Abstract
Aims
Cardiopulmonary bypass (CPB) during cardiac surgery is well known to be associated with the development of a systemic inflammatory response. The efficacy of parecoxib in attenuating this systemic inflammatory response is still unknown.
Methods
Patients undergoing elective mitral valve replacement with CPB were assessed, enrolled and randomly allocated to receive parecoxib (80 mg) or placebo. Blood samples were collected in EDTA vials for measuring serum cytokine concentrations, troponin T, creatinekinase myocardial‐brain isoenzyme CK‐MB concentrations and white cell counts.
Results
Compared with the control group, IL‐6 and IL‐8‐values in the parecoxib group increased to a lesser extent, peaking at 2 h after the end of CPB (IL‐6 31.8 pg ml−1 ± 4.7 vs. 77.0 pg ml−1 ± 14.1, 95% CI −47.6, −42.8, P < 0.001; IL‐8 53.6 pg ml−1 ± 12.6 vs. 105.7 pg ml−1 ± 10.8, 95% CI −54.8, −49.4, P < 0.001). Peak concentrations of anti‐inflammatory cytokine IL‐10 occurred immediately after termination of CPB and were higher in the parecoxib group (115.7 pg ml−1 ± 10.5 vs. 88.4 pg ml−1 ± 12.3, 95% CI 24.7, 29.9, P < 0.001). Furthermore, the increase in neutrophil counts caused by CPB during cardiac surgery was inhibited by parecoxib. The increases in serum troponin T and CK‐MB concentrations were also significantly attenuated by parecoxib in the early post‐operative days. Peak serum concentrations of CK‐MB in both groups occurred at 24 h post‐CPB (17.4 μg l−1 ± 5.2 vs. 26.9 μg l−1 ± 6.9, 95% CI −10.9, −8.1, P < 0.001). Peak troponin T concentrations occurred at 6 h post‐bypass (2 μg l−1 ± 0.62 vs. 3.5 μg l−1 ± 0.78, 95% CI −1.7, −1.3, P < 0.001).
Conclusion
Intra‐operative parecoxib attenuated the systemic inflammatory response associated with CPB during cardiac surgery and lowered the biochemical markers of myocardial injury.
Keywords: cardiopulmonary bypass, COX2, parecoxib, systemic inflammatory response
What is Already Known about This Subject
COX‐2 is up‐regulated during systemic inflammatory response invoked by cardiopulmonary bypass (CPB) and cardiac surgery. This systemic inflammation is often associated with the development of multiple organ injury. However, during cardiac surgery under CPB, the strategy aiming at the inhibition of COX‐2 has not been well evaluated in clinical trials.
What This Study Adds
The anti‐inflammatory effects of intra‐operative parecoxib exhibited in elective mitral valve replacement surgery indicate that short term use of COX‐2 inhibitors intra‐operatively is an effective strategy to control systemic inflammation associated with CPB.
Introduction
Cardiopulmonary bypass (CPB) is well known to be associated with a systemic inflammatory response that presents itself as a long standing issue and an intractable problem [1]. CPB during cardiac surgery provokes a vigorous inflammatory response via a variety of mechanisms [2,3], mainly including contact of blood to the foreign surfaces of the CPB circuit, surgical trauma and endotoxaemia. Various technological and pharmacological strategies have been developed aimed at reducing the inflammatory response following cardiac surgery, such as implementation of novel cardiac surgical techniques [4], improvement in the biocompatibility of the extracorporeal circuit [5], maintenance of haemodynamic stability [6] and the use of pharmacologic agents including aprotinin [7], free radical scavengers and anti‐oxidants [8]. Significant morbidity associated with CPB is becoming rare, but most patients still experience different degrees of organ dysfunction as the result of the activation of the inflammatory response.
COX‐2 is up‐regulated during the inflammatory response to CPB [b9,b10], resulting in increased concentrations of thromboxanes [b11] and vasoconstrictor prostaglandins [b12]. Inhibiting the inducible COX‐2 exhibits considerable potential to result in desirable anti‐inflammatory effects.
The highly selective COX‐2 inhibitor parecoxib, which is rapidly transformed to its active metabolite valdecoxib in vivo, is widely used to treat osteoarthritis and rheumatoid arthritis [b11,b13], as well as used to afford post‐operative pain relief in various cases, such as disc herniation discectomy [b14] and total abdominal hysterectomy [b15]. Nevertheless, the clinical efficacy of parecoxib in attenuating CPB associated systemic inflammation has not yet been well evaluated.
Here, we report that parecoxib attenuates the systemic inflammatory response associated with CPB and protects against myocardial injury.
Methods
Patients selection and study design
The study protocol was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology. Informed consent was obtained from patients or from their nearest relatives. No commercial entities providing equipment or devices had a role in any aspect of this study. Rheumatic heart disease patients below 65‐years‐old who were due to undergo elective mitral valve replacement with CPB were enrolled. (Figure 1) Exclusion criteria were pre‐operative creatine kinase myocardial‐brain isoenzyme (CK‐MB) > 175 u l−1, ejection fraction (EF) < 40%, aspartate aminotransferase (AST) > 150 U l−1 and creatinine concentration > 132 mmol l−1, repeat cardiac surgery and combined operations, immunosuppressive medication or immunodeficiency syndromes, neurologic or psychiatric disorders, peptic ulcer disease, severe uncontrolled hypertension, bronchial asthma, known allergy to NSAIDS, clinical or angiographic evidence of coronary heart disease and a previous history of cerebrovascular accident. Patients with plasma concentrations of IL‐6 > 20 pg ml−1, CRP concentrations >16 mg l−1 on the morning of surgery or both were also excluded. In addition, patients who underwent surgery for more than 5 h were excluded.
Figure 1.
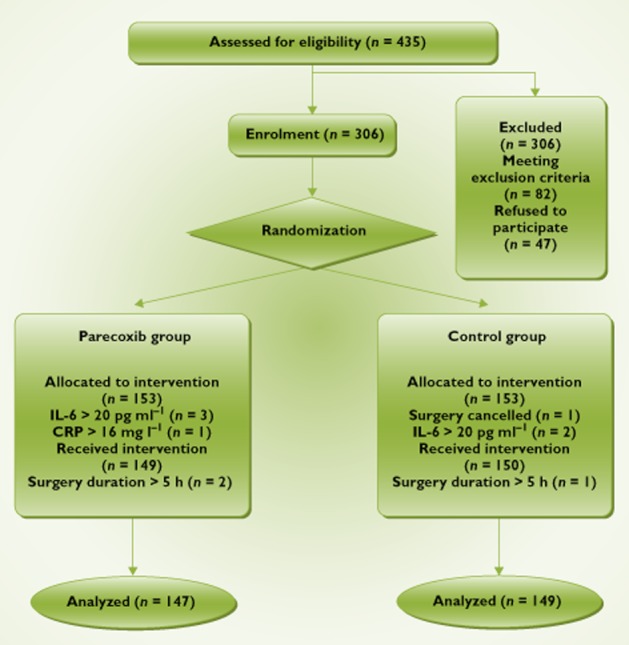
Flowchart of study design
Using a computer‐generated random code, 306 enrolled patients were randomly allocated to two groups by the nurses, and the procedure was blinded to researchers. In parecoxib (Pfizer) treatment group (n = 147), patients received 10 ml of 40 mg parecoxib i.v. at induction of anaesthesia, 5 ml of 20 mg parecoxib i.v. before CPB and 5 ml of 20 mg parecoxib i.v. at the end of surgery. The control group (n = 149) patients received 10 ml normal saline i.v. at induction of anaesthesia, 5 ml normal saline i.v. before CPB and 5 ml normal saline i.v. at end of surgery.
Anaesthesia and CPB
Similar anaesthesia and CPB management were carried out in all patients. Premedication consisted of standard cardiac drugs and i.m. injections of atropine 0.5 mg and phenobarbitone 0.1 g, administered 30 min prior to induction. Anaesthesia was induced with fentanyl 20–30 μg kg−1 and etomidate 200–300 μg kg−1. Muscular relaxation was achieved with vecuronium 0.1 mg kg−1 and mechanical ventilation was with 100% oxygen. Anaesthesia was maintained with i.v. administration of midazolam (0.1 mg kg−1 h−1), sufentanil (2.0–3.0 μg kg−1 h−1) and vecuronium (0.1 mg kg−1 h−1). Before induction of anaesthesia, a five‐lead ECG and pulse oximetry were routinely used as monitoring, and an arterial line was secured (radial artery) for continuous invasive blood pressure monitoring. After induction of anaesthesia an internal jugular venous catheter was inserted for central venous pressure measurement.
The extra corporeal circulation (ECC) was performed under moderate hypothermia (temperature between 30°C and 32°C) at continuous flow of 60–80 ml kg−1 min−1 using the STOCKERT SIII CPB machine fitted with membrane oxygenators. The priming solution for the circuit consisted of 500 ml Ringer's lactate, 800 ml gelatin and was supplemented with 2.5 ml kg−1 of a 15% mannitol solution, 0.3 ml kg−1 of magnesium and 10 mg furosemide. Cold cardioplegia, consisting of cold blood and crystalloid in the ratio of 4:1 was used for all patients. Prior to vascular cannulation, 350 IU kg−1 heparin was administered i.v. and activated clotting time (ACT) was determined after 3 min to achieve an ACT > 480 s. At the end of ECC, dexamethasone 10 mg and protamine sulphate in a ratio of 1.5:1 to heparin were administered. During weaning from the bypass, dopamine and dobutamine (5–20 μg kg−1 min−1) were given if systolic arterial pressure was below 90 mmHg. Epinephrine was supplemented with the inotropes if the arterial blood pressure was persistently low. If the haematocrit was less than 30% and the haemoglobin level was below 10 g dl−1, packed cells were transfused.
Similar operation techniques and cardioprotective strategies were used in all patients according to the surgeon's preference. After surgery, patients were transferred to the intensive care unit and weaned from ventilation when haemodynamically stable and re‐warmed. On transfer to the intensive‐care unit (ICU), an infusion of sufentanil was continued at 0.01–0.02 μg kg−1 h for sedation until recovery of spontaneous ventilation. Criteria for discharge from the ICU were: no mechanical ventilation and arterial oxygen pressure > 75 mmHg and oxygen saturation by pulse oximetry > = 95% or higher with FiO2 of 0.4 or less, normal arterial carbon dioxide pressure, stable haemodynamic state without or minimum inotropic support (< 5 μg kg−1 min−1), no newly occurred arrhythmia, normal chest X‐ray, no severe pain, no significant fever (rectal temperature < 38.8°C) and blood potassium > = 4.5 mEq l−1. Tramadol 1 mg kg−1 was given i.v. around 30 min to 1 h prior to extubation. After extubation, patients were allowed to use a morphine patient‐controlled analgesia (PCA) device for the next 24 h. The patients were usually discharged from the ICU after a 24 h stay if they met the above criteria. Otherwise, patients would stay in the ICU for another night. None of the physicians caring for the patients during and after the operation was involved in the study.
Blood sampling and processing
Blood samples were collected from an arterial line at different time points (T): before start of CPB (T1), at the end of CPB (T2), and at 2 h (T3), 12 h (T4), 24 h (T5) and 48 h(T6) thereafter. The blood samples were collected in two EDTA (ethylenediaminetetraacetic acid) vials and immediately sent to the clinical laboratory. One vial for each of time points T1 to T6 was sent for total white cell count determination in the haematology laboratory. Another blood sample at each of the time points T1 to T5 was centrifuged within 30 min at 1123 g min−1 for 5 min and plasma was stored at −80°C in labelled EP tubes until ELISA assay for serum cytokines.
Additional 2 ml arterial blood samples were obtained pre‐operatively and then 6, 24, 48 and 72 h post‐bypass for troponin T and CK‐MB measurements. All cardiac troponin‐T (cTnT) assays used heparinized plasma samples. CK‐MB assays used plasma samples treated with ethylenediaminetetraacetic acid. All samples were centrifuged within 20 min and the plasma was stored at −20°C until analysis. CK‐MB and cTnT concentrations were determined at the Analytical Unit of Union Hospital.
ELISA
Commercially available sandwich enzyme‐linked immunosorbent assay kits (eBioscience, San Diego, USA) were used to determine serum concentrations of cytokines (TNF‐α, IL‐1β, IL‐6, IL‐8, 1 L‐10) according to instruction. ELISA readings were done according to manufacturer's procedures and read with a Biotek ELx800 ELISA reader.
Post‐operative complications and peri‐operative variables
The frequencies of post‐operative complications (stroke, rhythm disturbance, peri‐operative myocardial infarction, atrial fibrillation, liver failure, renal failure, cerebrovascular accident and infection) were registered and compared between both groups. Neurological examination was performed daily by an intensivist. A new onset of focal neurological deficit lasting for more than 24 h was evaluated by a neurologist and confirmed by imaging. Serial ECGs were done every 6 h and concentrations of troponin T as per method described were determined. Myocardial infarction was defined in accordance to the recommendations of the 2007 Joint Task Force of the ESC/ACCF/AHA/WHF for the redefinition of myocardial infarction, as the appearance of new, pathological Q waves on the ECG or appearance of new left bundle branch block along with a five‐fold increase in the upper limit of laboratory normal (ULN) values of biochemical markers within the first 72 h after surgery [b16]. Liver dysfunction was graded using the Modified Child‐Pugh classification according to the degree of ascites, the plasma concentrations of bilirubin and albumin, the prothrombin time, and the degree of encephalopathy, if present. Renal dysfunction was defined as a 50% rise in preoperative levels of serum creatinine. The sternal wound was examined daily for signs and symptoms related to infection such as wound erythema and blanching, tenderness, pain, purulent discharge, fever (temperature > 38.0°C) and leucocytosis. In addition, we recorded peri‐operative variables including CPB time, aortic cross‐clamp time, total procedural time, post‐operative drainage fluid in first 24 h, blood transfusion, duration of ventilation, length stay in ICU, length stay in hospital and hospital death.
Statistical analysis
The sample sizes for the study were calculated with BiAS based on the IL‐6 concentration 2 h after the end of CPB as the primary outcome variable. On the basis of our previous pilot study, both groups obtained a SD of 5.2 pg ml−1, a difference of ≥2 pg ml−1 between treatment groups was anticipated to reveal a statistical significance. For a power of 0.9 and a two‐tailed α of 0.05, a sample size of 143 patients in each group was calculated to be appropriate.The sample size was based on the assumption of normal distribution and homogeneity of variances. After all data were collected, statistical analysis was performed using standard computer software (Statistical Analysis System, The SAS Institute, Cary, NC, USA). All categorical variables were tested by a chi‐square test. All normally distributed data (tested by Kolmogorov−Smirnov test) were analyzed using Student's t‐test. Repeated measures were analyzed using analysis of variance. P < 0.05 was considered statistically significant.
Results
Patient demographics and peri‐operative data
Four hundred and thirty‐five patients were assessed for study eligibility and 306 patients were enrolled (Figure 1). A female patient in the control group had her menses ahead of time just on the morning of operation, so the surgery was cancelled. Also on the morning of surgery, a patient in the parecoxib group with a serum CRP > 16 mg l−1 was excluded and another five patients (control group: n = 2; parecoxib group: n = 3) with serum IL‐6 > 20 pg l−1 were also excluded. Three patients (control group n = 1; parecoxib group n = 2) were excluded at the end of surgery due to the duration of the operation. There were no statistically significant differences between the two groups in their pre‐operative demographic and clinical profiles (Table 1). Moreover, there were no significant differences in their peri‐operative data (Table 2).
Table 1.
Patients demographic and clinical profiles
| Variables | Control group (n = 149) | Parecoxib group (n = 147) |
|---|---|---|
| Gender ratio (male/female) | 73/76 | 71/76 |
| Age (years) | 43.2 ± 14.3 | 44.7 ± 13.8 |
| Height (cm) | 158.6 ± 7.1 | 159.1 ± 7.4 |
| Weight (kg) | 57.9 ± 2.4 | 57.6 ± 2.3 |
| BMI (kg m−2) | 23.2 ± 3.9 | 23.3 ± 3.9 |
| EF (%) | 62.2 ± 6.9 | 61.2 ± 7.4 |
| Cardiac function (NYHA) | ||
| II (n) | 91 | 92 |
| III (n) | 58 | 55 |
| Diabetes (n) | 4 | 5 |
| Hypertension (n) | 10 | 7 |
| Smoker (n) | 18 | 20 |
| Prosthesis (n) | ||
| Bioprosthesis | 5 | 4 |
| Mechanical prosthesis | 144 | 143 |
| Pre‐operative platelet counts (103 μl−1) | 230.7 ± 68.5 | 228.0 ± 59.3 |
| Pre‐operative total leucocyte counts (103 μl−1) | 6.3 ± 1.0 | 6.3 ± 0.8 |
Data are presented as mean ± SD and as absolute numbers, respectively. BMI, body mass index; EF, ejection fraction; NYHA, New York Heart Association.
Table 2.
Peri‐operative variables
| Variables | Control group (n = 149) | Parecoxib group (n = 150) |
|---|---|---|
| CPB time (min) | 79.8 ± 3.9 | 81.9 ± 6.5 |
| Aortic cross‐clamp time (min) | 50.3 ± 3.1 | 54.5 ± 4.4 |
| Total procedural time (min) | 166.1 ± 5.3 | 172.6 ± 6.9 |
| Post‐operative drainage fluid in first 24 h (ml) | 452.2 ± 103.6 | 443.7 ± 115.1 |
| Blood transfusion (units) | 3.2 ± 1 | 2.9 ± 1.2 |
| Duration of ventilation (h) | 18.1 ± 7.3 | 20.5 ± 8.9 |
| Length stay in ICU (days) | 2.0 ± 0.7 | 2.2 ± 0.5 |
| Length stay in hospital (days) | 9.0 ± 2.7 | 9.2 ± 1.9 |
| Hospital deaths | 0 | 0 |
Data are presented as mean ± SD. CPB, cardiopulmonary bypass; ICU, intensive care unit.
Cytokine concentrations
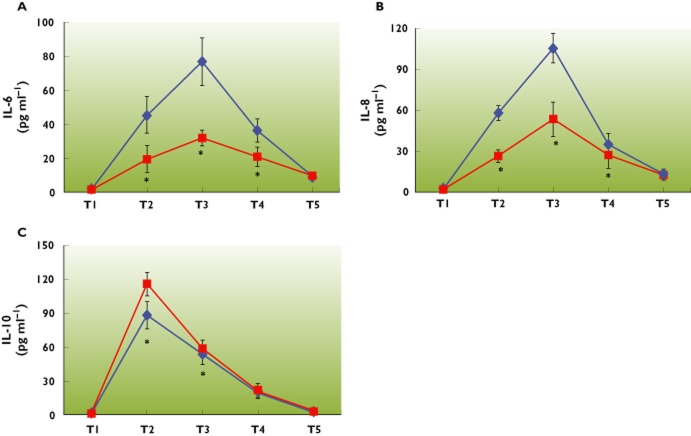
After CPB and cardiac surgery, all measured cytokines TNF‐α, IL‐1β, IL‐6, IL‐8 and IL‐10 plasma concentrations increased from baseline both in the control group and the parecoxib group. Interestingly, the increase of pro‐inflammatory cytokines IL‐6 and IL‐8 serum values was attenuated by parecoxib, and the serum concentrations of IL‐6 and IL‐8 in both groups peaked at 2 h after the end of CPB. (Figure 2A,B). On the contrary, the patients in the parecoxib group showed significantly higher plasma concentrations of the anti‐inflammatory cytokine IL‐10 at the end of CPB (Figure 2C).
Figure 2.
A, B, C) Effect of parecoxib on serum concentrations of pro‐inflammatory and anti‐inflammatory cytokines in patients undergoing mitral valve replacement surgery with CPB. Serum concentrations of pro‐inflammatory cytokines IL‐6 (A) and IL‐8 (B) increased after CPB in both groups and peaked at 2 h after CPB, evaluated by ELISA, showing that the peak concentrations were diminished by parecoxib. Serum concentrations of the anti‐inflammatory cytokine IL‐10 (C) were elevated and peaked earlier at the end of CPB, and the elevation was enhanced by parecoxib. Data are shown by means ± SD, *P < 0.05 vs. control group. Timepoints: T1, before start of CPB; T2, at the end of CPB; T3, 2 h after the CPB; T4, 12 h after the CPB; T5, 24 h after the CPB.  : control group;
: control group;  : parecoxib group
: parecoxib group
In addition, we could not find statistical differences in TNF‐α and IL‐1β concentrations between the two groups at all the time points (TNF‐α, P value, 0.39; IL‐1β, P value, 0.43), and the concentrations in both groups were low. The reason underlying this discrepancy may lie in the fact that both TNF‐α and IL‐1β are early mediators during the inflammatory response. Moreover, TNF‐α and IL‐1β have short half‐lives and we could not detect the peak concentrations.
Leucocyte counts
There was an increase in white blood cell counts during and after the CPB with a maximum at 24 h after the end of CPB in both the control and parecoxib groups (Table 3). When comparing the two groups, a significantly higher leucocytosis was seen in the control group (T2∼T5), and parecoxib reduced the peak leucocyte counts 24 h after CPB (control group, 21.2 ± 3.8 × 109 l−1; parecoxib group, 18.6 ± 2.9 × 109 l−1; P < 0.001). The elevation in white blood cells was dominated by an increase in circulating neutrophils, which showed a significant difference between the two groups, being higher in the control group at 24 h after CPB (control group, 16.3 ± 2.2 × 109 l−1; parecoxib group, 14.6 ± 3.0 × 109 l−1; P < 0.001). An unexpected finding was that neutrophil numerical counts in the parecoxib group were even higher at 48 h post‐CPB than in the control group, though there was no statistical difference (control group10.8 ± 2.8 × 109 l−1; parecoxib group11.0 ± 2.4 × 109 l−1; P > 0.5).
Table 3.
Leucocyte counts at different time points
| Time | Total leucocyte count | Neutrophil count | Monocytes count | |||
|---|---|---|---|---|---|---|
| (109 l−1) | (109 l−1) | (109 l−1) | ||||
| Control group | Parecoxib group | Control group | Parecoxib group | Control group | Parecoxib group | |
| T1 | 6.3 ± 1.0 | 6.3 ± 0.8 | 2.6 ± 0.6 | 2.5 ± 0.5 | 0.3 ± 0.12 | 0.4 ± 0.11 |
| T2 | 12.9 ± 1.9 | 8.9 ± 2.1* | 9.5 ± 1.6 | 7.0 ± 1.7* | 0.7 ± 0.11 | 0.6 ± 0.11 |
| T3 | 14.8 ± 2.3 | 11.9 ± 1.4* | 11.6 ± 2.3 | 9.1 ± 1.5* | 0.8 ± 0.13 | 0.9 ± 0.20 |
| T4 | 16.7 ± 2.4 | 14.2 ± 1.9* | 13.9 ± 3.0 | 11.8 ± 2.9* | 1.0 ± 0.15 | 1.2 ± 0.21 |
| T5 | 21.2 ± 3.8 | 18.6 ± 2.9* | 16.3 ± 2.2 | 14.6 ± 3.0* | 1.2 ± 0.16 | 1.3 ± 0.31 |
| T6 | 14.0 ± 3.7 | 14.9 ± 3.2 | 10.8 ± 2.8 | 11.0 ± 2.4 | 1.4 ± 0.25 | 1.3 ± 0.28 |
Data are shown by means ± SD, *P < 0.05 vs. control group. Timepoints: T1, before start of CPB; T2, at the end of CPB; T3, 2 h after the CPB; T4,12 h after the CPB; T5, 24 h after the CPB; T6, 48 h after the CPB.
Platelet counts
The mean pre‐operative platelet counts (103 μl−1 ± SD) were comparable in both the control (230.7 ± 68.5) and the parecoxib groups (228.0 ± 59.3). The decrease in platelet counts 2 h after bypass was not significant between the two groups. (210.3 ± 73.0 in the parecoxib group vs. 217.1 ± 45.9 in the control group).
Myocardial injury
Following cardiac surgery with CPB, CK‐MB values significantly increased in both groups from baseline and reached peak concentrations 24 h after CPB. When comparing the two groups, the CK‐MB peak concentrations were significantly reduced by parecoxib (17.4 μg l−1 ± 5.2 vs. 26.9 μg l−1 ± 6.9, 95% CI −10.9, −8.1, P < 0.001) (Figure 3). Pre‐operative troponin T concentrations in all patients were below the detectable level of the assay (<0.1 μg l−1). After bypass, there was significant release of troponin T in both groups with troponin T concentrations remaining above baseline over the 72 h period, thus indicating myocardial damage. Peak troponin T concentrations occurred at 6 h post‐bypass in both groups with a significantly lower release of the marker in the parecoxib group (2 μg l−1 ± 0.62 vs. 3.5 μg l−1 ± 0.78, 95% CI −1.7, −1.3, P < 0.001) (Figure 4).
Figure 3.
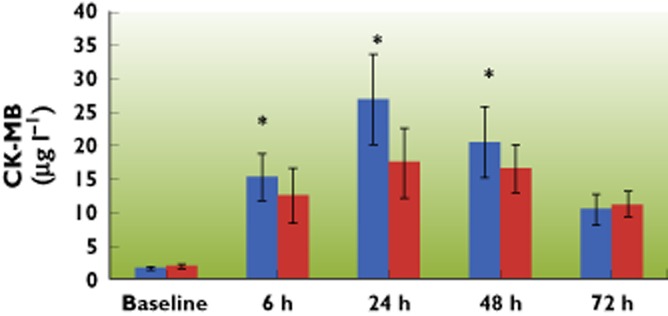
Serial changes following CPB in cardiac CK‐MB concentrations in arterial blood of patients over a 72 h period. CK‐MB increased following CPB in both groups, but the increase in the parecoxib group was lower. Peak serum concentrations of CK‐MB in both groups occurred at 24 h post‐CPB. Data are shown as means ± SD, *P < 0.05 vs. control group.  : control group;
: control group;  : parecoxib group
: parecoxib group
Figure 4.
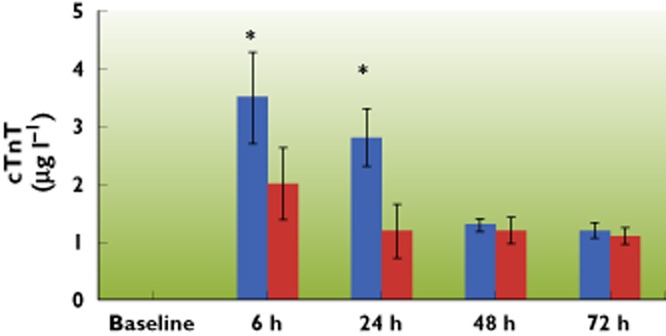
Serial changes following CPB in cTnT concentrations in arterial blood of patients over a 72 h period. Pre‐operative concentrations of cTnT were too low to detect in both groups, cTnT was elevated significantly following CPB and peaked at 6 h after CPB. The elevation of cTnT was also attenuated by parecoxib. Data are shown as means ± SD, *P < 0.05 vs. control group.  : control group;
: control group;  : parecoxib group
: parecoxib group
Post‐operative complications
Although inflammatory cytokine release and the neutrophil activation stimulated by CPB was inhibited by parecoxib, the frequency of post‐operative complications showed no significant differences between two groups (Table 4).
Table 4.
Post‐operative complications
| Complications | Control group | Parecoxib group |
|---|---|---|
| (n = 149) | (n = 147) | |
| Stroke | 2 | 0 |
| Arrhythmia | 2 | 4 |
| Peri‐operative MI | 0 | 0 |
| Atrial fibrillation | 3 | 4 |
| Liver failure | 0 | 0 |
| Renal failure | 1 | 0 |
| Cerebrovascular accidents | 0 | 0 |
| Infection | 0 | 0 |
Data are presented as the absolute number. MI, myocardial infarction.
Discussion
Although it has been demonstrated that COX‐2 expression is up‐regulated in many tissues after CPB and is involved in the pathogenesis of adverse events after cardiac surgery [b9,b17], the strategy aimed at targeting the inhibition of COX‐2 has not been adequately evaluated in clinical trials. Previous literature has documented that COX inhibitors, decreased the duration of post‐operative fever, chest pain, myalgias and malaise after CPB [b18]. In the present study, we have demonstrated that intra‐operative parecoxib could blunt the inflammatory cytokine response and inhibit the rise in neutrophil counts that follows CPB in patients undergoing elective mitral valve replacement. Intra‐operative parecoxib could also afford protection against myocardial injury, reflected by a decrease in the biochemical markers of cardiac injury, cTnT and CK‐MB. However, this was not translated in clinical outcomes probably due to the low rate of adverse cardiac complications that were observed in our study.
Prostanoids are important mediators in physiological responses, such as inflammation and thrombosis, synthesized by two isoforms of cyclo‐oxygenases. Inhibition of prostanoids synthesis has contributed to the anti‐inflammatory effects of cyclo‐oxygenase inhibitors. Hinson et al. demonstrated in a rat model of peritoneal inflammation that COX‐2 up‐regulation was involved in the production of PGE2 which in turn induced IL‐6 production [b19]. Some NSAIDS also possess certain cyclo‐oxygenase‐independent mechanisms by which they can influence inflammation [b20]. At the core of this effect lie the transcriptional factors NF‐κB and AP‐1 which regulate the expression of pro‐inflammatory mediators, cytokines, chemokines and cell adhesion molecules.
A plethora of pro‐inflammatory and anti‐inflammatory cytokines are released during the acute inflammatory response that follows CPB. It has been emphasized that the balance between pro‐inflammatory and anti‐inflammatory cytokines is essential for the clinical prognosis after CPB [b21]. Pro‐inflammatory cytokines, such as IL‐6, IL‐8, TNF‐α, IL‐1β, are strongly associated with post = operative complications [b22]. Serum IL‐6 concentrations correlate with post‐operative morbidity in paediatric patients undergoing open heart surgery [b23]. IL‐8 is a powerful neutrophil chemo‐attractant [b24] and a marker of tissue damage. The role of IL‐10 as an anti‐inflammatory cytokine has to be emphasized. It suppresses the production of pro‐inflammatory cytokines and inhibits neutrophil‐endothelial interaction [b25,b26]. In addition to its anti‐inflammatory properties, IL‐10 also exhibits an immunosuppressive function [b27]. The protection that parecoxib can provide against myocardial injury, that arises following operation trauma and CPB, might correlate with the regulation of the balance of cytokines.
Paradoxically, COX‐2 also possesses anti‐inflammatory properties at some phase during inflammation [b28] and is essential in the biosynthesis of omega‐3 polyunsaturated fatty acids (PUFA) derived mediators [b29]. These mediators, in turn, activate inflammation resolution programmes. Thus, if the activity of COX‐2 is inappropriately suppressed, the resolution of inflammation will be disrupted. This can be followed by chronic or perpetuated inflammation [b30,b31], and even resolution of chronic inflammation requires COX‐2 activity [b32]. Moreover, resolution of acute inflammation is an active, not passive, and programmed process [b33]. COX‐2 can be regarded as a ‘double‐edged sword’ that forms an integral part of the body defence mechanisms in response to insults. Study of resolution indices of inflammation may be important to elucidate this problem [b34].
Many studies have questioned the safety of the selective COX‐2 inhibitors or the COXIBS as they are commonly referred to. The first concerns were raised following the VIGOR (Vioxx GI Outcomes Research) study, by Bombardier et al., the results of which found an unacceptably higher risk of acute myocardial infarction in the rofecoxib group when compared with the naproxen group [b35]. Since then, others have demonstrated that the use of other COX‐2 inhibitors was also associated with a higher incidence of adverse events. Ott et al. conducted a study on 462 patients undergoing a coronary artery bypass graft (CABG) procedure who were allocated to receive either placebo or parecoxib/valdecoxib. In spite of noteworthy analgesic effects, use of parecoxib/valdecoxib was found to be associated with significant risk of sternal wound infections. They also reported a higher proportion of renal dysfunction, cerebrovascular and myocardial infarction in the parecoxib/valdecoxib treated group [b36]. The balance between the prothrombotic, COX‐1 derived thromboxane and the COX‐2 induced production of prostacyclin has been reported as the underlying cause of the increased adverse thrombotic effects of the COXIBS. Selective inhibition of the COX‐2 isoform leads to reduced prostacyclin production and the resulting unopposed action of thromboxane causes endothelial dysfunction and higher risk of arterial thrombosis [b37]. In spite of compelling evidence on the detrimental effects of the COXIBS that have been observed in some studies, the vast majority of this evidence is related to their long term usage, and very few studies have focussed on their short term use when started intra‐operatively or even post‐operatively [b38]. One study by Khalil et al. addressed the above and found that patients undergoing coronary artery bypass grafting had better ventilatory function after being administered a single dose of 40 mg parecoxib intravenously when compared with the placebo group, which showed a positive significance the same as in our study [b39].
Even if the selective COX‐2 inhibitors are associated with an increased risk of thrombosis, the presence of other endothelium‐derived substances that modulate the process, for example, nitric oxide, carbon monoxide and CD39 should lower this risk [b40]. Therefore, the presence of concomitant diseases that favour thrombotic events would put patients under selective COX‐2 inhibitors at higher risk. The imbalance hypothesis has been investigated in a recent study by Borgdorff et al. [b41]. These investigators showed that in the presence of arterial stenosis and high shear stress in a rat model, parecoxib pretreatment increased platelet aggregation as a result of selective inhibition of COX‐2. Valve replacement surgery is a commonly performed procedure and our subjects did not have any clinical or angiographic evidence of coronary artery disease. Inhibition of COX‐2 in a canine model subjected to orthotopic heart transplantation lead to an improvement in haemodynamics when compared with control [b42]. Therefore suppression of COX‐2 activity can limit the ischaemia‐reperfusion injury of ischaemic heart.
There are many limitations in our study. Since the use of parecoxib after CABG was associated with higher incidence of cardiovascular events [b32], we enrolled only those rheumatic heart disease patients undergoing elective mitral valve replacement. The results and benefits may not be applicable to other target groups. Similar studies can be carried out in other specific target groups with no underlying ischaemic heart disease to assess the reproducibility of the organ protection of the selective COX‐2 inhibitor parecoxib. In addition, the side‐effects and cardiovascular risks in patients undergoing elective mitral valve replacement need more clinical controlled trials to determine the safety of our intervention. Another limitation was that our study was a single centre research study, so more organizations should be taken into consideration to increase conviction.
Actually, a single therapeutic strategy will unlikely be sufficient to affectively abrogate CPB‐associated inflammation. More importantly, our intervention should aim at a controlled inflammatory response which would result in better outcomes. Therefore a combination of multiple pharmacological and mechanical therapeutic strategies aimed at controlling rather than at eliminating the systemic inflammatory response following CPB, may eventually prevent associated morbidity and improve clinical outcome.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (81070060 and 30930089). The authors thank Professor Yu Songlin (Department of Health Statistics, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) for his invaluable assistance in the statistical section.
Competing Interests
There are no competing interests to declare.
Authors' contributions
G Purusram, HQ Wang, RX Yuan and WL Xie performed the experiments and analyzed the data. P Gui and NG Dong provided useful advice and reviewed the manuscript. Q.P. Wu and G. Purusram conceived the study, participated in its design and coordination. H.Q. Wang wrote the manuscript. Q.P. Wu and S.L. Yao oversaw the experimental design and edited the manuscript. All authors of this paper have read and approved the final version of the manuscript.
References
- 1.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 2.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anaesthesiologist. Anaesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 4.Diegeler A, Doll N, Rauch T, Haberer D, Walther T, Falk V, Gummert J, Autschbach R, Mohr FW. Humoral immune response during coronary artery bypass grafting: a comparison of limited approach, ‘Off‐Pump’ technique, and conventional cardiopulmonary bypass. Circulation. 2000;102:III95–100. doi: 10.1161/01.cir.102.suppl_3.iii-95. [DOI] [PubMed] [Google Scholar]
- 5.Ranucci M, Mazzucco A, Pessotto R, Grillone G, Casati V, Porreca L, Maugeri R, Meli M, Magagna P, Cirri S, Giomarelli P, de Lorusso R, Jong A. Heparin‐coated circuits for high‐risk patients: a multicenter, prospective, randomized trial. Ann Thorac Surg. 1999;67:994–1000. doi: 10.1016/s0003-4975(99)00062-4. [DOI] [PubMed] [Google Scholar]
- 6.Bennett‐Guerrero E, Panah MH, Bodian CA, Methikalam BJ, Alfarone JR, DePerio M, Mythen MG. Automated detection of gastric luminal partial pressure of carbon dioxide during cardiovascular surgery using the Tonocap. Anaesthesiology. 2000;92:38–45. doi: 10.1097/00000542-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Soeparwata R, Hartman AR, Frerichmann U, Stefano GB, Scheld HH, Bilfinger TV. Aprotinin diminishes inflammatory processes. Int J Cardiol. 1996;26:S55–63. doi: 10.1016/0167-5273(96)02573-9. [DOI] [PubMed] [Google Scholar]
- 8.Dingchao H, Zhiduan Q, Liye H, Xiaodong F. The protective effects of high‐dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac Cardiovasc Surg. 1994;42:276–278. doi: 10.1055/s-2007-1016504. [DOI] [PubMed] [Google Scholar]
- 9.Hindman BJ, Moore SA, Cutkomp J, Smith T, Ross‐Barta SE, Dexter F, Brian JE., Jr Brain expression of inducible cyclooxygenase 2 messenger RNA in rats undergoing cardiopulmonary bypass. Anesthesiology. 2001;95:1380–1388. doi: 10.1097/00000542-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Erez E, Erman A, Snir E, Raanani E, Abramov D, Sulkes J, Boner G, Vidne BA. Thromboxane production in human lung during cardiopulmonary bypass: beneficial effect of aspirin? Ann Thorac Surg. 1998;65:101–106. doi: 10.1016/s0003-4975(97)01040-0. [DOI] [PubMed] [Google Scholar]
- 11.Jean YH, Wen ZH, Chang YC, Hsieh SP, Tang CC, Wang YH, Wong CS. Intra‐articular injection of the cyclooxygenase‐2 inhibitor parecoxib attenuates osteoarthritis progression in anterior cruciate ligament‐transected knee in rats: role of excitatory amino acids. Osteoarthritis Cartilage. 2007;15:638–645. doi: 10.1016/j.joca.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Shafique T, Johnson RG, Dai HB, Weintraub RM, Sellke FW. Altered pulmonary microvascular reactivity after total cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106:479–486. [PubMed] [Google Scholar]
- 13.Laine L, White WB, Rostom A, Hochberg M. COX‐2 selective inhibitors in the treatment of osteoarthritis. Semin Arthritis Rheum. 2008;38:165–187. doi: 10.1016/j.semarthrit.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Riest G, Peters J, Weiss M, Dreyer S, Klassen PD, Stegen B, Bello A, Eikermann M. Preventive effects of perioperative parecoxib on post‐discectomy pain. Br J Anaesth. 2008;100:256–262. doi: 10.1093/bja/aem345. [DOI] [PubMed] [Google Scholar]
- 15.Ng A, Smith G, Davidson AC. Analgesic effects of parecoxib following total abdominal hysterectomy. Br J Anaesth. 2003;90:746–749. doi: 10.1093/bja/aeg139. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF. Task force for the redefinition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 17.Metais C, Li J, Simons M, Sellke FW. Serotonin‐induced coronary contraction increases after blood cardioplegia‐reperfusion: role of COX‐2 expression. Circulation. 1999;100:II328–334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 18.Livelli FD, Johnson RA, McEnany MT, Sherman E, Newell J, Block PC, DeSanctis RW. Unexplained in‐hospital fever following cardiac surgery: natural history, relationship to postpericardiotomy syndrome, and a prospective study of therapy with indomethacin versus placebo. Circulation. 1978;57:968–975. doi: 10.1161/01.cir.57.5.968. [DOI] [PubMed] [Google Scholar]
- 19.Hinson RM, Williams JA, Shacter E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase‐2. Proc Natl Acad Sci USA. 1996;93:4885–4890. doi: 10.1073/pnas.93.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase‐independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 21.McBride WT, Armstrong MA, Crockard AD, McMurray TJ, Rea JM. Cytokine balance and immunosuppressive changes at cardiac surgery: contrasting response between patients and isolated CPB circuits. B J Anaesth. 1995;75:724–733. doi: 10.1093/bja/75.6.724. [DOI] [PubMed] [Google Scholar]
- 22.Wan S, DeSmet JM, Barvais L, Goldstein M, Vincent JL, LeClerc JL. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:806–811. doi: 10.1016/S0022-5223(96)70068-5. [DOI] [PubMed] [Google Scholar]
- 23.Hauser GJ, Ben‐Ari J, Colvin MP, Dalton HJ, Hertzog JH, Bearb M, Hopkins RA, Walker SM. Interleukin‐6 levels in serum and lung lavage fluid of children undergoing open heart surgery correlate with postoperative morbidity. Intensive Care Med. 1998;24:481–486. doi: 10.1007/s001340050600. [DOI] [PubMed] [Google Scholar]
- 24.Baggiolini M, Clark‐Lewis I. Interleukin‐8, a chemotactic and inflammatory cytokine. FEBS. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 25.Hayward R, Nossuli TO, Scalia R, Lefer AM. Cardioprotective effect of interleukin‐10 in murine myocardial ischemia‐reperfusion. Eur J Pharmacol. 1997;334:157–163. doi: 10.1016/s0014-2999(97)01149-7. [DOI] [PubMed] [Google Scholar]
- 26.Journois D, Israel‐Biet D, Pouard P, Rolland B, Silvester W, Vouhe P, Safran D. High volume, zero balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology. 1996;85:965–976. doi: 10.1097/00000542-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 27.O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL‐10 or its antagonists in human disease. Immunol Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 28.Gilroy DW, Colville‐Nash PR, Willis D, Chivers J, Paul‐Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti‐inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 29.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid‐derived mediators with anti‐inflammatory actions generated fromomega‐3 fatty acids via cyclooxygenase 2‐nonsteroidal anti‐inflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I(2) and E(2) mediate the protective effects of cyclooxygenase‐2 in a mouse model of immune‐mediated liver injury. Hepatology. 2007;45:159–169. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
- 31.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 32.Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 34.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 35.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos‐Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 36.Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, Hubbard RC, Hsu PH, Saidman LJ, Mangano DT. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1481–1492. doi: 10.1016/s0022-5223(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 37.Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, Gertz BJ. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation. 2001;104:2280–2288. doi: 10.1161/hc4401.100078. [DOI] [PubMed] [Google Scholar]
- 38.Ganne S, Rao U, Gelb AW. To use or not to use: the dilemma of NSAIDs and craniotomy. Eur J Anaesthesiol. 2009;26:625–626. doi: 10.1097/EJA.0b013e32832a21ad. [DOI] [PubMed] [Google Scholar]
- 39.Khalil MW, Chaterjee A, Macbryde G, Sarkar PK, Marks RRD. Single dose parecoxib significantly improves ventilatory function in early extubation coronary artery bypass surgery: a prospective randomized double blind placebo controlled trial. Br J Anaesth. 2006;96:171–178. doi: 10.1093/bja/aei298. [DOI] [PubMed] [Google Scholar]
- 40.Marcus AJ, Broekman MJ, Pinsky DJ. COX inhibitors and thromboregulation. New Engl J Med. 2002;347:1025–1026. doi: 10.1056/NEJMcibr021805. [DOI] [PubMed] [Google Scholar]
- 41.Borgdorff P, Tangelder GJ, Paulus WJ. Cyclooxygenase‐2 inhibitors enhance shear stress‐induced platelet aggregation. J Am Coll Cardiol. 2006;48:817–823. doi: 10.1016/j.jacc.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 42.Oshima K, Takeyoshi I, Tsutsumi H, Mohara J, Ohki S, Koike N, Nameki T, Matsumoto K, Morishita Y. Inhibition of cyclooxygenase‐2 improves cardiac function following long‐term preservation. J Surg Res. 2006;135:380–384. doi: 10.1016/j.jss.2006.03.044. [DOI] [PubMed] [Google Scholar]