐aminobutyric acid; GrRH, gonadotropin releasing hormone; HbA1c, haemoglobin A1c; HLE, human leucocyte elastase; HMG, CoA‐hepatic 3‐hydroxy‐3‐methylglutaryl reductase; HDL, high‐density lipoprotein; HRT, hormone replacement therapy; INR, International Normalized Ratio; LH, luteinizing hormone; LDL, low‐density lipoprotein; LTB4, leukotriene B4; MOAI, monoamine oxidase inhibitors; NADH, dehydrogenase; NEFA, non‐esterified fatty acid; NK, natural killer cells; n.m., not mentioned; n.k., not known; NRCT, non‐randomized controlled trial; OCPs, Oral contraceptives; OS, observational study; OLS, open label study; RCT, randomized controlled trial; RRC, retrospective review of cases; SSRIs, Selective serotonin re‐uptake inhibitors; SR, systematic review; SRS, spontaneous reporting scheme; TG, triglicerides; TNF, tumour necrosis factor; TSH, Thyroid stimulating hormone; UCT, uncontrolled trial; VLDL, very low density lipoprotein; 5‐HETE, 5‐hydroxyeicosatetraenoic.
The following drugs interacted with HMPs (Table 2): anaesthetics [16], anti‐arrhythmic agents [15,27], antibiotics [19,34,36,39,40,42,46,48,49,52,53], anticoagulants [13,15,16,18,24,26,27,29,33,34,36,37,39,40,44–46,49,50,52,53], anticholinergics [17,22], antidepressants [25,26,33,45], antidiabetics [26,27,38,40,43,47,50–53], antihypertensives [16,29,33,41,43–46,49,53], anti‐inflammatory agents [13,16,35,46,52,53], antimicrobials [31], antineoplastics [11,14,31,36,39–43,51–53], antiplatelet agents [13,15,24,25,27,29,33,34,36,37,39,40,44,45,49,50,52,53], anti‐epileptic drugs [13,41,45], antivirals [31,40,45,51,52], calcium channel blockers [24,26,30], cholesterol lowering agents [10,13,24,26,27,40,41,44,45,50,53], CYP‐450 metabolized agents [18,21,25,29,32,34,40,42,45,48,49,53], diuretics [26,30,43], hormonal agents [18,24,26,31,40,41,43,45,49,53,54], hypoglycaemics [9,10,19,20,28], immunnosupressants [31,34,37,40,43,46,48] laxatives [27,37,50], leukotriene inhibitors [14], sedatives [12,13,23,25,45], selective serotonin re‐uptake inhibitors [23,33] and vasolidators [30,44].
Table 2.
The found herb–drug interactions for each class of medication
| Class of medication | Herb | Type of interaction | |
|---|---|---|---|
| Synergism | Antagonism | ||
| Alzheimer's agents | Saffron (Crocus sativus) | x | |
| Anaesthetics | Ginkgo (Ginkgo biloba) | x | |
| Analgesics | Ginkgo (Ginkgo biloba) | x | |
| Green tea (Camellia sinensis) | x | ||
| Anti‐arrhythmias | Ginseng (Panax ginseng) | x | x |
| Antibiotics | Rosemary (Rosmarinus officinalis) | x | |
| Saw palmetto (Serenoa repens) | x | ||
| St John's wort (Hypericum perforatum) | x | ||
| Anticoagulants and antiplatelet agents | Ginkgo (Ginkgo biloba) | x | |
| Ginseng (Panax ginseng) | x | ||
| Guggul (Commifora mukul) | x | ||
| Lavender (Lavandula angustifolia Miller) | x | ||
| Noni (Morinda citrifolia) | x | ||
| Reishi mushroom (Ganoderma lucidum) | x | ||
| Saw palmetto (Serenoa repens) | x | ||
| Senna (Cassia senna) | x | ||
| Turmeric (Curcuma longa) | x | ||
| Antidiabetics | Aloe (Aloe vera) | x | |
| Cinnamon (Cinnamomum spp.) | x | ||
| Fenugreek (Trigonella foenum‐graecum) | x | ||
| Ginseng (Panax ginseng) | x | ||
| Gymnema (Gymnema sylvestre) | x | ||
| Turmeric (Curcuma longa) | x | ||
| Antihypertensives | Black cohosh (Cimicifuga racemosa) | x | |
| Chia (Salvia hispanica) | x | ||
| Mistletoe (Viscum album) | x | ||
| Saffron (Crocus sativus) | x | ||
| Stevia (Stevia rebaudiana) | x | ||
| Anti‐inflamatory agents | Aloe (Aloe vera) | x | |
| Saw palmetto (Serenoa repens) | x | ||
| Reishi mushroom (Ganoderma lucidum) | x | ||
| Antilipaemics | Alfalfa (Medicago sativa) | x | |
| Fenugreek (Trigonella foenum‐graecum) | x | ||
| Ginseng (Panax ginseng) | x | ||
| Green tea (Camellia sinensis) | x | ||
| Gymnema (Gymnema sylvestre) | x | ||
| Antineoplastics | Black cohosh (Cimicifuga racemosa) | x | |
| St John's wort (Hypericum perforatum) | x | ||
| Thyme (Thymus vulgaris) | x | ||
| Anti‐oxidants | Chia (Salvia hispanica) | x | |
| Antiseizures | Green tea (Camellia sinensis) | x | |
| Lavender (Lavandula angustifolia Miller) | x | ||
| Antivirals | Aloe (Aloe vera) | x | |
| Green tea (Camellia sinensis) | x | ||
| Maitake mushroom (Grifola frondosa) | x | ||
| St John's wort (Hypericum perforatum) | x | ||
| Anxiolytics | Rosemary (Rosmarinus officinalis) | x | |
| β‐adrenoceptor blockers | Green tea (Camellia sinensis) | x | |
| Guggul (Commifora mukul) | x | ||
| Turmeric (Curcuma longa) | x | ||
| Cholinergic agents | Butterbur (Petasites hybridus) | x | |
| Mistletoe (Viscum album) | x | ||
| CNS depressants | Kava (Piper methysticum) | x | |
| Mistletoe (Viscum album) | x | ||
| Cytochrome P450‐metabolized agents | Echinacea spp. | x | x |
| Green tea (Camellia sinensis) | x | ||
| St John's wort (Hypericum perforatum) | x | ||
| Dopamine agonists and antagonists | Kava (Piper methysticum) | x | |
| Diuretics | Ginseng (Panax ginseng) | x | |
| Mistletoe (Viscum album) | x | ||
| Stevia (Stevia rebaudiana) | x | ||
| Gastroprotective agents | Belladonna (Herbae pulvis standardisatus) | x | |
| Hepatotoxic agents | Echinacea spp. | x | |
| Green tea (Camellia sinensis) | x | ||
| Noni (Morinda citrifolia) | x | ||
| Umckaloabo (Pelargonium sidoides) | x | ||
| Hormonal agents | Chasteberry (Vitex agnus‐castus) | x | |
| Ginseng (Panax ginseng) | x | ||
| Green tea (Camellia sinensis) | x | ||
| Red clover (Trifolium pratense) | x | x | |
| Saffron (Crocus sativus) | x | ||
| Saw palmetto (Serenoa repens) | x | x | |
| Hypoglycaemics | Alfalfa (Medicago sativa) | x | |
| Bitter melon (Momordica charantia) | x | ||
| Immunomodulators | Saw palmetto (Serenoa repens) | x | |
| St John's wort (Hypericum perforatum) | x | ||
| Laxatives | Umckaloabo (Pelargonium sidoides) | x | |
| Leukotriene inhibitors | Boswellia (Boswellia serrata) | x | |
| Monoamine oxidase inhibitors | Saffron (Crocus sativus) | x | |
| Sedatives | Calendula (Calendula officinalis) | x | |
| Green tea (Camellia sinensis) | x | ||
| Lavender (Lavandula angustifolia Miller) | x | ||
| Lemon balm (Melissa officinalis) | x | ||
| Selective serotonin re‐uptake inhibitors | Saffron (Crocus sativus) | x | |
CNS, central nervous system.
These interactions were thought to cause a wide variety of clinical outcomes such as altered hormone concentrations [18], apraxia, death, haematoma, hyphaema, permanent neurological deficit [16], bradycardia and vasodilation [53], cardiovascular complications, hepatotoxicity [37], coma, sedation, lethargy, drowsiness [25], coronary vasoconstriction, tachycardia, diaphoresis, abdominal pain, muscle weakness [47], gastrointestinal upset [41], haemorrhage, bleeding [34], increased body weight, diarrhoea, decreased blood haemoglobin and altered calcium serum concentrations, hypoglycaemia [10,38], increased liver enzyme levels [17], mania, headache, tremor and insomnia [26], microcytic anaemia, ischaemic stroke [45], organ fibrosis and death, cardiotoxicity, hypovolaemic shock, inflammatory reaction [43], potassium depletion, hypokalaemia [50], rhabdomyolysis, shock and death [42], severe intra‐operative and cerebral haemorrhage, hypertension, nausea or vomiting [49], transplant rejection, unwanted pregnancy, mania, orofacial dystonia, delayed emergence from anaesthesia, cardiovascular collapse [31].
Thirteen SRs were based on less than 10 primary reports [9–13,19,27–30,47,48,54], 32 were based on less than 100 primary reports [14–18,20–25,31–46,49–53] and one SR was based on more than 100 primary reports [26]. The SRs included human studies [9–11,16–18,23–27,29,31,33,34,36–38,40–47,49–54], animal studies [12,13,15,19–24,27–30,32–35,39,40,42–46,48,51–53] or in vitro experiments [14,18,19,23,25,33–37,39–46,48,51–53]. Some HMPs acted as inhibitors/antagonists [16,21,22,31] while others acted as agonists/synergists [9–13,17,20,28–30,34–36,38–40,47,50,51,54]. In nine SRs, HMPs acted both as inhibitors and synergists [14,15,18,19,23–27,32,33,37,41–46,48,49,52,53]. For 39 HMPs, only minor concerns were raised regarding interactions [9–15,17–24,27–30,32–42,44,46–48,50–54], five raised some concerns [16,25,26,45,49] and two raised serious concerns [31,43].
Only three SRs were of excellent methodological quality [16,25,31], 10 had minor deficits [10,17,18,20,21,24,27,28,48,50] and 20 had major methodological flaws [9,11–15,19,22,23,26,29,30,32–47,49,51–54] (Table 3). Conflicts of interest of the authors were mentioned in only one SR [31]. The source of funding was mentioned in only two SRs [20,21].
Table 3.
Quality ratings for included systematic reviews of HMPs
| Study (year) [ref] | Search methods? (a) | Search comprehensive? (b) | Inclusion criteria? (c) | Bias avoided? (d) | Conclusions supported? (e) | Sum |
|---|---|---|---|---|---|---|
| Armbruer (2012) [40] | −1 | −1 | −1 | −1 | 0 | −4 |
| Barrette (2012) [41] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2003) [9] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2003) [10] | 1 | 1 | 0 | −1 | 1 | 2 |
| Basch (2004) [11] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2006) [12] | 0 | 1 | 0 | −1 | 0 | 0 |
| Basch (2004) [13] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2004) [14] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2005) [42] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2012) [43] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2012) [44] | −1 | −1 | −1 | −1 | 0 | −4 |
| Basch (2012) [45] | −1 | −1 | −1 | −1 | 0 | −4 |
| Brendler (2006) [15] | 0 | 1 | 0 | −1 | 0 | 0 |
| Brendler (2012) [46] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ceurvels (2012) [47] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ernst (2005) [16] | 1 | 1 | 1 | 1 | 1 | 5 |
| Giles (2005) [17] | 0 | 1 | 0 | −1 | 1 | 1 |
| Keifer (2007) [48] | 1 | 1 | 0 | −1 | 1 | 2 |
| Nelsen (2002) [18] | 1 | 1 | −1 | −1 | 1 | 1 |
| Sweeney (2005) [19] | −1 | −1 | −1 | −1 | 1 | −4 |
| Tiffany (2002) [20] | 1 | 1 | 0 | −1 | 0 | 1 |
| Ulbricht (2003) [21] | 1 | 1 | 0 | −1 | 1 | 2 |
| Ulbricht (2004) [22] | −1 | −1 | −1 | −1 | 1 | −4 |
| Ulbricht (2005) [23] | 0 | 1 | 0 | −1 | 0 | 0 |
| Ulbricht (2005) [24] | 0 | 1 | 0 | −1 | 1 | 1 |
| Ulbricht (2005) [25] | 1 | 1 | 1 | −1 | 1 | 3 |
| Ulbricht (2006) [49] | −1 | −1 | −1 | −1 | 1 | −4 |
| Ulbricht (2007) [27] | 1 | 1 | 0 | −1 | 1 | 2 |
| Ulbricht (2007) [28] | 1 | 1 | 0 | −1 | 0 | 1 |
| Ulbricht (2007) [50] | 1 | 1 | 0 | −1 | 1 | 2 |
| Ulbricht (2008) [39] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2009) [29] | 0 | 1 | 0 | −1 | 0 | 0 |
| Ulbricht (2009) [35] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2009) [26] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2009) [51] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2010) [52] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2010) [30] | 0 | 1 | 0 | −1 | 0 | 0 |
| Ulbricht (2010) [37] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2010) [34] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2010) [32] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2011) [33] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2011) [36] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2011) [38] | −1 | −1 | −1 | −1 | 0 | −4 |
| Ulbricht (2011) [53] | −1 | −1 | −1 | −1 | 0 | −4 |
| Vora (2012) [54] | −1 | −1 | −1 | −1 | 0 | −4 |
| Whitten (2006) [31] | 1 | 1 | 1 | 1 | 1 | 5 |
Scoring: each question is scored as 1, 0 or −1; A score of 0 or below means the review has major flaws, 1–2 minor flaws and 3–5 minimal or no flaws.
1 means that: (a) the review states the databases used, date of most recent searches and some mention of search terms; (b) the review searches at least 2 databases and looks at other sources; (c) the review states the criteria used for deciding which studies to include in the overview; (d) the review reports how many studies were identified by searches, numbers excluded and appropriate reasons for excluding them; (e) the conclusions made by the author(s) are supported by the data and/or analysis reported in the review.
0 means that the above mentioned criteria were partially fulfilled.
−1 means that none of the above criteria was fulfilled.
Discussion
This article was aimed at providing an overview of SRs of herb–drug interactions. Forty‐six SRs could be included. Thirty‐nine of the HMPs submitted to SRs did not interact with drugs [9–20,22–24,27–30,32–42,44,46–48,50–54]. Eight HMPs had the potential for such interactions [21,25,26,31,43,45,49]. The interactions caused mostly mild to severe adverse effects (AEs). The HMPs implicated were ginkgo, ginseng, green tea, kava, mistletoe, saw palmetto and St John's wort (Tables 1, 2 and 4).
Table 4.
The most clinically important herb–drug interactions
| HMP | Synthetic drug | Clinical outcome |
|---|---|---|
| Ginkgo | Anticoagulants, anti‐inflammatory agents, antihypertensives, anaesthetics | Haemorrhage, apraxia, haematoma, hyphaema, permanent neurological deficit, death |
| Ginseng | Antidepressants, antidiabetics, anticoagulants, calcium channel blockers, cholesterol lowering agents, diuretics, hormonal agents | Inhibition of platelet aggregation, reduced platelet adhesiveness, hypoglycaemia, changes in blood pressure and heart rate, mania, headache, tremor, insomnia |
| Kava | Antidepressants, antiplatelets, CYP‐450 metabolized agents, sedatives | Coma, sedation, lethargy, drowsiness |
| St John's wort | Antineoplastics, antimicrobials, antiretrovirals, hormonal agents, immunnosupressants | Transplant rejection, unwanted pregnancy, delayed emergence from anaesthesia, CVD collapse |
The most common interacting drugs were anticoagulants [13,15,16,18,24,26,27,29,33,34,36,37,39,40,44–46,49,50,52,53] and antiplatelet agents [13,15,24,25,27,29,33,34,36,37,39,40,44,45,49,50,52,53]. The most probable mechanisms of these interactions involve an inhibition of thromboxane synthesis and cyclooxygenase. Some herbs acted as inhibitors/antagonists of drugs [16,21,22,31] whereas others acted as agonists/synergists [9–13,17,20,28–30,34–36,38–40,47,50,51,54]. In nine SRs, there was a bimodal mode of action causing both antagonism and synergism [14,15,18,19,23–27,32,33,37,41–46,48,49,52,53].
The methodological quality of the included SRs was frequently inadequate (Table 3). Many of the articles that scored poorly on our quality rating were monograph‐type publications which are not designed as typical systematic reviews. As these articles do contribute relevant information and are relatively frequent in the literature about herbal medicine, we decided to include them in our overview.
Thirty‐nine HMPs were reported not to interact with synthetic drugs. However, this information may be unreliable because of the frequently poor quality of the primary data that missed detection of herb–drug interactions and subsequent AEs. In 10 SRs, herb–drug interactions were hypothetical as the primary research was based on in vitro and/or animal studies. This overview suggests that the quality of research on herb−drug interactions is often wanting. It also reveals that there is still paucity of such investigations. As a consequence, therapeutic decisions can be hampered. To make progress in this area, we need more effective monitoring systems, better implementation of existing regulations, better quality of reporting and more reliable SRs.
The present analysis has several limitations. Although comprehensive searches were conducted, there is no guarantee that all relevant SRs were located. Furthermore, any overview of SRs is susceptible to publication bias. As we only included SRs, our overview cannot provide information on HMPs for which no SR is available.
Conclusion
In conclusion, the majority of SRs revealed moderately severe or minor interactions between HMPs and drugs. Some HMPs, however, do interact with drugs posing severe health threats. Due to the limited quality and scarcity of the primary data, these conclusions should be treated with caution.
Appendix
Appendix 1 Search strategy for MEDLINE
| 1 | (herb$ adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or heal$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or therap$ or tincture$ or tisane$ or treatment$)).ti,ab. |
| 2 | Herbal$.ti,ab. |
| 3 | (plant$ adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or heal$ or herb$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or therap$ or tincture$ or tisane$ or treatment$)).ti,ab. |
| 4 | (phytodrug$ or phytomed$ or phytopharmac$ or phytother$ or phytochemical$).ti,ab. |
| 5 | ((natural$ or naturo$) adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or herb$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or therap$ or tincture$ or tisane$ or treatment$)).ti,ab. |
| 6 | (botanical$ adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or heal$ or herb$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or therap$ or tincture$ or tisane$ or treatment$)).ti,ab. |
| 7 | (traditional adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or herb$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or tincture$ or tisane$)).ti,ab. |
| 8 | (Chinese adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or herb$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or tincture$ or tisane$)).ti,ab. |
| 9 | (Ethnobotan$ or pharmacogno$ or Ethnopharmaco$ or ethnomedic$).ti,ab. |
| 10 | (Ayur ved$ or Ayurved$ or kampo or siddha or unani).ti,ab. |
| 11 | (folk adj3 (caplet$ or capsule$ or compound$ or cream$ or decoction$ or drug$ or essence$ or extract$ or formul$ or herb$ or Infus$ or juice$ or medic$ or mixture$ or powder$ or prepar$ or prescri$ or product or products or remed$ or supplement$ or tablet$ or tea or teas or tincture$ or tisane$)).ti,ab. |
| 12 | exp Ethnobotany/ |
| 13 | exp Phytotherapy/ |
| 14 | exp Plants, Medicinal/ |
| 15 | exp Herb−Drug Interactions/ |
| 16 | exp Plant Exudates/ |
| 17 | exp Materia Medica/ |
| 18 | exp Herbal Medicine/ |
| 19 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 |
| 20 | complicat$.ti,ab. |
| 21 | (safe or safety or risk$).ti,ab. |
| 22 | Side effect$.ti,ab. |
| 23 | (tolerate or tolerability or tolerance or hypersensativ$ or aggravat$).ti,ab. |
| 24 | (Adverse adj3 (effect$ or event$ or Interaction$ or outcome$ or Reaction$ or response$)).ti,ab. |
| 25 | (Uninten$ adj3 (effect$ or event$ or Interaction$ or outcome$ or Reaction$ or response$)).ti,ab. |
| 26 | (Unwanted adj3 (effect$ or event$ or Interaction$ or outcome$ or Reaction$ or response$)).ti,ab. |
| 27 | (Unexpected adj3 (effect$ or event$ or Interaction$ or outcome$ or Reaction$ or response$)).ti,ab. |
| 28 | (Undesir$ adj3 (effect$ or event$ or Interaction$ or outcome$ or Reaction$ or response$)).ti,ab. |
| 29 | (harm$ adj3 (effect$ or event$ or Interaction$ or outcome$ or Reaction$ or response$)).ti,ab. |
| 30 | (Toxic$ or Adulterat$ or Contaminat$ or Poison$ or hepatotoxic$).ti,ab. |
| 31 | (Aftereffect or after effect).ti,ab. |
| 32 | exp Drug Toxicity/ |
| 33 | exp Drug Contamination/ |
| 34 | 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 |
| 35 | Review$.ti. |
| 36 | 19 and 34 and 35 |
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Hunt KJ, Ernst E. Patients' use of CAM: results from the Health Survey for England 2005. Focus Altern Complement Ther. 2010;15:101–3. [Google Scholar]
- 2.Merritt‐Charles L. The prevalence of herbal medicine use among surgical patients in Trinidad. Focus Altern Complement Ther. 2011;16:266–70. [Google Scholar]
- 3.Paul JH, Seaforth CE. Harmful plants in Caribbean folk medicine. Focus Altern Complement Ther. 2011;16:261–5. [Google Scholar]
- 4.Ernst E. Possible interactions between synthetic and herbal medicinal products. Part 1: a systematic review of the indirect evidence. Perfusion. 2000;13:4–6. [Google Scholar]
- 5.Ernst E, Pittler MH, Wider B, Boddy K. 2nd. Elsevier Mosby; 2006. The Desktop Guide to Complementary and Alternative Medicine edn. Edinburgh: [Google Scholar]
- 6.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs an updated systematic review. Drugs. 2009;69:1777–98. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Izzo AA, Di Carlo G, Borrelli F, Ernst E. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol. 2005;98:1–14. doi: 10.1016/j.ijcard.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 8.Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44:1271–8. doi: 10.1016/0895-4356(91)90160-b. [DOI] [PubMed] [Google Scholar]
- 9.Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am J Health Syst Pharm. 2003;60:356–9. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- 10.Basch E, Ulbricht C, Harrison M, Sollars D, Smith M, Dennehy C, Szapary P. Alfalfa (Medicago sativa L.):a clinical decision support tool. J Herb Pharmacother. 2003;3:69–90. doi: 10.1300/j157v03n02_09. [DOI] [PubMed] [Google Scholar]
- 11.Basch E, Ulbricht C, Hammerness P, Bevins A, Sollars D. Thyme (Thymus vulgaris L.), thymol. J Herb Pharmacother. 2004;4:49–67. [PubMed] [Google Scholar]
- 12.Basch E, Bent S, Foppa I, Haskmi S, Kroll D, Mele M, Szapary P, Ulbricht C, Vora M, Yong S. Marigold (Calendula officinalis L.): an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2006;6:135–59. doi: 10.1080/j157v06n03_08. [DOI] [PubMed] [Google Scholar]
- 13.Basch E, Foppa I, Liebowitz R, Nelson J, Smith M, Sollars D, Ulbricht C. Lavender (Lavandula angustifolia Miller) J Herb Pharmacother. 2004;4:63–78. [PubMed] [Google Scholar]
- 14.Basch E, Boon H, Davies‐Heerema T, Foppo I, Hashmi S, Hasskarl J, Sollars D, Ulbricht C. Boswellia: an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2004;4:63–83. [PubMed] [Google Scholar]
- 15.Brendler T, Gruenwald J, Ulbricht C, Basch E. Devil's Claw (Harpagophytum procumbens DC): an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2006;6:89–126. [PubMed] [Google Scholar]
- 16.Ernst E, Canter PH, Thompson Coon J. Does Ginkgo biloba increase the risk of bleeding? A systematic review of case reports. Perfusion. 2005;18:52–6. [Google Scholar]
- 17.Giles M, Ulbricht C, Khalsa KP, Kirkwood CD, Park C, Basch E. Butterbur: an evidence‐based systematic review by the natural standard research collaboration. J Herb Pharmacother. 2005;5:119–43. [PubMed] [Google Scholar]
- 18.Nelsen J, Ulbricht C, Barrette EP, Sollars D, Tsouronis C, Rogers A, Basch S, Hashmi S, Bent S, Basch E. Red clover (Trifolium pratense) monograph: a clinical decision support tool. J Herb Pharmacother. 2002;2:49–72. doi: 10.1080/j157v02n03_06. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney B, Vora M, Ulbricht C, Basch E. Evidence‐based systematic review of dandelion (Taraxacum officinale) by Natural Standard Research Collaboration. J Herb Pharmacother. 2005;5:79–93. [PubMed] [Google Scholar]
- 20.Tiffany N, Boon H, Ulbricht C, Basch E, Bent S, Barrette EP, Smith M, Sollars D, Dennehy CE, Szapary P. Horse chestnut:a multidisciplinary clinical review. J Herb Pharmacother. 2002;2:71–85. [PubMed] [Google Scholar]
- 21.Ulbricht C, Basch E, Vora M, Sollars D, Rogers A, Basch S, Smith M, Moffet H, Hammerness P. Chaparral monograph: a clinical decision support tool. J Herb Pharmacother. 2003;3:121–31. doi: 10.1080/j157v03n01_08. [DOI] [PubMed] [Google Scholar]
- 22.Ulbricht C, Basch E, Hammerness P, Vora M, Wylie J, Woods J. An evidence‐based systematic review of belladonna by the Natural Standard Research Collaboration. J Herb Pharmacother. 2004;4:61–90. [PubMed] [Google Scholar]
- 23.Ulbricht C, Brendler T, Gruenwald J, Kligler B, Keifer D, Abrams TR, Woods J, Boon H, Kirkwood CD, Hackman DA, Basch E, Lafferty HJ. Lemon balm (Melissa officinalis L.): an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2005;5:71–114. [PubMed] [Google Scholar]
- 24.Ulbricht C, Basch E, Szapary P, Hammerness P, Axentsev S, Boon H, Kroll D, Garraway L, Vora M, Woods J. Guggul for hyperlipidemia: a review by the Natural Standard Research Collaboration. Complement Ther Med. 2005;13:279–90. doi: 10.1016/j.ctim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Ulbricht C, Basch E, Boon H, Ernst E, Hammerness P, Sollars D, Tsourounis C, Woods J, Bent S. Safety review of kava (Piper methysticum) by the Natural Standard Research Collaboration. Expert Opin Drug Saf. 2005;4:779–94. doi: 10.1517/14740338.4.4.779. [DOI] [PubMed] [Google Scholar]
- 26.Ulbricht C, Basch E, Brigham A, Bryan K, Costa D, Dacey C, Foppa I, Giese N, Hawkins EB, Montalbano JK, Tanguay‐Colucci S, Varghese M, Vora M, Weissner W. An evidence‐based systematic review of ginseng interactions by the Natural Standard Research Collaboration. Natural Med J. 2009;1:1–13. [Google Scholar]
- 27.Ulbricht C, Basch E, Burke D, Cheung L, Ernst E, Giese N, Foppa I, Hammerness P, Hashmi S, Kuo G, Miranda M, Mukherjee S, Smith M, Sollars D, Tanguay‐Colucci S, Vijayan N, Weissner W. Fenugreek (Trigonella foenum‐graecum L. Leguminosae): an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2007;7:143–77. doi: 10.1080/15228940802142852. [DOI] [PubMed] [Google Scholar]
- 28.Ulbricht C, Dam C, Milkin T, Seamon E, Weissner W, Woods J. Banaba (Lagerstroemia speciosa L.): an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2007;7:99–113. [PubMed] [Google Scholar]
- 29.Ulbricht C, Chao W, Nummy K, Rusie E, Tanguay‐Colucci S, Iannuzzi CM, Plammoottil JB, Varghese M, Weissner W. Chia (Salvia hispanica): a systematic review by the Natural Standard Research Collaboration. Rev Recent Clin Trials. 2009;4:168–74. doi: 10.2174/157488709789957709. [DOI] [PubMed] [Google Scholar]
- 30.Ulbricht C, Isaac R, Milkin T, Poole EA, Rusie E, Grimes Serrano JM, Weissner W, Windsor RC, Woods J. An evidence‐based systematic review of stevia by the Natural Standard Research Collaboration. Cardiovasc Hematol Agents Med Chem. 2010;8:113–27. doi: 10.2174/187152510791170960. [DOI] [PubMed] [Google Scholar]
- 31.Whitten DL, Myers SP, Hawrelak JA, Wohlmuth H. The effect of St John's wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol. 2006;62:512–26. doi: 10.1111/j.1365-2125.2006.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulbricht C, Costa D, Grimes Serrano JM, Guilford J, Isaac R, Seamon E, Varghese M. An evidence‐based systematic review of spearmint by the natural standard research collaboration. J Dietary Suppl. 2010;7:179–215. doi: 10.3109/19390211.2010.486702. [DOI] [PubMed] [Google Scholar]
- 33.Ulbricht C, Conquer J, Costa D, Hollands W, Iannuzzi C, Isaac R, Jordan JK, Ledesma N, Ostroff C, Grimes Serrano JM, Shaffer MD, Varghese M. An evidence‐based systematic review of saffron (Crocus sativus) by the Natural Standard Research Collaboration. J Dietary Suppl. 2011;8:58–114. doi: 10.3109/19390211.2011.547666. [DOI] [PubMed] [Google Scholar]
- 34.Ulbricht C, Abrams TR, Brigham A, Ceurvels J, Clubb J, Curtiss W, DeFranco Kirkwood C, Giese N, Hoehn K, Iovin R, Isaac R, Rusie E, Serrano G, Varghese M, Weissner W, Windsor RC. An evidence‐based systematic review of rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J Dietary Suppl. 2010;7:351–413. doi: 10.3109/19390211.2010.525049. [DOI] [PubMed] [Google Scholar]
- 35.Ulbricht C, Chao W, Costa D, Nguyen Y, Seamon E, Weissner W. An evidence‐based systematic review of green‐lipped mussel (Perna canaliculus) by the Natural Standard Research Collaboration. J Dietary Suppl. 2009;6:54–90. doi: 10.1080/19390210802690191. [DOI] [PubMed] [Google Scholar]
- 36.Ulbricht C, Conquer J, Costa D, Hamilton W, Higdon ER, Isaac R, Rusie E, Rychlik I, Grimes Serrano JM, Tanguay‐Colucci S, Theeman M, Varghese M. An evidence‐based systematic review of senna (Cassia senna) by the Natural Standard Research Collaboration. J Dietary Suppl. 2011;8:189–238. doi: 10.3109/19390211.2011.573186. [DOI] [PubMed] [Google Scholar]
- 37.Ulbricht C, Abrams TR, Conquer J, Costa D, Serrano G, Iovin R, Yen Nguyen RS, Rusie E, Tran D, Weissner W, Windsor RC. An evidence‐based systematic review of umckaloabo (Pelargonium sidoides) by the Natural Standard Research Collaboration. j Dietary Suppl. 2010;7:283–302. doi: 10.3109/19390211.2010.507116. [DOI] [PubMed] [Google Scholar]
- 38.Ulbricht C, Abrams TR, Basch E, Davies‐Heerema T, Foppa I, Hammerness P, Rusie E, Tanguay‐Colucci S, Taylor S, Varghese M, Weissner W, Woods J. An evidence‐based systematic review of Gymnema (Gymnema sylvestre R. Br.) by the Natural Standard Research Collaboration. J Dietary Suppl. 2011;8:311–30. doi: 10.3109/19390211.2011.597977. [DOI] [PubMed] [Google Scholar]
- 39.Ulbricht C, Basch E, Dacey C, Dith S, Hammerness P, Hashmi S, Seamon E, Vora M, Weissner W. An evidence‐based systematic review of blessed thistle (Cnicus benedictus) by the Natural Standard Research Collaboration. J Dietary Suppl. 2008;5:422–237. [Google Scholar]
- 40.Armbruester N, Bryan K, Costa D, Giese N, Gruenwald J, Iovin R, Isaac R, Seamon E, Grimes Serrano JM, Tanguay‐Colucci S, Ulbricht C, Weissner W, Windsor R, Yoon H, Zhang J. 2012. pp. 1–70. Cinnamon (Cinnamomum spp.). Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/cassia.asp (last accessed 21 January 2012),
- 41.Barrette EP, Basch E, Bent S, Burke D, Costa D, Foppa I, Giese N, Goble M, Grimes Serrano JM, Hammerness P, Mendoza V, Miranda M, Smith M, Ulbricht C. 2012. Black cohosh (Cimicifuga racemosa [L.] Nutt.). Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/cimicifuga.asp (last accessed 21 January 2012)
- 42.Basch E, Ulbricht C, Basch S, Dalton S, Ernst E, Foppa I, Szapary P, Tiffany N, Orlando CW, Vora M. An evidence‐based systematic review of echinacea (E. angustifolia DC, E. pallida, E. purpurea) by the Natural Standard Research Collaboration. J Herb Pharmacother. 2005;5:57–88. [PubMed] [Google Scholar]
- 43.Basch E, Conquer J, Dominguez R, Giese N, Grimes Serrano JM, Hackman D, Heller M, Isaac R, Joseph A, Linardakis N, McGarry M, Schadde S, Scully L, Seamon E, Shaffer M, Ulbricht C, Weissner W. 2012. pp. 1–70. Mistletoe (Viscum album L.). Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/mistletoe.asp (last accessed 21 January 2012),
- 44.Basch E, Dacey C, Ernst E, Foppa I, Grimes Serrano JM, Hammerness P, Kerbel B, Nummy K, Seamon E, Shaffer M, Tanguay‐Colucci S, Vora M, Ulbricht C, Weissner W. 2012. Hawthorn (Crataegus laevigata,C. oxyacantha,C.monogyna,C. pentagyna). Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/crataegus.asp (last accessed 21 January 2012).
- 45.Basch E, Conquer J, Culwell S, Dacey C, Grimes Serrano JM, Guilford J, Hammerness P, Higdon ER, Jingst S, Kats J, McDermott YH, Rusie E, Ulbricht C, Weissner W, Windsor R. 2012. Green tea (Camellia sinensis). Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/camellia.asp (last accessed 21 January 2012).
- 46.Brendler T, Abrams TT, Brigham A, Bryan JK, Diem‐Che W, Ceurvels J, Giese N, Grimes Serrano JM, Gruenwald J, Khanzada F, Lee DS, Markowitz JS, Seamon E, Weissner W. 2012. Noni (Morinda citrifolia). Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/noni.asp (last accessed 21 January 2012).
- 47.Ceurvels J, Davis M, Clubb J, Giese N, Goodfriend J, Tanguay‐Colucci S, Weissner W. 2012. Blue cohosh (Caulophyllum thalictroides) Natural Standard Professional Monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/caulophyllum.asp (last accessed 21 January 2012).
- 48.Keifer D, Ulbricht C, Abrams TR, Basch E, Giese N, Giles M, DeFranco Kirkwood C, Miranda M, Woods J. Peppermint (Mentha piperita): an evidence‐based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2007;7:91–143. doi: 10.1300/j157v07n02_07. [DOI] [PubMed] [Google Scholar]
- 49.Ulbricht C, Basch E, Bent S, Boon H, Corrado M, Foppa I, Hashmi S, Hammerness P, Kingsbury E, Smith M, Szapary P, Vora M, Weissner W. Evidence‐based systematic review of saw palmetto by the Natural Standard Research Collaboration. J Soc Integr Oncol. 2006;4:170–86. doi: 10.2310/7200.2006.016. [DOI] [PubMed] [Google Scholar]
- 50.Ulbricht C, Armstrong J, Basch E, Basch S, Bent S, Dacey C, Dalton S, Foppa I, Giese N, Hammerness P, Kirkwood C, Sollars D, Tanguay‐Colucci S, Weissner W. An evidence‐based systematic review of Aloe vera by the Natural Standard Research Collaboration. J Herb Pharmacother. 2007;7:279–323. doi: 10.1080/15228940802153339. [DOI] [PubMed] [Google Scholar]
- 51.Ulbricht C, Weissner W, Basch E, Giese N, Hammerness P, Rusie‐Seamon E, Varghese M, Woods J. Maitake mushroom (Grifola frondosa): systematic review by the Natural Standard Research Collaboration. J Soc Integr Oncol. 2009;7:66–72. [PubMed] [Google Scholar]
- 52.Ulbricht C, Abrams TR, Bent S, Boon H, Costa D, Dacey C, Guilford J, Giese N, Grimes Serrano JM, Hackman DA, Scully L, Rusie E, Shaffer M, Varghese M, Vijarian N, Weissner W, Welch S, Wong D, Woods J. Reishi mushroom (Ganoderma lucidum): systematic review by the Natural Standard Research Collaboration. J Soc Integr Oncol. 2010;8:148–59. [Google Scholar]
- 53.Ulbricht C, Basch E, Barrette E, Boon H, Chao W, Costa D, Higdon ER, Isaac R, Lynch M, Papaliodis G, Serrano J, Varghese A, Vora M, Windsor R, Woods J. Turmeric (Curcuma longa): an evidence‐based systematic review by the natural standard research collaboration. Altern Complement Ther. 2011;17:225–36. [Google Scholar]
- 54.Vora K, Dennehy C, Ulbricht C, Basch E, Vora M. 2012. Chasteberry (Vitex agnus‐castus) natural standard professional monograph. Available at http://www.naturalstandard.com/databases/herbssupplements/all/vitex-agnus-castus.asp (last accessed 21 January 2012).
- 55.Ernst E, Posadzki P, Lee MS. Complementary and alternative medicine (CAM) for sexual dysfunction and erectile dysfunction in older men and women: an overview of systematic reviews. Maturitas. 2011;70:37–41. doi: 10.1016/j.maturitas.2011.06.011. [DOI] [PubMed] [Google Scholar]


