Abstract
The underlying cause of sarcopenia and dynapenia (age‐related strength loss) are not fully elucidated, but may be the result, or combination, of alterations in lifestyle or inflammatory and endocrine profiles. What is clear is that functional ability is limited and mortality risk is elevated. Mechanistically, muscle atrophy is the result of the prolonged periods of net negative muscle protein balance, brought about by the imbalance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB). Contractile loading of skeletal muscle, through resistive‐type exercise and amino acid ingestion both act as a strong stimulus for MPS and, when combined, can induce a net positive protein balance and muscle hypertrophy. Given that MPS in older muscles displays a blunted response to anabolic stimuli compared with the young, the combined effect and manipulation of contractile and nutrient interventions to optimize muscle anabolism could be extremely important for counteracting sarcopenia. Specifically, the dose, absorption kinetics, leucine content, but less‐so the timing of ingestion, are important determinants of the mRNA translational signalling response regulating MPS. In addition, resistance exercise‐induced rates of MPS and hypertrophy appear to be dependent on exercise volume (to achieve maximal muscle fibre recruitment), as opposed to the absolute load that is lifted. A number of recent studies in young adults lend weight to this notion by showing that contraction can be manipulated; allowing low load weight lifting to effectively stimulate rates of MPS to a level comparable with traditional high loads, a finding with important implications for older adults interested in undertaking resistance exercise.
Keywords: amino acids, muscle contraction, muscle protein metabolism, sarcopenia
Introduction
Age‐related skeletal muscle atrophy, or sarcopenia (translated as poverty of the flesh), occurs predominantly in type II muscle fibres and is accompanied by declining strength. This ageing phenomenon contributes to impairments in physical function, thereby lowering the quality of life in the elderly. In addition to the predictive relationship between sarcopenia and disability, sarcopenia can increase the prevalence of age‐associated complications including rheumatoid and osteoarthritis, vascular disease, type II diabetes and osteoporosis that require extensive health care resources. Given the well‐described expansion of older populations in society, concerns over the continued availability of health care resources to counteract age‐related disease are well founded. Therefore, investigations into nutrient and exercise interactions to prevent muscle wasting in the elderly are of great importance.
At the cellular level, muscle atrophy stems from prolonged periods of net negative protein balance, brought about by dampened rates of muscle protein synthesis (MPS), elevated rates of muscle protein breakdown (MPB), or a combination of the two, the extent of which depends on the specific wasting condition (i.e. sarcopenia vs. cachexia). Currently, it is unclear whether sarcopenia is the result of alterations in basal, fasted protein kinetics (i.e. a reduction in MPS and elevated MPB) or a diminished muscle protein synthetic response to normally robust anabolic stimuli, such as food intake and muscle contractile activity. Certainly, a growing number of studies point to the latter of these mechanisms as a primary contributor to sarcopenia and are discussed herein. Importantly, sarcopenia does not appear to be an inevitable consequence of ageing. Muscle wasting in the elderly is dependent on the quality of ageing. For example, relatively ‘well‐preserved’ elderly muscles in older individuals who maintain a physically active lifestyle appear able to maintain the ability to mount a strong anabolic response to contractile loading and food intake that is comparable with the young. Furthermore, basal, fasted rates of MPS and MPB do not appear different between healthy old and young muscles [1,2,3,4,5]. On the other hand, muscle unloading/disuse elevates systemic and local markers of inflammation and oxidative stress, which may contribute toward blunting basal, fasted protein kinetics and anabolic sensitivity in older adults who do not maintain a physically active lifestyle or, more likely, suffer from repeated bouts of protracted hospitalization. Empirical support for the notion that maintaining a physically active lifestyle can delay sarcopenia is apparent from a number of studies showing that a short period of immobilization can hasten the decline in muscle protein synthesis in the elderly [6–9]. This model of sarcopenia has been termed the ‘acute catabolic crises’ [b10]. In summary, with advancing age, it becomes increasingly likely that even a brief, clinically mandated period of bed rest or reduced ambulation (Breen & Phillips, unpublished observations) could initiate a serious decline in muscle strength and functional capacity, that is, a ‘tipping point’ from which some older adults may not fully recover.
Recent evidence points to differences in muscle protein metabolism between older men and women. Specifically, Smith and co‐workers [b11,b12] found that the basal rate of MPS was greater in old women than in old men and that in response to meal ingestion older men, but not older women, were able to increase the rate of MPS in response to meal ingestion. These data are somewhat paradoxical when one considers that older women generally have less lean mass and more body fat than older men and indications are that post‐absorptive and post‐prandial rates of MPS are reduced in obese young adults [b13]. Clearly, the discrepant findings may be due to differences between young and old muscles. In support of this notion, there appears to be no difference in basal rates of MPS between healthy young and middle‐aged men and women [b14]. For the purpose of the current review and in the absence of conclusive evidence, we will consider older men and women a homogeneous population when discussing nutrient and exercise strategies to counteract sarcopenia.
Mechanisms of action for MPS
In order for resistance exercise or nutrition to affect the rate of MPS, an increase in the rate of mRNA translation initiation, as the primary mechanism, is required (54). This process involves the assembly of the components of the translational machinery, specifically the mRNA that directs protein assembly and the ribosome where assembly occurs. The mammalian target of rapamycin (mTOR) controls MPS and cell growth via a complex interaction with downstream signalling targets, including eukaryotic initiation factor 2 (eIF2), the 4E binding protein complex (4E‐BP1) and ribosomal protein S6 kinase (S6K1) [b15,b16]. Given that acute changes in MPS are thought to be predictive of long term adaptations in muscle size/strength it is not surprising that researchers have found that the protein synthetic response to resistance exercise is abolished when mTOR is blocked using the pharmacological agent, rapamycin [b16]. The activation of mTOR increases with resistance exercise and occurs in a manner distinct from growth factors like insulin growth factor (IGF)‐1 [b17,b18]. For example, the activation of mTOR following resistance exercise is thought to occur independent of the protein kinase B/Akt signalling pathway and is independent of the circulating hormone concentration [b19]. Like resistance exercise, amino acids, in particular the branched‐chain amino acid (BCAA) leucine, increase mTOR signalling [b20,b21]. The extent of the intramuscular signalling response with protein ingestion mirrors essential amino acid (EAA) availability in the blood and muscle [b22], but in contrast, does not have a strong relationship with the temporal time course of feeding‐induced MPS [b22].
Nutrient‐exercise interaction for optimal muscle anabolism
Feeding protein results in a substantial hyperaminoacidaemia that promotes a net inward gradient for amino acid transport to the intracellular free amino acid pool [b22–b24]. The resulting influx of amino acids (AA), particularly the branched‐chain amino acid (BCAA) leucine, into the free amino pool provides the signal [b25,b26] and building blocks [b22–b24] (up to a point of saturation), for the stimulation of MPS. Thus, periodic feeding induces shifts in net protein balance (NPB) from negative during fasting where MPS < MPB, to positive after feeding where MPS > MPB [b27]. Adequate nutrition therefore assists in the maintenance of muscle protein mass [b27,b28]. The underlying basis for the anabolic state induced by resistance exercise is also a pronounced stimulation of MPS [b29]. Combining the resistance exercise‐induced rise in MPS with feeding results in an additive effect on MPS and marked increase in NPB (MPS > > MPB) [23,30,31,32]. Repeated bouts of resistance exercise together with feeding therefore result in periodic increases in accretion of proteins and, ultimately, muscle hypertrophy [b33]. Understanding the leucine content of the food being consumed, the digestion pattern of the protein, along with kinetic measures of protein turnover and their interaction with exercise can allow for practical recommendations to be made for older adults in whom muscle mass is compromised.
Protein source
The amplitude and duration of the stimulation in MPS is dependent on the ingested protein source. Seminal work from the laboratory of Boirie [b34–b36] showed that there are two types of protein contained in milk, characterized by the resulting patter of aminoacidaemia. Specifically, fast proteins, the whey fraction in milk (∼20%), are acid soluble, resulting in rapid appearance in the circulation. In contrast, slow proteins, the casein fraction in milk (∼80%), are acid insoluble and clot in the splanchnic region, resulting in a slower absorption through the intestine and more prolonged aminoacidaemia. Thus, casein proteins better support the synthesis of proteins in the splanchnic region, whereas, whey proteins better support MPS. Acutely, we have demonstrated that whey proteins promote greater protein accretion than equivalent amounts of casein and soy proteins after resistance exercise [b37]. Furthermore, when practiced over time, milk protein ingestion results in greater gains in lean muscle mass than isonitrogenous and isoenergetic soy ingestion [b38]. Interestingly whey, casein and soy proteins have a similar EAA content and are considered to be ‘high‐quality’ protein sources (based on a PDCAAS ≥ 1.0). However, whey protein has greater leucine content than casein and soy proteins. The rapid absorption kinetics of whey induce a rapid leucinemia which may ‘trigger’ the intramuscular signalling processes required for MPS [b20,b39]. This thesis may, in part, explain the greater resting and post‐exercise rates of MPS after whey protein ingestion, compared with casein and soy [b40–b42]. Furthermore, alterations in the leucine threshold required for anabolism may explain the apparent ‘anabolic resistance’ to AA ingestion in elderly muscle [b43,b44]. In a series of recent studies of older adults we have demonstrated that whey protein ingestion elevates fed state MPS to a greater extent than dose‐matched soy and casein ingestion and that this pattern of response remains apparent when feeding is synergistically combined with resistance exercise [43,45,46]. The efficacy of long term leucine supplementation as a lone intervention to promote hypertrophy is not supported. A recent, well‐controlled study from Verhoeven and colleagues, showed no hypertrophy in older adults consuming an additional 7.5 g of leucine each day [b47]. These data and more recent findings from our laboratory [b48] suggest that supplemental leucine should be given in addition to a full complement of EAAs (as substrate to support MPS) in order to maximize muscle anabolic potential. It could be theorized that co‐ingesting additional leucine with a protein dose insufficient to maximize MPS (i.e. 5–10 g during breakfast), may elevate the acute muscle protein synthetic response. This thesis is yet to be examined in detail, but a hypothetical example is highlighted in Figure 1.
Figure 1.
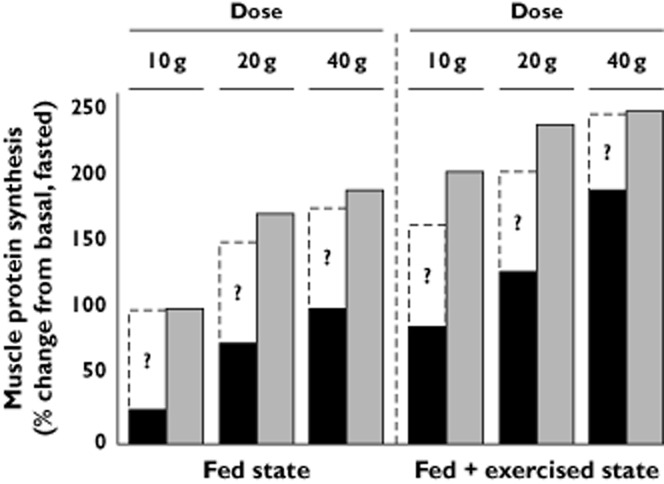
Comparison of the dose–response of muscle protein synthesis to graded intact protein ingestion alone and in combination with resistance exercise in old and young. For the purpose of this figure, intact protein is defined as a high quality protein with rapid absorption kinetics and resistance exercise is defined as high load low intensity lifting. Dashed columns indicate the potential for anabolic interventions to be implemented to potentiate acute MPS rates. For example, in the fed state, ingesting additional leucine may enhance the effect of an intact protein dose on MPS in the elderly. In addition, nutrient interventions coupled with appropriate contractile manipulation (i.e. low load high intensity exercise, slow cadence or elevated volume lifting) may enhance feeding + exercise‐induced rates of MPS. These findings are based on data from references [22,30,32,43,45,46,49,75].  , old;
, old;  , young
, young
Protein dose–response
A dose–response for MPS with graded protein ingestion has been demonstrated in three previous studies, only one of which was conducted in older adults. Cuthbertson et al. [b49] were the first to show that oral ingestion of 10 g crystalline EAA, which equates to ∼25 g of high quality protein (i.e. milk, soy or chicken), resulted in maximal stimulation of MPS in young and old adults at rest. Similarly, recent work from our laboratory [b43] showed that 20 g of whey protein ingestion resulted in maximal stimulation of MPS in the elderly. However, in contrast to the work of Cuthbertson et al. [b49] our findings indicated that a < 20 g of protein ingestion was insufficient to mount a robust increase in MPS compared with the fasted state (Figure 1). Thus, the lack of sensitivity of older muscles to low‐doses of AA is consistent with the phenomenon of ‘anabolic resistance’ with advancing age. In the context of resistance exercise, Moore et al. [b30] showed that 20 g of egg protein ingestion induced maximal stimulation of MPS in young men. Consuming an additional 20 g of egg protein (40 g total) resulted in an 11% greater rate of MPS, but a concomitant increase in leucine oxidation over that seen with the 20 g protein dose. Thus, at intakes of protein of 20 g and higher, a greater proportion of the ingested leucine was being oxidized for fuel with minimal further stimulation of MPS [b30]. In contrast, our highlighted work [b43] suggested that older muscles were responsive to a greater dose of amino acids than the young following resistance exercise, as MPS was greater after 40 g of whey, compared with 20 g (Figure 1).
With regard to the frequency of protein ingestion to maintain elevated rates of MPS, data in young adults [b22,b32] showed that MPS was transiently elevated with feeding, peaking early and remaining elevated at 3 h post‐feeding. Resistance exercise appears to sensitize the myofibrillar, but not non‐myofibrillar proteins, within the muscle, thereby increasing the amplitude and duration of the synthetic response. The finding of enhanced sensitivity of previously exercised muscle to subsequent amino acid feeding has yet to be examined in the elderly. It appears that further protein intake is required somewhere between 3–5 h after the initial bolus (dependent on whether muscle contraction is present) in order to maintain higher rates of MPS.
Thus, in line with recommendations from Paddon‐ Jones [b50], we advise that older adults should distribute their daily protein equally across three or more daily meals. For example, given our findings that the elderly require more protein to increase MPS above rest than the young, in a 75 kg individual consuming ∼60 g of protein daily (based on the RDA of 0.8 g·kg−1), this would mean consuming ∼20 g of protein with each meal, as opposed to a typical feeding regimen in which the elderly typically ingest smaller amounts of protein with breakfast (∼8 g) and lunch (∼12 g) and the majority of dietary protein with dinner (∼40 g) [b51].
Timing of protein ingestion with resistance exercise
Data in older populations concerning the optimal timing of protein intake to maximize resistance exercise‐induced muscle protein anabolism are scarce [b52–b54]. In young adults evidence to support pre‐exercise feeding for optimizing the anabolic response is equivocal at best [b55–b57]. Based on the heightened anabolic signalling response following resistance exercise the presence of a post‐exercise ‘window of opportunity’, in which to provision of amino acids will, theoretically, induce the greatest anabolic response, has been touted [b57,b58]. A single study [b52] showed that resistance training (over 12 weeks) followed by immediate protein ingestion resulted in greater gains in muscle cross‐section and isokinetic strength, compared with a delayed protein intake. However, many studies have found that protein ingestion in close proximity to resistance exercise has little additive effect on muscle anabolism in the young and elderly [5,54,55,59,60]. Indeed, it has recently been shown that dividing protein intake over the morning prior to and evening after training, is more effective for lean mass accretion than consuming a single bolus of protein immediately after exercise [b61]. Furthermore, provided adequate dietary protein is consumed, protein supplements taken in close proximity to exercise do not augment hypertrophy in the elderly [b62]. At the muscle protein synthetic level, recent evidence from our laboratory in healthy, young adults suggests that muscles remain sensitive to protein ingestion at 24 h after low or high load resistance exercise performed to failure [b63], as evidenced by an increase in MPS. Thus, protein feeding during late exercise recovery still maintains the capacity to be synergistic to exercise‐mediated rates that have been shown to exist in the fasted state during this time [b64,b65]. Taken together, these data give equivocal insight into the most appropriate time for older adults to consume protein in the context of resistance exercise to optimize muscle anabolism. It appears that protein ingestion at doses of at least 20 g at intervals over 24 h post‐exercise may be able to elicit a potent anabolic response in the elderly and promote muscle protein accretion for hypertrophy.
Contractile regulation of muscle anabolism
Manipulation of contractile factors including volume, intensity and time under tension can alter the acute muscle protein synthetic response to resistance exercise. It was recently demonstrated that low load high intensity lifting at 30% of their one repetition maximum (1RM) to failure can elicit similar rates of myofibrillar MPS as traditional, high load low intensity lifting at 90% of 1RM in trained young men [b66]. Moreover, the MPS response at 24 h post‐exercise was sustained only with the low load high intensity lifting. Importantly, preliminary evidence suggests low and high load lifting performed to failure produces equivalent hypertrophy when practiced over time [b67]. Combined with the knowledge that three sets of resistance exercise stimulate greater rates of MPS than one set [b68], these data suggest that post‐resistance exercise MPS is not entirely load dependent [b69,b70], but appears to be related to exercise volume (defined as repetitions x sets x load) and, thus, achieving maximal muscle fibre activation (particularly of type II fibres) [b71]. Further support for this notion was recently demonstrated by Burd et al. [b72] who manipulated the time under tension of muscle contraction during resistance exercise by comparing 6 vs. 1 s contractions. The authors showed that the slower contraction mode was accompanied by greater muscle activation than normal contraction and stimulated greater rates of myofibrillar and mitochondrial MPS. Collectively, these findings may be of relevance to older adults striving to increase lean mass through resistance exercise as they typically display difficulty lifting high load weights due to sarcopenic co‐morbidities, such as rheumatoid and osteoarthritis as well as a blunted acute muscle protein synthetic response to high load exercise intensities [b73]. Thus, in older muscles we suggest that a minimum contractile threshold must be surpassed, either by increasing exercise intensity and/or volume, in order to initiate an MPS response. Support for this notion was demonstrated very recently by Kumar et al. [b74], who showed that low load (40% 1RM) resistance exercise did not elevate MPS in the elderly when three sets of 14 repetitions (i.e. non‐fatiguing) were completed. However, when the volume was doubled to six sets of 14 repetitions, a robust increase in MPS was noted.
Conclusions and guidelines
In summary, older adults seeking non‐pharmacological interventions to assist in the maintenance or addition of muscle mass would be best advised to implement a combination of resistance‐type exercise combined with a source of amino acids/protein. Given the thesis that maximal muscle fibre recruitment, achieved by completing an exercise protocol of sufficiently high intensity or volume, is the centrally important mechanism for achieving hypertrophy, older adults performing resistance exercise may be best advised to perform low load lifting to near failure or, alternatively, with a greater number of sets (for example, six sets) or a slower lifting cadence (for example, 6 s) than a typical exercise regimen. First and foremost, this approach will almost certainly reduce the number of injuries/complaints and increase compliance in older adults, although it remains to be seen as to which regimen is most effective for maintaining or restoring muscle mass and strength in older adults. In addition, low load resistance exercise performed to achieve a high volume or intensity may confer other benefits not typically found when lifting heavier loads, such as improved aerobic capacity, as evidenced in the acute phase by an increase in the synthesis of mitochondrial proteins [b72]. Focusing on protein intake, to achieve sustained periods of NPB it appears the elderly may be required to distribute dietary protein equally with each meal throughout the day, as opposed to ingesting the majority of their dietary protein with dinner. To potentiate resistance exercise‐induced rates of MPS it is recommended that the elderly ingest a high quality, rapidly digested, leucine‐rich protein source (for example, whey, chicken or soy) in close proximity to resistance exercise. However, immediate post‐exercise protein intake may not be required to optimize the hypertrophic response to training in the elderly. Instead, consistent protein ingestion over the day after training may be all that is required to facilitate exercise‐induced hypertrophy in older adults.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and SMP had support from the Dairy Farmers of Canada and the US Dairy Research Institute as well as Nestec (Nestle) in the previous 3 years.
SMP is supported by research grants from Natural Sciences and Engineering Research Council of Canada, Canadian Institutes of Health Research and Nestec Ltd, Vevey, Switzerland. All authors read and approved the final manuscript.
References
- 1.Pennings B, Koopman R, Beelen M, Senden JM, van Saris WH, Loon LJ. Exercising before protein intake allows for greater use of dietary protein‐derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93:322–331. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 2.Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci. 2011;66:681–688. doi: 10.1093/gerona/glr036. [DOI] [PubMed] [Google Scholar]
- 3.Dillon EL, Casperson SL, Durham WJ, Randolph KM, Urban RJ, Volpi E, Ahmad M, Kinsky MP, Sheffield‐Moore M. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO‐induced hyperemia. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1408–1417. doi: 10.1152/ajpregu.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Symons TB, Sheffield‐Moore M, Mamerow MM, Wolfe RR, Paddon‐Jones D. The anabolic response to resistance exercise and a protein rich meal is not diminished by age 15. J Nutr Health Aging. 2011;15:376–381. doi: 10.1007/s12603-010-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrando AA, Lane HW, Stuart CA, Davis‐Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 7.Ferrando AA, Paddon‐Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clinical. Nutrition. 2010;29:18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Paddon‐Jones D, Sheffield‐Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 9.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 10.English KL, Paddon‐Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65‐80 year old men and women. PLoS ONE. 2008;3:e1875. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith GI, Villareal DT, Sinacore DR, Shah K, Mittendorfer B. Muscle protein synthesis response to exercise training in obese, older men and women. Med Sci Sports Exerc. 2012;44:1259–1266. doi: 10.1249/MSS.0b013e3182496a41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94:3044–3050. doi: 10.1210/jc.2008-2216. [DOI] [PubMed] [Google Scholar]
- 14.Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia‐hyperaminoacidemia in middle‐aged adults. J Appl Physiol. 2009;107:1308–1315. doi: 10.1152/japplphysiol.00348.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 16.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction‐induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3‐kinase independent activation of mTORC1. J Appl Physiol. 2011;110:561–568. doi: 10.1152/japplphysiol.00941.2010. [DOI] [PubMed] [Google Scholar]
- 18.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin like growth factor receptor is not necessary for load‐induced skeletal muscle hypertrophy. J Physiol. 2008;586:283–291. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise‐induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol. 2009;587:5239–5247. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 21.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- 22.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time‐dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 23.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 24.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose‐response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 26.Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- 27.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24‐h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003;284:E76–89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- 28.Phillips SM. Protein requirements and supplementation in strength sports. Nutrition. 2004;20:689–695. doi: 10.1016/j.nut.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268:E514–520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 30.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 31.Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- 32.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- 34.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dangin M, Boirie Y, Guillet C, Beaufrere B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132:3228S–3233. doi: 10.1093/jn/131.10.3228S. [DOI] [PubMed] [Google Scholar]
- 36.Dangin M, Boirie Y, Garcia‐Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. American journal of physiology Endocrinology and. Metabolism. 2001;280:E340–348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy‐protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 38.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat‐free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 39.Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, The GPJ. leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–1109. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- 40.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 41.Pennings B, Boirie Y, Senden JM, Gijsen AP, van Kuipers H, Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 42.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Breen L, Burd NA, Hector AJ, Churchward‐Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114511007422. [DOI] [PubMed] [Google Scholar]
- 44.Pennings B, de Groen B, Lange A, Gijsen AP, Zorenc AH, van Senden JM, Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol. Metab. 2012;302:E992–999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Churchward‐Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab. 2012;9:57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr. 2012;108:958–962. doi: 10.1017/S0007114511006271. [DOI] [PubMed] [Google Scholar]
- 47.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, van Dendale P, Loon LJ. Long‐term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 48.Churchward‐Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 50.Paddon‐Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tieland M, Borgonjen‐Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community‐dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr. 2012;51:173–179. doi: 10.1007/s00394-011-0203-6. [DOI] [PubMed] [Google Scholar]
- 52.Esmark B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onambele‐Pearson GL, Breen L, Stewart CE. Influences of carbohydrate plus amino acid supplementation on differing exercise intensity adaptations in older persons: skeletal muscle and endocrine responses. Age. 2010;32:125–138. doi: 10.1007/s11357-009-9129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports. 2011;21:e372–383. doi: 10.1111/j.1600-0838.2011.01318.x. [DOI] [PubMed] [Google Scholar]
- 55.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens‐Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid‐carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197–206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 56.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab. 2007;292:E71–76. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 57.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol. 2009;106:1730–1739. doi: 10.1152/japplphysiol.90395.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E‐BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid‐carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 60.Candow DG, Chilibeck PD, Facci M, Abeysekara S, Zello GA. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol. 2006;97:548–556. doi: 10.1007/s00421-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 61.Burk A, Timpmann S, Medijainen L, Vahi M, Oopik V. Time‐divided ingestion pattern of casein‐based protein supplement stimulates an increase in fat‐free body mass during resistance training in young untrained men. Nutr Res. 2009;29:405–413. doi: 10.1016/j.nutres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 63.Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Philips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- 64.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 65.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low‐load high volume resistance exercise stimulates muscle protein synthesis more than high‐load low volume resistance exercise in young men. PLoS ONE. 2010;5:e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell CJ, Churchward‐Venne TA, West DD, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training‐mediated hypertrophic gains in young men. J Appl Physiol. 2012;113:71–77. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010;588:3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holm L, van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E257–269. doi: 10.1152/ajpendo.00609.2009. [DOI] [PubMed] [Google Scholar]
- 70.Ratamess NA, Alvar BA, Evetoch TK, Housh TJ, Kibler WB, Kraemer WJ, Triplett NT. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 71.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex‐based differences. J Appl Physiol. 2009;106:1692–1701. doi: 10.1152/japplphysiol.91351.2008. [DOI] [PubMed] [Google Scholar]
- 72.Burd NA, Andrews RJ, West DW, Little JP, Cochran AJ, Hector AJ, Cashaback JG, Gibala MJ, Potvin JR, Baker SK, Phillips SM. Muscle time under tension during resistance exercise stimulates differential muscle protein sub‐fractional synthetic responses in men. J Physiol. 2012;590:351–362. doi: 10.1113/jphysiol.2011.221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009;106:2026–2039. doi: 10.1152/japplphysiol.91481.2008. [DOI] [PubMed] [Google Scholar]
- 74.Kumar V, Atherton PJ, Selby A, Rankin D, Williams J, Smith K, Hiscock N, Rennie MJ. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci. 2012;67:1170–1177. doi: 10.1093/gerona/gls141. [DOI] [PubMed] [Google Scholar]
- 75.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–1138. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]


