Abstract
Even‐number, medium‐chain dicarboxylic acids (DAs), naturally occurring in higher plants, are a promising alternative energy substrate. Unlike the homologous fatty acids, DAs are soluble in water as salts. They are β‐oxidized, providing acetyl‐CoA and succinyl‐CoA, the latter being an intermediate of the tricarboxylic acid cycle. Sebacic acid and dodecanedioic acid, DAs with 10 and 12 carbon atoms respectively, provide 6.6 and 7.2 kcal g−1 each; therefore, their energy density is intermediate between glucose and fatty acids. Dicarboxylic acids have been proved to be safe in both experimental animals and humans, and their use has recently been proposed in diabetes. Studies in animals and humans with type 2 diabetes showed that oral administration of sebacic acid improved glycaemic control, probably by enhancing insulin sensitivity, and reduced hepatic gluconeogenesis and glucose output. Moreover, dodecanedioic acid intake reduced muscle fatigue during exercise in subjects with type 2 diabetes, suggesting an improvement of energy utilization and ‘metabolic flexibility’. In this article, we review the natural sources of DAs, their fate in animals and humans and their effect in improving glucose metabolism in type 2 diabetes.
Keywords: dicarboxylic acids, dodecanedioic acid, sebacic acid, type 2 diabetes
Introduction
Type 2 diabetes mellitus is often associated with obesity and insulin resistance. In all these pathological conditions, the presence of an increased deposition of intracellular triglycerides in insulin‐sensitive tissues, such as the skeletal muscle and the liver, led to the hypothesis of a defect in mitochondrial substrate oxidation [1–4]. This hypothesis was supported by the finding of multiple defects of metabolic fluxes through the tricarboxylic acid (TCA) and oxidative phosphorylation in skeletal muscle, as assessed by magnetic resonance spectroscopy [5,6]. These findings were confirmed by the evidence that the rate of incorporation of 13C into the C4‐glutamate pool was slower in the offspring of patients with insulin‐resistant type 2 diabetes than in control subjects, reflecting a 30% decrease in the basal rate of muscle substrate oxidation [7]. This impairment of the TCA flux was associated with an increased deposition of intramyocytic triglycerides [7].
In addition, the plasticity of the mitochondria, which is their capacity to adapt to different degrees of energy requirements through fission (division of a mitochondrion into two mitochondria) and fusion (fusion of two mitochondria to form a new one) [8–10], is reduced in type 2 diabetes mellitus, with subsequent metabolic inflexibility [11,12]. Metabolic inflexibility is a characteristic feature of diabetic individuals, who are incapable of adapting their metabolism to different energy requirements by switching from glucose to fatty acid oxidation as a consequence of a defective response to insulin.
In a recent study, Gaster [13] found that myotubes from patients with diabetes express a significantly lower acetate oxidation (30%) compared with myotubes from lean individuals, both in the basal state and during acute insulin stimulation. Also, the insulin‐induced increment in acetate oxidation tended to be lower in myotubes from patients with diabetes but did not reach statistical significance between groups [13].
It is not known whether the mitochondrial function is impaired as a primary, heritable defect or whether it is a consequence of insulin resistance.
In any case, an energy substrate providing TCA intermediates might be a potentially valuable fuel in those pathological conditions associated with insulin resistance, including type 2 diabetes mellitus.
Even‐number dicarboxylic acids (DAs) have been shown to be a suitable energy substrate [14–22], with characteristics intermediate between glucose and fatty acids. In fact, they are β‐oxidized like fatty acids but, like glucose, their salts are soluble in water, thanks to their short‐to‐medium chains and the presence of two terminal carboxylic groups. Their end product of β‐oxidation is succinic acid, which enters the TCA.
This review focuses on our studies of the effects of even‐number, medium‐chain DAs, specifically sebacic and dodecanedioic acids, as possible alternative energy substrates in diabetes. It is divided into sections describing the natural source of DAs, their formation from free fatty acids though ω‐oxidation, their cellular fate through β‐oxidation, and the benefits of medium‐chain DA intake in type 2 diabetes.
Dicarboxylic acids in nature
Medium‐ and long‐chain DAs are present in higher plants and animals deriving from the ω‐oxidation of fatty acids [23,24].
In higher plants, DAs are components of natural protective polymers, cutin and suberin, a support biopolyester that waterproofs the leaves and fruits, regulating the flow of nutrients and minimizing the harmful impact of pathogens [25]. Dihydroxy C16 fatty acids, 18‐hydroxy‐9,10‐epoxy C18 fatty acids and trihydroxy C18 fatty acids are the major components of cutin, while suberin is mainly composed of ω‐hydroxy fatty acids and C16‐C18 dicarboxylic acids. Dicarboxylic acids are β‐oxidized in specialized plant peroxisomes (glyoxysomes), where the glyoxylate cycle, whose intermediates derive from the degradation of reserve or structural lipids, takes place [26].
Sebacic acid, named from the Latin sebum (tallow) in reference to its use in the manufacture of candles, is a minor component of the royal jelly [27], a yellowish material secreted by the mandibular and hypopharyngeal glands of worker bees to nourish the queen bee, for whose longevity it is essential.
Fatty acid ω‐oxidation
In animals and humans, medium‐chain DAs are even numbered, with a chain length from 6 to 12 carbon atoms, and include adipic (C6), suberic (C8), sebacic (C10) and dodecanedioic (C12) acids (Figure 1). They derive from the β‐oxidation of longer chain DAs [28], which are formed by ω‐oxidation from free fatty acids of the same chain length inside the microsomial membranes or originate from a vegetable‐rich diet. However, a direct ω‐oxidation of a medium‐chain fatty acid, lauric acid, to dodecanedioic acid has been also demonstrated [29,30].
Figure 1.
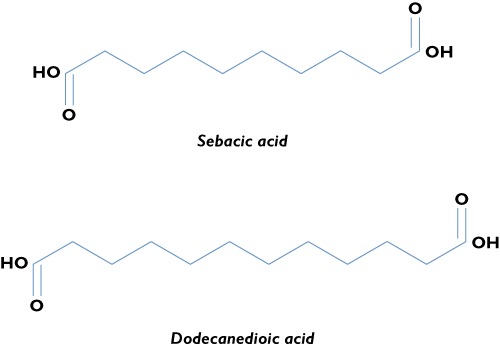
Structural formulae of sebacic and dodecanedioic acids
Dicarboxylic acid β‐oxidation
β‐Oxidation of DAs takes place in both mitochondria and peroxisomes [31–33]. Four different mitochondrial pathways for DA transportation have been shown [33], and they include an electrophoretic transport via an inner membrane anion channel, a passive diffusion, a tributyltin‐mediated transport and a transport via the dicarboxylate carrier, which operates for short‐chain Das, such as oxalate, malonate and succinate. This transportation does not require the carnitine shuttle (carnitine palmitoyltransferase 1, carnitine palmitoyltransferase 2 and carnitine acetyltransferase). However, a previous study [32] demonstrated that sebacic and dodecanedioic acids consume carnitine when entering the mitochondria.
In any case, once in the mitochondria DAs follow the same fate as free fatty acids, being degraded to acetyl‐CoA through the β‐oxidation (Figure 2). A characteristic of DAs, however, is that they produce succinyl‐CoA at the end of the β‐oxidation.
Figure 2.
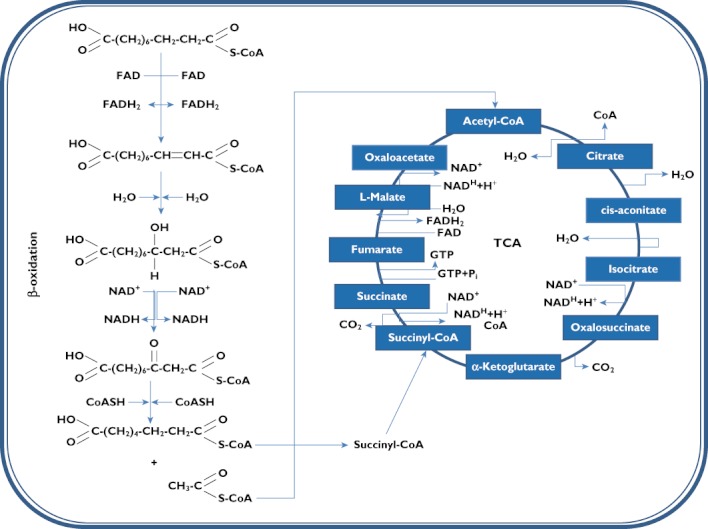
Fate of sebacic acid though the intramitochondrial β‐oxidation pathway and tricarboxylic acid cycle (TCA)
Succinyl‐CoA, like other TCA intermediates, is an entry pathway for other metabolites into the TCA (Figure 2) and is involved in a variety of important biological actions. Glucogenic amino acids, i.e. isoleucine, methionine and valine, are broken down into propionyl‐CoA. Propionyl‐CoA is converted into d‐methylmalonyl‐CoA by propionyl‐CoA carboxylase. d ‐Methylmalonyl‐CoA is racemized into l‐methylmalonyl‐CoA by methylmalonyl‐CoA racemase. Methylmalonyl mutase produces succinyl‐CoA, which enters into the TCA. Succinyl‐CoA is an intracycle regulator, inhibiting citrate synthase and α‐ketoglutarate dehydrogenase.
Use of DAs in diabetes
As shown by acute, subacute and chronic studies in rodents [34], sebacic and dodecanedioic acids are safe and well tolerated in humans [15–18].
Being slightly soluble in water, a certain percentage of circulating dicarboxylic acids, varying from 16 to 46% of the amount administered for sebacic acid [22] and to less than 4% for dodecanedioic acid [35], is lost in urine. Dicarboxylic acids are bound to plasma albumin, although to a much lower extent than the monocarboxylic acids of the same chain length, in equilibrium with their soluble fraction [35].
Experimentally, the tissue uptake of DAs was estimated to be about 490 mg min−1 for sebacic acid [22] and 900 mg min−1 for dodecanedioic acid in a man of average bodyweight (70 kg) [14]. As the glucose uptake measured by the euglycaemic hyperinsulinaemic clamp is usually between 7 and 10 mg (kg bodyweight)−1 min−1, in a man of average bodyweight its uptake ranges between 490 and 700 mg min−1; thus, it is very close to that of sebacic acid and lower than that of dodecanedioic acid. The caloric density of sebacic acid is 6.6 kcal g−1 and that of dodecanedioic acid is 7.2 kcal g−1.
Dicarboxylic acid triglycerides
The studies with DAs using intravenous injection were performed with sodium salt formulations in order to improve their water solubility. In order to reduce by half the amount of inorganic salts administered together with DAs, glycerol esters (DA triglycerides) were synthesized. The triglyceride of dodecanedioic acid is still soluble in water as salt and shows a very low excretion in the urine, corresponding to approximately 0.67% of the intake. Kinetic studies in rats have shown that this DA‐C12 triglyceride has a large volume of distribution (approximately 0.5 l (kg bodyweight)−1) and a rapid plasma clearance rate. Once hydrolysed by the organism into glycerol and free dodecanedioic acid, this latter is taken up by the tissues with a maximal transport rate of 0.636 mmol min−1[36].
Sebacic acid in diabetes
When the diet of db/db mice was supplemented with 15% of the energy deriving from sebacic acid over a period of 6 weeks, glycaemic control was significantly improved in comparison with the control group [37]. Indeed, fasting plasma glucose and glycated haemoglobin dropped to values 40 and 25%, respectively, lower than in control animals (P < 0.01) and became comparable to that of lean healthy db/+ animals. The insulin‐mediated glucose clearance was increased, suggesting an improvement of insulin sensitivity. Fasting concentrations of circulating non‐esterified fatty acids were significantly lowered by sebacic acid, probably as a consequence of the increased antilipolytic action of insulin. The transcriptomic analysis of the liver revealed an upregulation of 288 genes and a downregulation of 182 genes among 24 600 tested. In particular, the gluconeogenetic genes, coding for pyruvate carboxykinase (Pck1) and fructose 1,6‐bisphosphatase (fbp1), were greatly downregulated (−75 and −38%, respectively), suggesting that sebacic acid decreased gluconeogenesis.
Increased glucose production and increased gluconeogenesis from glucogenic amino acids are reported to occur in decompensated type 2 diabetes [38]. Dietary supplementation of sebacic acid reproduced the picture determined by partly silencing Pck1 expression (∼50%) in the liver of db/db mice [39], which allowed a net reduction of plasma glucose in this model of type 2 diabetes. Hypoglycaemic effects have been also observed in db/db mice by the administration of troglitazone, with a 38% inhibition of fbp1 expression [40]. Furthermore, it has recently been demonstrated that teglicar, a new antidiabetic drug acting through the selective and reversible inhibition of the liver isoform of carnitine palmitoyl‐transferase 1, reduces gluconeogenesis and improves glucose homeostasis in diabetic mice [41]. Indeed, the reduction of hepatic gluconeogenesis, which largely contributes to liver glucose output, is considered nowadays as a key pharmacological target in the treatment of diabetes.
In type 2 diabetic subjects [42], the ingestion of a mixed meal (450 kcal) with the addition of 10 g of sebacic acid or with 23 g of sebacic acid substituting the meal lipid content resulted in a net reduction of the glucose peak and, with the larger amount, a significant decrease of the area under the curve of plasma glucose concentration. The circulating levels of insulin in response to the meal were reduced with both amounts of sebacic acid. The rate of glucose appearance was decreased by about 18% (P < 0.05) with 23 g sebacic [42].
In vitro studies with L6 cells showed that sebacic acid resulted in a significantly (P < 0.01) increased uptake of glucose in the presence of insulin, associated with a 1.74 ± 0.27‐fold increase of glucose transporter GLUT4 protein expression [42], further supporting a direct action of this diacid in improving insulin resistance other than in reducing gluconeogenesis.
Dodecanedioic acid in diabetes
In humans, muscle glycogen stores represent an essential energy source during submaximal exercise, and depletion of muscle glycogen stores promotes muscle fatigue [43–46].
The skeletal muscle of metabolically healthy individuals has the capacity to switch easily from glucose to fat oxidation and vice versa in relation to the energy request and the insulin secretion; this phenomenon has received the name of ‘metabolic flexibility’[11]. There is a large body of literature showing that metabolic flexibility is impaired in subjects with type 2 diabetes as a consequence of their state of insulin resistance. Muscle fatigue, and consequently a decline in skeletal muscle performance, is a characteristic feature of diabetes [47] and derives from the inability to adjust the rate of ATP production to the energy requirements during a continuous activity.
The oral administration of 40 g dodecanedioic acid in subjects with type 2 diabetes before a bout of moderate exercise reduces muscle fatigue, thus allowing completion of the exercise. Dodecanedioic acid intake does not promote insulin secretion but is associated with a significant increase of plasma non‐esterified fatty acids, indicating increased triglyceride hydrolysis [48]. The tissue uptake in this study was high, with a total uptake of 47% of ingested dodecanedioate, and the oxidation of the diacid equalled 69% of the diacid taken up by the tissues, while the remaining fraction (53%) was possibly converted to glycogen in the liver. It is likely that dodecanedioic acid is able to overcome the muscular ‘metabolic inflexibility’, providing intermediate substrates for mitochondrial oxidation and ATP synthesis.
Perspectives
Future studies are needed to elucidate the fate of DAs in different tissues and organs in diabetes, as well as the potential for use of these diacids in enteral nutrition and as a natural food ingredient in patients with type 2 diabetes in daily life and during physical exercise.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–14. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Boden G. Fatty acid‐induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177–81. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 3.Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–46. doi: 10.1146/annurev.nutr.22.010402.102912. [DOI] [PubMed] [Google Scholar]
- 4.Mingrone G, Rosa G, Di Rocco P, Manco M, Capristo E, Castagneto M, Vettor R, Gasbarrini G, Greco AV. Skeletal muscle triglycerides lowering is associated with net improvement of insulin sensitivity, TNF‐alpha reduction and GLUT4 expression enhancement. Int J Obes Relat Metab Disord. 2002;26:1165–72. doi: 10.1038/sj.ijo.0802053. [DOI] [PubMed] [Google Scholar]
- 5.Krssak M, Petersen KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 6.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin‐resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin‐resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorzano A, Liesa M, Palacín M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–54. doi: 10.1016/j.biocel.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 10.Zorzano A, Liesa M, Palacín M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch Physiol Biochem. 2009;115:1–12. doi: 10.1080/13813450802676335. [DOI] [PubMed] [Google Scholar]
- 11.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63:363–8. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 12.Mingrone G, Manco M, Calvani M, Castagneto M, Naon D, Zorzano A. Could the low level of expression of the gene encoding skeletal muscle mitofusin‐2 account for the metabolic inflexibility of obesity? Diabetologia. 2005;48:2108–14. doi: 10.1007/s00125-005-1918-9. [DOI] [PubMed] [Google Scholar]
- 13.Gaster M. Reduced TCA flux in diabetic myotubes. A governing influence on the diabetic phenotype? Biochem Biophys Res Commun. 2009;387:651–5. doi: 10.1016/j.bbrc.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 14.Bertuzzi A, Mingrone G, Gandolfi A, Greco AV, Salinari S. Disposition of dodecanedioic acid in humans. J Pharmacol Exp Ther. 2000;292:846–52. [PubMed] [Google Scholar]
- 15.Mingrone G, De Gaetano A, Greco AV, Capristo E, Benedetti G, Castagneto M, Gasbarrini G. Comparison between dodecanedioic acid and long‐chain triglycerides as an energy source in liquid formula diets. JPEN J Parenter Enteral Nutr. 1999;23:80–4. doi: 10.1177/014860719902300280. [DOI] [PubMed] [Google Scholar]
- 16.Mingrone G, De Gaetano A, Greco AV, Benedetti G, Capristo E, Castagneto M, Gasbarrini G. Plasma clearance and oxidation of dodecanedioic acid in humans. JPEN J Parenter Enteral Nutr. 1996;20:38–42. doi: 10.1177/014860719602000138. [DOI] [PubMed] [Google Scholar]
- 17.Greco AV, Mingrone G. Dicarboxylic acids, an alternate fuel substrate in parenteral nutrition: an update. Clin Nutr. 1995;14:143–8. doi: 10.1016/s0261-5614(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 18.Raguso CA, Mingrone G, Greco AV, Tataranni PA, De Gaetano A, Castagneto M. Dicarboxylic acids and glucose utilization in humans: effect of sebacate. JPEN J Parenter Enteral Nutr. 1994;18:9–13. doi: 10.1177/014860719401800109. [DOI] [PubMed] [Google Scholar]
- 19.Mingrone G, Greco AV, Castagneto M, De Gaetano A, Tataranni PA, Raguso C. Kinetics and thermogenesis of medium‐chain monocarboxylic and dicarboxylic acids in man: sebacate and medium‐chain triglycerides. JPEN J Parenter Enteral Nutr. 1993;17:257–64. doi: 10.1177/0148607193017003257. [DOI] [PubMed] [Google Scholar]
- 20.Mingrone G, Tacchino RM, Castagneto M, Finotti E, Greco AV. Use of even‐numbered carbon atom dicarboxylic salts in parenteral nutrition as fuel substrate. JPEN J Parenter Enteral Nutr. 1992;16:32–8. doi: 10.1177/014860719201600132. [DOI] [PubMed] [Google Scholar]
- 21.Greco AV, Mingrone G, Raguso C, Tataranni A, Finotti E, Tacchino RM, Capristo E, De Gaetano A, Castagneto M. Metabolic effects and disposition of sebacate, an alternate dicarboxylic fuel substrate. Ann Nutr Metab. 1992;36:1–11. doi: 10.1159/000177693. [DOI] [PubMed] [Google Scholar]
- 22.Mingrone G, Greco AV, Bertuzzi A, Arcieri‐Mastromattei E, Tacchino RM, Marino F, Finotti E, Castagneto M. Tissue uptake and oxidation of disodium sebacate in man. JPEN J Parenter Enteral Nutr. 1991;15:454–9. doi: 10.1177/0148607191015004454. [DOI] [PubMed] [Google Scholar]
- 23.Verkade PE, Van der Lee J. Researches on fat metabolism II. Biochem J. 1934;28:31–40. doi: 10.1042/bj0280031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolattukudy PE, Walton TJ, Kushwaha RP. Biosynthesis of the C18 family of cutin acids: omega‐hydroxyoleic acid, omega‐hydroxy‐9,10‐epoxystearic acid, 9,10,18‐trihydroxystearic acid, and their delta12‐unsaturated analogs. Biochemistry. 1973;12:4488–98. doi: 10.1021/bi00746a029. [DOI] [PubMed] [Google Scholar]
- 25.Kolattukudy PE. Polyesters in higher plants. Adv Biochem Eng Biotechnol. 2001;71:1–49. doi: 10.1007/3-540-40021-4_1. [DOI] [PubMed] [Google Scholar]
- 26.Penfield S, Graham S, Graham IA. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem Soc Trans. 2005;33:380–3. doi: 10.1042/BST0330380. [DOI] [PubMed] [Google Scholar]
- 27.Moutsatsou P, Papoutsi Z, Kassi E, Heldring N, Zhao C, Tsiapara A, Melliou E, Chrousos GP, Chinou I, Karshikoff A, Nilsson L, Dahlman‐Wright K. Fatty Acids Derived from Royal Jelly Are Modulators of Estrogen Receptor Functions. PLoS ONE. 2010;5:e15594. doi: 10.1371/journal.pone.0015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolvraa S, Gregersen N. In vitro studies on the oxidation of medium‐chain dicarboxylic acids in rat liver. Biochim Biophys Acta. 1986;876:515–25. doi: 10.1016/0005-2760(86)90039-1. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson A, Arey H, Pedersen JI. The effect of high‐fat diets on microsomal lauric acid hydroxylation in rat liver. Biochim Biophys Acta. 1986;879:209–14. doi: 10.1016/0005-2760(86)90104-9. et al. [DOI] [PubMed] [Google Scholar]
- 30.Shet M, Fisher CW, Holmans PL, Estabrook RW. The omega‐hydroxlyation of lauric acid: oxidation of 12‐hydroxlauric acid to dodecanedioic acid by a purified recombinant fusion protein containing P450 4A1 and NADPH‐P450 reductase. Arch Biochem Biophys. 1996;330:199–208. doi: 10.1006/abbi.1996.0243. [DOI] [PubMed] [Google Scholar]
- 31.Mortensen PB. C6–C10‐dicarboxylic aciduria in starved, fat‐fed and diabetic rats receiving decanoic acid or medium‐chain triacylglycerol. An in vivo measure of the rate of beta‐oxidation of fatty acids. Biochim Biophys Acta. 1981;664:349–55. doi: 10.1016/0005-2760(81)90057-6. [DOI] [PubMed] [Google Scholar]
- 32.Kølvraa S, Gregersen N. In vitro studies on the oxidation of medium‐chain dicarboxylic acids in rat liver. Biochim Biophys Acta. 1986;876:515–25. doi: 10.1016/0005-2760(86)90039-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Hinch B, Beavis AD. Mechanisms for the transport of alpha,omega‐dicarboxylates through the mitochondrial inner membrane. J Biol Chem. 1996;271:338–44. doi: 10.1074/jbc.271.41.25338. [DOI] [PubMed] [Google Scholar]
- 34.Greco AV, Mingrone G, Arcieri Mastromattei E, Finotti E, Castagneto M. Toxicity of disodium sebacate. Drugs Exp Clin Res. 1990;16:531–6. [PubMed] [Google Scholar]
- 35.Bertuzzi A, Finotti E, Mingrone G, Greco AV. Sebacic acid binding to human plasma albumin. Biochem Pharmacol. 1993;45:697–702. doi: 10.1016/0006-2952(93)90145-m. [DOI] [PubMed] [Google Scholar]
- 36.de Gaetano A, Mingrone G, Castagneto M, Benedetti G, Greco AV, Gasbarrini G. Kinetics of dodecanedioic acid triglyceride in rats. Am J Physiol. 1999;276:E497–502. doi: 10.1152/ajpendo.1999.276.3.E497. [DOI] [PubMed] [Google Scholar]
- 37.Membrez M, Chou CJ, Raymond F, Mansourian R, Moser M, Monnard I, Ammon‐Zufferey C, Mace K, Mingrone G, Binnert C. Six weeks' sebacic acid supplementation improves fasting plasma glucose, HbA1c and glucose tolerance in db/db mice. Diabetes Obes Metab. 2010;12:1120–6. doi: 10.1111/j.1463-1326.2010.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turk D, Alzaid AA, Dinneen SF, Nair KS, Rizza RA. The effects of non‐insulin‐dependent diabetes mellitus on the kinetics of onset of insulin action in hepatic and extrahepatic tissues. J Clin Invest. 1995;95:755–62. doi: 10.1172/JCI117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez‐Valadés AG, Méndez‐Lucas A, Vidal‐Alabró A, Blasco FX, Chillon M, Bartrons R, Bermúdez J, Perales JC. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 2008;57:2199–210. doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiwara T, Okuno A, Yoshioka S, Horikoshi H. Suppression of hepatic gluconeogenesis in long‐term troglitazone treated diabetic KK and C57BL/KsJ‐db/db mice. Metabolism. 1995;44:486–90. doi: 10.1016/0026-0495(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 41.Conti R, Mannucci E, Pessotto P, Tassoni E, Carminati P, Giannessi F, Arduini A. Selective reversible inhibition of liver carnitine palmitoyl‐transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes. 2011;60:644–51. doi: 10.2337/db10-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iaconelli A, Gastaldelli A, Chiellini C, Gniuli D, Favuzzi A, Binnert C, Macé K, Mingrone G. Effect of oral sebacic Acid on postprandial glycemia, insulinemia, and glucose rate of appearance in type 2 diabetes. Diabetes Care. 2010;33:2327–32. doi: 10.2337/dc10-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casey A, Short AH, Curtis S, Greenhaff PL. The effect of glycogen availability on power output and the metabolic response to repeated bouts of maximal, isokinetic exercise in man. Eur J Appl Physiol Occup Physiol. 1996;72:249–55. doi: 10.1007/BF00838647. [DOI] [PubMed] [Google Scholar]
- 44.Broberg S, Sahlin K. Hyperammoniemia during prolonged exercise: an effect of glycogen depletion? J Appl Physiol. 1988;65:2475–7. doi: 10.1152/jappl.1988.65.6.2475. [DOI] [PubMed] [Google Scholar]
- 45.Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967;71:129–39. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- 46.Bergstrom J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210:309–10. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- 47.Panagiotis H, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- 48.Salinari S, Bertuzzi A, Gandolfi A, Greco AV, Scarfone A, Manco M, Mingrone G. Dodecanedioic acid overcomes metabolic inflexibility in type 2 diabetic subjects. Am J Physiol Endocrinol Metab. 2006;291:E1051–8. doi: 10.1152/ajpendo.00631.2005. [DOI] [PubMed] [Google Scholar]


