Abstract
Aim
To assess the pharmacokinetics, pharmacodynamics, safety and tolerability of the 5‐lipoxygenase‐activating protein inhibitor, GSK2190915, after oral dosing in two independent phase I studies, one in Western European and one in Japanese subjects, utilizing different formulations.
Method
Western European subjects received single (50–1000 mg) or multiple (10–450 mg) oral doses of GSK2190915 or placebo in a dose‐escalating manner. Japanese subjects received three of four GSK2190915 doses (10–200 mg) plus placebo once in a four period crossover design. Blood samples were collected for GSK2190915 concentrations and blood and urine were collected to measure leukotriene B4 and leukotriene E4, respectively, as pharmacodynamic markers of drug activity.
Results
There was no clear difference in adverse events between placebo and active drug‐treated subjects in either study. Maximum plasma concentrations of GSK2190915 and area under the curve increased in a dose‐related manner and mean half‐life values ranged from 16–34 h. Dose‐dependent inhibition of blood leukotriene B4 production was observed and near complete inhibition of urinary leukotriene E4 excretion was shown at all doses except the lowest dose. The EC50 values for inhibition of LTB4 were 85 nm and 89 nm in the Western European and Japanese studies, respectively.
Conclusion
GSK2190915 is well‐tolerated with pharmacokinetics and pharmacodynamics in Western European and Japanese subjects that support once daily dosing for 24 h inhibition of leukotrienes. Doses of ≥50 mg show near complete inhibition of urinary leukotriene E4 at 24 h post‐dose, whereas doses of ≥150 mg are required for 24 h inhibition of blood LTB4.
Keywords: asthma, FLAP, GSK2190915, leukotrienes, pharmacodynamics, pharmacokinetics
What is Already Known about This Subject
Leukotrienes are potent inflammatory molecules that have been shown to be important mediators in asthma and cardiovascular diseases.
There are no potent leukotriene synthesis inhibitors on the market, but cysteinyl leukotriene 1 receptor antagonists have shown benefit in asthma and allergic diseases.
5‐ lipoxygenase‐activating protein (FLAP) inhibitors reduce both cysteinyl leukotrienes and the pro‐inflammatory neutrophil activator leukotriene B4 (LTB4) and therefore should have therapeutic benefit in any disease in which leukotrienes play a pathological role.
What This Study Adds
These studies show that the novel FLAP inhibitor, GSK2190915, is well‐tolerated with no clear difference in adverse events between placebo and active drug‐treated subjects.
The pharmacokinetic and pharmacodynamic profile of GSK2190915 supports once daily dosing for 24 h inhibition of both cysteinyl leukotriene and LTB4 production.
Based on the excellent safety, pharmacokinetic and pharmacodynamic profile of GSK2190915, this molecule was successfully evaluated in phase IIa allergen challenge and exercise challenge studies and is now being studied in multiple phase IIb clinical trials in asthma.
Introduction
5‐lipoxygenase‐activating protein (FLAP) in concert with 5‐lipoxygenase (5‐LO) is required in cells for the production of 5‐hydroxyeicosatetraenoic acid (5‐HETE), 5‐oxo‐eicosatetraenoic acid (5‐oxo‐ETE) and the leukotrienes (LTs) including leukotriene A4 (LTA4), the precursor of both leukotriene B4 (LTB4) and the cysteinyl LTs (cysLTs) namely leukotrienes C4, D4 and E4 (LTC4, LTD4 and LTE4) [1,2]. Leukotrienes have been implicated in asthma, chronic obstructive pulmonary disease (COPD), arthritis, cardiovascular disease and cancer, and the FLAP gene has been linked to the risk of both asthma and cardiovascular diseases in multiple ethnic cohorts [3–6]. Association analyses identified a significant association between increased risk of asthma and two SNPs within the FLAP gene (SG13S114 in intron 1 and SG13S41 in intron 4) [5]. In addition, the SG13S114 SNP showed association with aspects of lung function (bronchial hyper‐responsiveness and FEV1). These SNPs have been implicated as determinants of LTB4 production, suggesting that LTB4 is an important determinant in asthma pathogenesis. Therefore, molecules which inhibit LTB4 production might have added benefit in the treatment of asthma.
Three different FLAP inhibitors, including MK‐886, MK‐591 and BAY X1005, have shown efficacy in phase II clinical studies in asthmatics. However none of these molecules was developed further [7–9]. GSK2190915 is similar in potency to MK‐591 and 10‐times and 100‐times more potent than MK‐886 and BAY X1005, respectively, in inhibiting LTB4 production in human whole blood (Bain, unpublished data). Only one LT synthesis inhibitor, the 5‐LO inhibitor zileuton (trade name Zyflo®), is approved for the treatment of asthma. However, it requires large multiple daily doses, exhibits incomplete inhibition of lung CysLT production and has a less than optimal safety profile which necessitates liver function testing [b10]. Three selective antagonists of the LTD4‐activated CysLT1 receptor, namely montelukast, zafirlukast and pranlukast, have been approved for the treatment of asthma [b11,b12]. Recently, CysLT1R antagonists were shown to have equivalent clinical efficacy as inhaled corticosteroids as first‐line controller therapy and similar efficacy to long acting β‐adrenoceptor agonists as add on therapy to inhaled glucocorticoid in mild asthmatics [b13]. However, none of the selective CysLT1 antagonists directly block the production of the inflammatory mediators LTB4, 5‐HETE or 5‐oxo‐ETE or the action of the CysLTs at receptors other than CysLT1. Therefore, oral FLAP inhibitors that completely inhibit all 5‐lipoxygenase products may provide additional benefits in asthma and other diseases, such as cardiovascular disease, where these bioactive lipids have been strongly suggested to play a pathological role.
To address the untapped clinical potential of inhibiting leukotriene production, we developed several novel FLAP inhibitors including AM103 and GSK2190915 (formerly known as AM803) [14,15,16,17,18]. In preclinical pharmacological studies, both compounds were potent, selective FLAP inhibitors [b16,b17]. GSK2190915, 3‐[3‐tert‐butylsulfanyl‐1‐[4‐(6‐ethoxy‐pyridin‐3‐yl)‐benzyl]‐5‐(5‐methyl‐pyridin‐2‐ylmethoxy)‐1H‐indol‐2‐yl]‐2,2‐dimethyl‐propionic acid, potently inhibited production of LTB4 from ionophore‐challenged human blood and reduced rat bronchoalveolar lavage fluid (BALF) CysLTs and LTB4 concentrations [b17]. After oral dosing in rats, GSK2190915 was well absorbed (∼30–50% bioavailability) and showed a half‐life of ∼2–3 h. The major routes of metabolism were oxidation, dehydrogenation and acyl glucuronidation (unpublished data). Approximately 10% of the total absorbed drug was excreted in the bile and no drug was found in the urine [b17]. In in vivo pharmacodynamic assays, GSK2190915 demonstrated prolonged inhibition of ionophore‐challenged leukotriene production in blood and bronchoalveolar lavage fluid when compared with AM103 [b16,b17]. GSK2190915 inhibited calcium ionophore‐challenged LTB4 production in human blood with a 50% inhibitory concentration (IC50) of 76 nm but showed no inhibition of cyclo‐oxygenase‐1 or ‐2 activities in blood up to concentrations of 10 000 nm [b17]. Here we present the pharmacodynamic, pharmacokinetic and safety profile of GSK2190915 in healthy adult Western European and Japanese adults.
Methods
Study design
Two independent randomized, double‐blind, placebo controlled, dose escalating studies were conducted using GSK2190915, one in Western European subjects and one in Japanese subjects. The Western European study was conducted in healthy adult subjects (EURDACT2007‐00484872) and performed in two phases which consisted of a single dose phase and a multiple dose phase. The single dose phase was a parallel group design in which five cohorts of eight subjects each received single doses (50, 150, 300, 600 and 1000 mg) of GSK2190915 or placebo (six dosed with GSK2190915 and two with placebo in each cohort). A starting dose of 50 mg was chosen based on regulatory guidelines for predicted safety margins and because it was predicted to have minimal or no effect on leukotriene suppression at 24 h post‐dose. Dose escalation was stopped at 1000 mg since it was determined, based on leukotriene inhibition, to be several multiples above the likely clinical dose. The multiple dose phase consisted of four cohorts of eight subjects each who received repeat doses (10, 50, 150 and 450 mg) of GSK2190915 or placebo, once daily for 11 days (six dosed with GSK2190915 and two with placebo in each cohort). The starting dose for the multiple dose phase was lowered to 10 mg from that of the single dose phase in order to explore further a minimally active pharmacological dose. In total, 54 subjects from the single dose and multiple dose phases were dosed with GSK2190915 and a total of 18 subjects were dosed with placebo. The Japanese study was performed in 13 healthy subjects (NCT00955383) and utilized a four period crossover design. Single doses (10, 50, 150 and 200 mg) of GSK2190915 or placebo were given such that each subject was to receive three of the four active doses plus placebo once. The doses selected for this study were based on information gained from the Western European study with the intention of (i) exploring doses with submaximal and maximal pharmacological activity, (ii) obtaining safety and tolerability data for the maximum dose likely to be used (200 mg) in subsequent studies based on suppression of LTB4 and LTE4 in the repeat dose phase of the Western European study and (iii) including some overlapping doses such that a rough pharmacokinetic comparison between Western European and Japanese subjects could be performed. In both studies, GSK2190915 (10 mg ml−1 in vehicle) or matching placebo was dosed orally in solution after an overnight fast. The vehicle in the Western European study contained 1% (w/w) Lutrol L‐44 (Poloxamer 124) in a 10 mm sodium carbonate buffer, pH 9−10, sweetened with sucralose (5 mg 100 ml−1). In the Japanese study, Lutrol was substituted by 2% (w/w) ethanol due to the lack of availability of Lutrol in time to meet study timelines. Blood samples for pharmacokinetic and pharmacodynamic (LTB4) analysis and urine samples for pharmacodynamic analysis (LTE4) were taken at frequent intervals during the studies. Physical examinations, vital signs, electrocardiograms, and blood and urine tests were also performed for safety and adverse events were recorded.
Subjects
The Western European study was conducted in healthy adult subjects and approved by the Ethics Committee BEBO Foundation (Assen, the Netherlands). Seventy‐two subjects were randomized and completed the study, 54 were dosed with GSK2190915 and 18 with placebo. The Japanese study was approved by the Ethics Committee of the Medical Association North Rhine (Ethikkommission der Ärztekammer Nordrhein) (Düsseldorf, Germany). Thirteen subjects were randomized, two subjects were unable to participate in the final (fourth) period for personal reasons, with one replacement subject participating in period 4 only. Both studies were conducted under European Clinical Trial Directive and in adherence with ICH GCP (E6) and the principles of the Declaration of Helsinki. Written informed consent was obtained from each subject prior to study participation. To be eligible for the studies, subjects had to be healthy (as judged by screening physical examination, 12‐lead ECG, vital signs, blood and urine laboratory tests and no significant current or previous medical history), non‐smoking adults (aged 18 to 65 years for the Western European study and aged 20 to 65 years for the Japanese study) and not taking any prescription or over the counter medicines or herbal remedies (with the exception of the oral contraceptive pill or paracetamol for minor ailments). Females in the repeat dose and Japanese groups were of non‐childbearing potential. Subjects in the Japanese study were required to be born in Japan with four ethnic Japanese grandparents and able to speak Japanese. The demographics of the subjects from both studies are shown in Table 1.
Table 1.
Demographics Summary
| Study population | Western European | Japanese | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosing regimen | Single dose | Multiple dose | Single dose | |||||||||
| Dose (mg) | Placebo | 50 | 150 | 300 | 600 | 1000 | Placebo | 10 | 50 | 150 | 450 | 10, 50, 100, 200 and Placebo |
| n | 10 | 6 | 6 | 6 | 6 | 6 | 8 | 6 | 6 | 6 | 6 | 13 |
| Age (years) | ||||||||||||
| Mean | 45 | 61 | 50 | 40 | 53 | 54 | 48 | 51 | 61 | 63 | 60 | 31 |
| Range | 18–65 | 53–63 | 20–64 | 21–62 | 18–64 | 20–65 | 22–62 | 26–64 | 57–65 | 61–64 | 54–65 | 23–53 |
| Gender | 5M | 3M | 3M | 3M | 3M | 3M | 8M | 5M | 4M | 4M | 5M | 12M |
| 5F | 3F | 3F | 3F | 3F | 3F | 1F | 2F | 2F | 1F | 1F | ||
| Weight (kg) | ||||||||||||
| Mean | 73.1 | 78.6 | 73.9 | 74.5 | 82.1 | 72.3 | 79.9 | 76.6 | 73.6 | 75.0 | 77.0 | 64.4 |
| range | 52–96 | 63–89 | 57–92 | 58–87 | 74–95 | 54–92 | 64–100 | 61–86 | 65–88 | 52–89 | 73–82 | 52–87 |
| BMI (kg m−2) | ||||||||||||
| Mean | 24.1 | 26.9 | 24.4 | 24.6 | 28.9 | 24.2 | 25.2 | 25.8 | 26.5 | 25.0 | 25.9 | 21.6 |
| Range | 18–29 | 22–29 | 20–27 | 20–28 | 25–35 | 20–28 | 22–29 | 19–29 | 22–30 | 22–28 | 25–27 | 19–27 |
| Ethnicity | 1 Asian 9 Caucasian |
6 Caucasian | 6 Caucasian | 6 Caucasian | 1 Asian 5 Caucasian |
6 Caucasian | 1 Asian 1 Black 6 Caucasian |
6 Caucasian | 6 Caucasian | 6 Caucasian | 1 Asian 5 Caucasian |
13 Japanese |
BMI = body mass index; F = Female; M = male; N = number of subjects.
Measurements
Pharmacokinetics
Blood samples for measurement of GSK2190915 plasma concentrations were collected by venepuncture into heparinized tubes at pre‐dose and at various times post‐dosing (between 0.25 and 72 h post‐dose). In the Western European multiple‐dose study, blood was also collected at various times post‐dosing on day 3 (between 1 and 12 h post‐dose) and day 11 (between 0.25 and 72 h post‐dose) and at pre‐dose on all other days. The blood was centrifuged within 30 min of draw and the plasma stored at −80°C until analyzed by liquid chromatography‐tandem mass spectroscopy (LC‐MS/MS) using a validated method. Results were calculated using peak area ratios of analyte to internal standard. Calibration curves for GSK2190915 in human plasma ranged from 10 to 10 000 ng ml−1 and were generated using a weighted (1/x2) linear least‐squares regression. The lower limit of quantitation for GSK2190915 in human plasma was 10 ng ml−1. The precision and accuracy was determined from quality control samples and determined to be within acceptance criteria (%CV of ≤ 6% and bias of ≤ 15%). The structure of GSK2190915 has been published previously [b17].
Pharmacodynamics
Blood LTB4 analysis
Blood samples for determination of ex vivo LTB4 production were collected by venepuncture into heparinized tubes at pre‐dose and at various times post‐dosing on day 1 (between 0.5 and 72 h post‐dose). In the Western European multiple dose study, blood was collected pre‐dosing and at various times post‐dosing (between 0.5h and 72 h) on day 11. Analysis of ex vivo LTB4 production was performed as described previously [b14]. For each subject, their day 1 pre‐dose ionophore‐stimulated blood LTB4 concentration (ng ml−1) was set to 100% and this was defined as their baseline. An unstimulated blood LTB4 concentration was determined (usually <5% of stimulated LTB4) and this was set as 0%. At all time points subsequent to dosing, the concentration of LTB4 after ionophore challenge was normalized to the day 1 pre‐dose simulated (100%) and unstimulated (0%) value for that subject.
Urinary LTE4 analysis
Urinary LTE4 was measured in pre‐dose spot samples the day before dosing and on the morning of dosing, then as pooled 0–3, 3–6, 6–9 and 9–12 h samples and later as spot collections at 24, 48 and 72 h after dosing as described previously [b14]. The lower limit of detection was approximately 1 pg LTE4 mg−1 creatinine and samples below this were arbitrarily given this designation. Urinary LTE4 concentrations are expressed as % change from the individual's pre‐dose values. In the Western European subjects, the mean pre‐dose values were ∼38 and ∼65 pg LTE4 mg−1 creatinine for the single‐dose and multiple dose phases, respectively, and for the Japanese subjects the mean predose value was ∼65 pg LTE4 mg−1 creatinine.
Data analysis
Pharmcokinetics
Pharmacokinetic parameters were calculated using the non‐compartmental extravascular plasma input model in WinNonlin (Pharsight Mountain View, CA). The area under the curve (AUC) was calculated using the trapezoidal method. The AUC extrapolated to infinity (AUC(0,∞)) was calculated as the sum of AUC(0,t) and Ct/λz where Ct was the observed plasma concentration obtained from the log‐linear regression analysis of the last quantifiable time point and λz was the terminal phase rate constant. The apparent terminal half‐life (t1/2) was calculated as loge 2 divided by λz. The maximum observed concentration (Cmax) and the time of its occurrence (tmax) were obtained directly from the concentration−time data. The apparent clearance (CL/F) was calculated as GSK2190915 dose divided by AUC(0,∞). The apparent volume of distribution (Vz/F) was calculated as dose divided by AUC(0,∞) multiplied by λz. An exploratory dose proportionality statistical analysis was performed on log‐transformed Cmax and loge‐transformed AUC using the power model within SAS version 9.1. The extent of accumulation after repeat dosing was calculated as AUC(0,24 h) on day 11 divided by AUC(0,24 h) on day 1. Graphs of GSK2190915 concentration vs. time are expressed as mean data.
Pharmacodynamics
Graphs of % inhibition vs. time are expressed as median and interquartile range. The relationship between GSK2190915 plasma concentration and LTB4 inhibition was determined in subjects who had measurable GSK2190915 plasma concentrations in both the single‐ and multiple dose studies using non‐linear regression analysis applying a sigmoid Emax model in GraphPad Prism®. Graphs for pharmacokinetic and pharmacodynamic descriptions were generated using GraphPad Prism® or SAS or S‐Plus software.
Results
Study in Western European subjects
Pharmacokinetics of GSK2190915
The demographics of the subjects in the Western European study are shown in Table 1. Following oral dosing in Western European subjects, GSK2190915 reached maximum plasma concentrations at ∼2 h post‐dose at all doses following both single and multiple dose administration (Figure 1A–C). Thereafter, an initial rapid decrease in plasma concentrations was followed by a slower terminal elimination phase in the majority of subjects. The values for Cmax, AUC(0,24 h), AUC(0,∞), tmax), CL/F, Vz/F and t1/2 for the different doses of GSK2190915 in Western Europeans are listed in Table 2. After single dosing, the mean half‐life ranged from 17 to 27 h over the 10 mg to 1000 mg dose range. After multiple dosing of 10 mg to 450 mg, the mean half‐life ranged from 23 to 34 h suggesting a slight prolongation of half‐life after multiple dosing. The plasma profiles of GSK2190915 demonstrated dose‐dependent increases up to 1000 mg after single doses and an approximate two‐fold accumulation after multiple days of dosing relative to day 1 (Figure 1A–C). The AUC/dose appears lower for the 300 mg single dose relative to the other cohorts, and this correlated with a higher mean clearance (15.5 l h−1 for the 300 mg single dose group as compared with 7–12.7 l h−1 for the other cohorts). The CL/F and Vz/F appeared independent of dose. We noted considerable inter‐individual variability in Cmax and AUC in both the single and multiple dose phases, with maximum concentrations up to 9‐fold different between subjects within one dose level (Table 2). Results of exploratory dose proportionality analysis indicated there were no obvious deviations from dose proptionality for Cmax, AUC(0,24 h) or AUC(0,∞).
Figure 1.
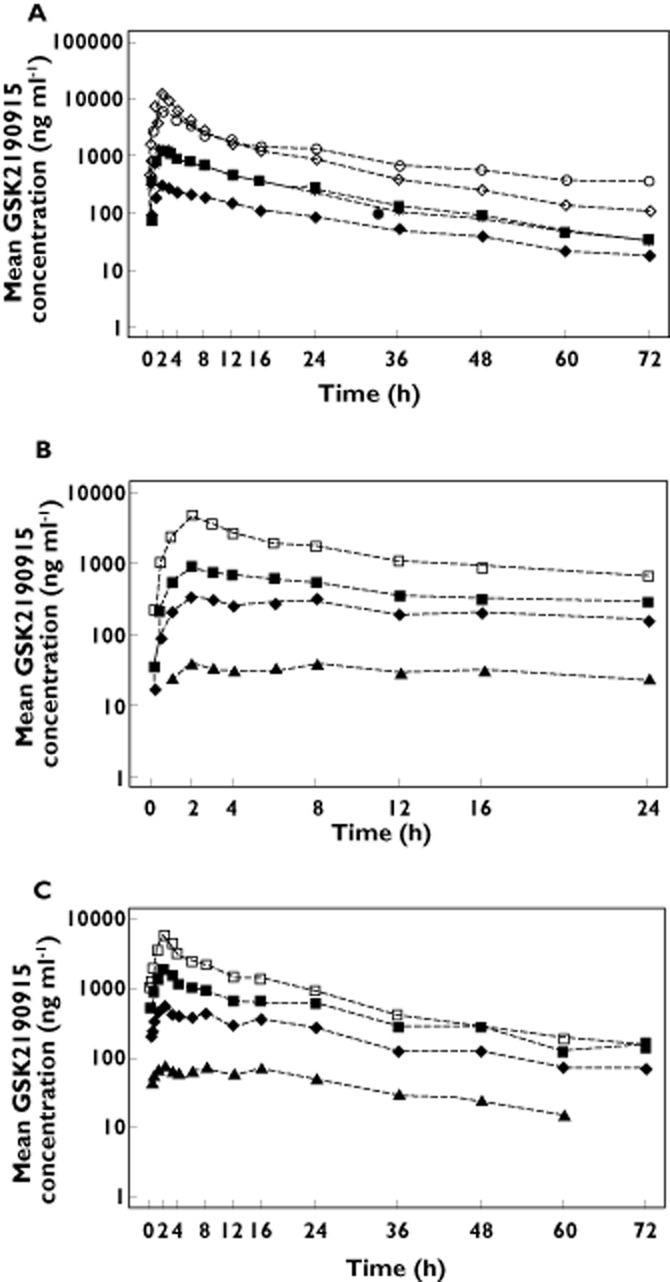
Pharmacokineticsof GSK2190915. Mean plasma concentration vs. time profiles for GSK2190915 in healthy Western European subjects on (A) day 1 after single dose administration of 50 mg, ( ), 150 mg (
), 150 mg ( ), 300 mg (
), 300 mg ( ), 600 mg (
), 600 mg ( ) or 1000 (
) or 1000 ( ) mg. Mean plasma concentration vs. time profiles for GSK2190915 in healthy Western European subjects on (B) day 1 or (C) day 11 following multiple dose administration of 10 mg (
) mg. Mean plasma concentration vs. time profiles for GSK2190915 in healthy Western European subjects on (B) day 1 or (C) day 11 following multiple dose administration of 10 mg ( ), 50 mg, (
), 50 mg, ( ), 150 mg (
), 150 mg ( ) or 450 mg (
) or 450 mg ( ).
).
Table 2.
Western European GSK2190915 PK summary. Geometric mean (%CV) of AUC(0,24 h), AUC(0,24 h)/D, AUC(0,∞), AUC(0,∞)/D, Cmax, Cmax/D, CL/F, Vz/F and t1/2 and median tmax (minimum – maximum)
| Dose | AUC(0,24 h) (ng ml−1 h) | AUC(0,24 h)/D (ng*hr/mL/mg) | AUC(0,∞) (ng ml−1 h) | AUC(0,∞)/D (ng*hr/mL/mg) | Cmax (ng ml−1) | Cmax/D (ng*hr/mL/mg) | tmax (h) | t1/2 (h) | CL/F (l h−1) | Vz/F (l) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single dose (n = 6 per dose group) | |||||||||||
| 50 mg | 3416 (41) | 68.3 | 5651 | 113 | 286 (52) | 5.7 | 2.00 (2.00–3.00) | 20.6 (21) | 8.85 (48) | 272 (76) | |
| 150 mg | 12514 (30) | 83.4 | 17980 | 120 | 1244 (28) | 8.3 | 2.00 (1.97‐3.02) | 17.3 (21) | 8.34 (30) | 217 (67) | |
| 300 mg | 14361 (29) | 47.9 | 19356 | 64 | 1638 (66) | 5.5 | 2.00 (2.00–6.00) | 17.6 (43) | 15.5 (25) | 418 (142) | |
| 600 mg | 47051 (88) | 78.4 | 78029 | 130 | 5668 (95) | 9.4 | 2.01 (2.00–3.02) | 26.9 (60) | 7.69 (129) | 348 (225) | |
| 1000 mg | 63815 (45) | 63.8 | 78628 | 78 | 10665 (46) | 10.7 | 2.00 (2.00–2.00) | 17.4 (21) | 12.7 (49) | 334 (104) | |
| Multiple dose (n = 6 per dose group) | |||||||||||
| 10 mg | Day 1 | 687 (16) | 69 | N/C | N/C | 41 (21) | 4.1 | 2.00 (2.00–8.07) | – | – | |
| Day 11 | 1421 (43) | 142 | N/C | N/C | 77 (37) | 7.7 | 12.00 (2.00–16.00) | 29.5 (39) | 7.04 (43) | 315 (119) | |
| 50 mg | Day 1 | 4936 (38) | 99 | N/C | N/C | 366 (36) | 7.3 | 2.51 (2.00–8.00) | – | – | |
| Day 11 | 8325 (27) | 167 | N/C | N/C | 568 (9) | 11.4 | 2.03 (2.00–8.00) | 33.8 (28) | 6.01 (27) | 293 (23) | |
| 150 mg | Day 1 | 9540 (48) | 64 | N/C | N/C | 821 (53) | 5.5 | 2.00 (2.00–4.00) | – | – | |
| Day 11 | 18883 (49) | 126 | N/C | N/C | 1790 (39) | 11.9 | 2.01 (2.00–2.15) | 26.5 (25) | 7.94 (49) | 313 (71) | |
| 450 mg | Day 1 | 25490 (158) | 57 | N/C | N/C | 2758 (269) | 6.1 | 2.00 (2.00–2.00) | – | – | |
| Day 11 | 44102 (53) | 98 | N/C | N/C | 5009 (75) | 11.1 | 2.00 (2.00–2.00) | 23.2 (16) | 10.2 (53) | 395 (261) | |
AUC = Area under the curve; Cmax = maximum concentration; CL/F = apparent clearance; D = dose; N/C = not calculated; t1/2 = half‐life; tmax = time to maximum plasma concentration; Vz/F = apparent volume of distribution.
Pharmacodynamics of GSK2190915: inhibition of LTB4 synthesis in ex vivo calcium ionophore‐challenged blood
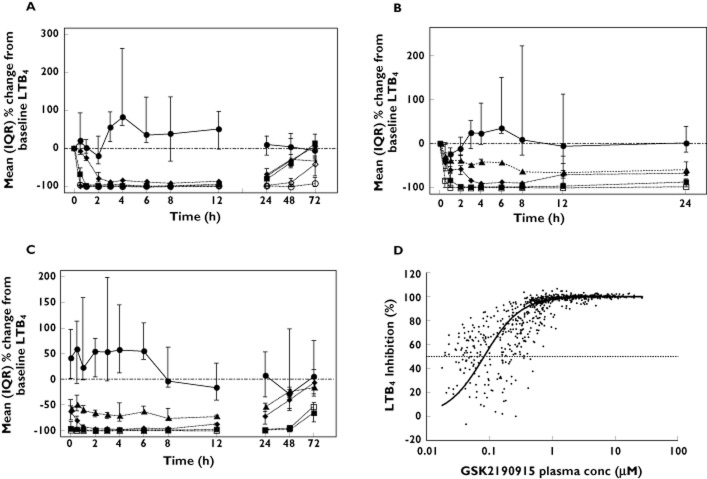
After a single dose of 50 mg, GSK2190915 showed a maximum 80% inhibition of LTB4 production at ∼3 h post‐dose and this level of inhibition was maintained through 12 h post‐dose (Figure 2A). Even at 24 h post‐dose, 50 mg GSK2190915 showed 60% inhibition of LTB4 production. After single doses of >150 mg, GSK2190915 showed a more rapid onset of LTB4 inhibition which occurred at ∼1 h post‐dose (Figure 2A). Single doses of 150 or 300 mg GSK2190915 resulted in 90–100% inhibition from 1–12 h post‐dose and maintained 75% inhibition of LTB4 production at 24 h (Figure 2A). Single doses of ≥ 300 mg showed ≥ 90% inhibition of LTB4 production through 48 h post‐dose.
Figure 2.
LTB4 synthesis in ex vivo calcium ionophore‐challenged blood from Western European subjects. Median (plus interquartile range) percentage changes from baseline LTB4 in (A) the single dose study following placebo ( ) or 50 mg (
) or 50 mg ( ), 150 mg (
), 150 mg ( ), 300 mg (
), 300 mg ( ), 600 mg (
), 600 mg ( ) or 1000 mg (
) or 1000 mg ( ) GSK2190915. Median (plus interquartile range) percentage changes from baseline LTB4 on (B) day 1 or (C) day 11 following multiple dose administration of placebo (
) GSK2190915. Median (plus interquartile range) percentage changes from baseline LTB4 on (B) day 1 or (C) day 11 following multiple dose administration of placebo ( ) or 10 mg (
) or 10 mg ( ), 50 mg, (
), 50 mg, ( ), 150 mg (
), 150 mg ( ) or 450 mg (
) or 450 mg ( ) GSK2190915. (D) Percent inhibition of LTB4 synthesis ex vivo in blood vs. plasma concentration. Included are the data from all dosed subjects in the single dose and multiple dose studies with measurable concentrations of GSK2190915. The EC50 and EC90 values of 85 nm and 360 nm, respectively, were generated from a non‐linear regression analysis of the data.
) GSK2190915. (D) Percent inhibition of LTB4 synthesis ex vivo in blood vs. plasma concentration. Included are the data from all dosed subjects in the single dose and multiple dose studies with measurable concentrations of GSK2190915. The EC50 and EC90 values of 85 nm and 360 nm, respectively, were generated from a non‐linear regression analysis of the data.
Multiple doses of GSK2190915 that ranged from 10 mg to 450 mg were administered to explore doses with lower pharmacodynamic activity. Following dosing with 10 mg, there was a maximum 60% inhibition of LTB4 production that occurred at 8 h post‐dose on day 1 and which increased to 75% inhibition at 8 h post‐dose on day 11 (Figure 2B, C). Even at 24 h post‐dose, 10 mg GSK2190915 showed approximately 50% inhibition of LTB4 production on day 1 and day 11. 50 mg GSK2190915 showed a maximum 90% inhibition of LTB4 production that occurred at 4 h post‐dose on day 1 and which increased to 100% inhibition at 4 h post‐dose on day 11 (Figure 2B, C). By 24 h post‐dose, 50 mg GSK2190915 maintained 65% and 75% inhibition of LTB4 production on day 1 and day 11, respectively. Doses of 150 and 450 mg showed complete inhibition of LTB4 by 2 h post‐dose and this was maintained for 48 h post‐dose on day 11 (Figure 2C). By 72 h post the last dose, 150 and 450 mg GSK2190915 still showed 60–65% inhibition of LTB4 production (Figure 2C). Non‐linear regression analysis of GSK2190915 plasma concentrations plotted against LTB4 inhibition, using all data from the single dose and multiple dose phases, generated EC50 and EC90 values for inhibition of LTB4 production of ∼85 nm and ∼360 nm, respectively (Figure 2D).
Pharmacodynamics of GSK2190915: inhibition of urinary LTE4 production
A decrease in urinary LTE4 excretion represents inhibition of leukotriene synthesis within the lung, which is important to demonstrate for a compound being developed for the treatment of asthma. In Western European subjects, single oral doses of GSK2190915 showed early dose‐dependent decreases in urinary LTE4 (Figure 3A). However, by 12 h post‐dose, all single doses of GSK2190915 (50 mg to 1000 mg) showed near complete inhibition of urinary LTE4 excretion which continued through 36 h post‐dose (Figure 3A). On day 1 of the multiple‐dose phase, 10 mg GSK2190915 showed a maximal 40% inhibition of urinary LTE4 excretion at 16 h post‐dose which increased to ∼80% inhibition at 12 h post‐dose following 11 days dosing (Figure 3B, C). Following 11 days dosing with ≥ 50 mg GSK2190915, approximately 90% inhibition of urinary LTE4 was seen and this inhibition persisted for 48 h post‐dose (Figure 3C).
Figure 3.
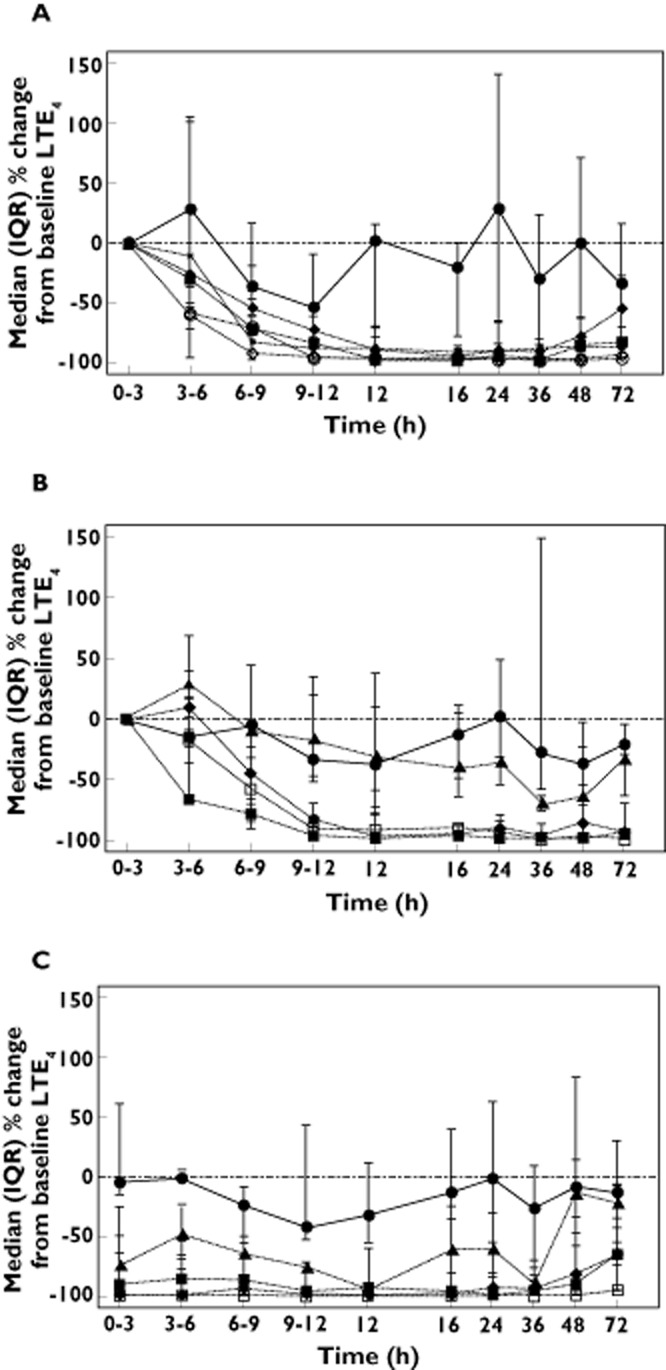
UrinaryLTE4 excretion in Western European subjects. Median (plus interquartile range) of LTE4 excretion in urine as a percentage of the pre‐dose value in (A) the single dose study following placebo ( ) or 50 mg (
) or 50 mg ( ), 150 mg (
), 150 mg ( ), 300 mg (
), 300 mg ( ), 600 mg (
), 600 mg ( ) or 1000 mg (
) or 1000 mg ( ) GSK2190915. Median (plus interquartile range) of LTE4 excretion in urine as a percentage of the pre‐dose value on (B) day 1 or (C) day 11 following multiple dose administration of placebo (
) GSK2190915. Median (plus interquartile range) of LTE4 excretion in urine as a percentage of the pre‐dose value on (B) day 1 or (C) day 11 following multiple dose administration of placebo ( ) or 10 mg (
) or 10 mg ( ), 50 mg, (
), 50 mg, ( ), 150 mg (
), 150 mg ( ) or 450 mg (
) or 450 mg ( ) GSK2190915.
) GSK2190915.
Study in Japanese subjects
Pharmacokinetics of GSK2190915
Following single oral dosing in Japanese subjects, maximum GSK2190915 plasma concentrations were reached at 4–6 h post‐dose (Figure 4A, Table 3). The values for Cmax, AUC(0,24 h), AUC(0,∞), tmax, CL/F, Vz/F and t1/2 for the different doses of GSK2190915 in the Japanese subjects are listed in Table 3. Plasma concentrations were slightly higher in Japanese subjects as compared with Western European subjects, on average ∼1.2 and ∼1.5 times higher based on geometric mean Cmax and AUC values, respectively. The t1/2 for the Japanese subjects was similar to that observed for the Western European subjects and averaged 16 to 23 h. There was no deviation from dose proportionality for AUC(0,∞). However a statistically significant departure from dose proportionality was observed for Cmax, with increasing dose of GSK2190915 yielding slightly larger than dose proportional increases in Cmax. The CL/F was independent of dose.
Figure 4.
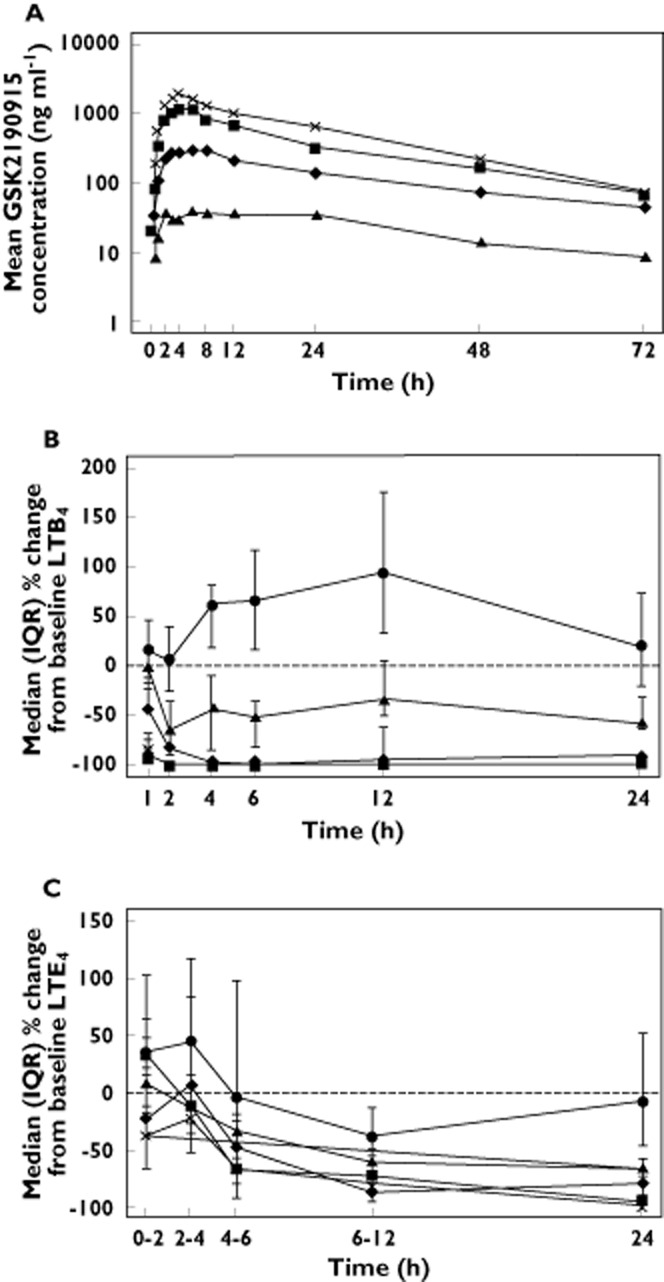
Pharmacokineticsand pharmacodynamics of GSK2190915 in healthy Japanese subjects. (a) Mean plasma concentration vs. time profiles for GSK2190915 following single dose administration of 10 mg ( ), 50 mg (
), 50 mg ( ), 150 mg (
), 150 mg ( ) or 200 mg (
) or 200 mg ( ) GSK2190915. (B) Median (plus interquartile range) percentage changes from baseline plasma LTB4 following single dose administration of placebo (
) GSK2190915. (B) Median (plus interquartile range) percentage changes from baseline plasma LTB4 following single dose administration of placebo ( ) or 10 mg (
) or 10 mg ( ), 50 mg (
), 50 mg ( ), 150 mg (
), 150 mg ( ) or 200 mg (
) or 200 mg ( ) GSK2190915. (C) Median (plus interquartile range) of LTE4 excretion in urine as a percentage of the pre‐dose value following single dose administration of placebo (
) GSK2190915. (C) Median (plus interquartile range) of LTE4 excretion in urine as a percentage of the pre‐dose value following single dose administration of placebo ( ) or 10 mg (
) or 10 mg ( ), 50 mg (
), 50 mg ( ), 150 mg (
), 150 mg ( ) or 200 mg (
) or 200 mg ( ) GSK2190915.
) GSK2190915.
Table 3.
Japanese GSK2190915 PK summary. Geometric mean (%CV) of AUC(0,24 h), AUC(0,∞), AUC(0,∞)/D, Cmax, Cmax/D, CL/F, Vz/F and t1/2 and median tmax (minimum – maximum)
| Dose | AUC(0,24 h) (ng ml−1 h) | AUC(0,∞) (ng ml−1 h) | AUC(0,∞)/D (ng ml−1 h) | Cmax (ng ml−1) | Cmax/D (ng ml−1) | tmax (h) | t1/2 (h) | CL/F (l h−1) | Vz/F (l) |
|---|---|---|---|---|---|---|---|---|---|
| Single (n = 9, except for 200 mg dose group where n = 8) | |||||||||
| 10 mg | 825 (16) | 1752 (31) | 175 | 45 (21) | 4.5 | 6.00 (2.00–24.00) | 19.7 (17) | 5.71 (31) | 162 (27) |
| 50 mg | 5155 (45) | 10256 (64) | 205 | 372 (49) | 7.4 | 6.00 (2.00–12.00) | 23.0 (23) | 4.88 (65) | 162 (42) |
| 150 mg | 16956 (49) | 31973 (38) | 213 | 1462 (53) | 9.7 | 4.00 (2.00–6.00) | 18.8 (24) | 4.69 (38) | 127 (44) |
| 200 mg | 26271 (39) | 41021 (39) | 205 | 2128 (54) | 10.6 | 4.00 (2.00–6.00) | 16.0 (19) | 4.88 (39) | 113 (35) |
AUC = Area under the curve; Cmax = maximum concentration; CL/F = apparent clearance; D = Dose;; t1/2 = half‐life; tmax = time to maximum plasma concentration; Vz/F = apparent volume of distribution.
Pharmacodynamics of GSK2190915. Inhibition of LTB4 synthesis in ex vivo calcium ionophore‐challenged blood and urinary LTE4 excretion
In healthy Japanese subjects, GSK2190915 showed a rapid onset and dose‐dependent inhibition of ex vivo calcium ionophore‐stimulated blood LTB4 (Figure 4B). Following a single dose of 10 mg, approximately 50–60% inhibition of LTB4 production was observed from 2–24 h post‐dose. Doses of 50 to 200 mg GSK2190915 resulted in 90–100% inhibition from 1–12 h post‐dose and maintained at least 85% inhibition of LTB4 production at 24 h post‐dose (Figure 4B). Similar to that observed in the Western European subjects, non‐linear mixed effects analysis of LTB4 inhibition vs. plasma drug concentrations in the Japanese subjects generated an EC50 value of ∼89 nm for inhibition of LTB4 production (data not shown).
In Japanese subjects, GSK2190915 showed dose‐dependent decreases in urinary LTE4 (Figure 4C). Following a single dose of 10 mg, there was a 30–50% decrease in urinary LTE4 that persisted for 24 h (Figure 4C). Following single doses of 50 mg and higher, there was a ≥ 80% decrease from baseline in urinary LTE4 at 12 h post‐dose which persisted for at least 24 h post‐dose (Figure 4C).
Clinical safety
No clinically significant changes in laboratory tests, physical examination, 12‐lead electrocardiograms or vital signs were observed in either study. There were no serious adverse events (AEs) and there were no adverse events that led to study withdrawal. Overall, there were 80 adverse events reported by 39 subjects in the two phases of the Western European study. All adverse events were mild in severity with 25 of the adverse events being judged possibly related to the study medication (Table 4). The remaining 55 adverse events were judged not related or unlikely to be related to the study medication by the investigator. The most frequent adverse event was diarrhoea (eight GSK2190915 and three placebo) with six (one placebo and five GSK2190915) reported in the 150 mg single dose cohort. Other adverse events which were reported at least three times during the study were headache (five GSK2190915 and four placebo), nasopharyngitis (four GSK2190915, one placebo) venepuncture pain/bruising (four GSK2190915 and one placebo), flatulence (four GSK2190915, none placebo), dyspepsia (four GSK2190915, none placebo), influenza like illness (three GSK2190915, one placebo), nausea (three GSK2190915, none placebo) and abdominal pain (one GSK2190915, two placebo). There was no dose response in adverse events (either in number of events or number of subjects reporting events) nor was there any clear difference between the number of adverse events in placebo vs. active drug‐treated subjects (Table 4). As a result, none of the TEAEs was considered relevant. In the Japanese study there were three adverse events reported (diarrhoea, nasopharyngitis and venepuncture hematoma) all following the 50 mg dose. All adverse events were mild with only the report of diarrhoea being considered related to GSK2190915 by the investigator.
Table 4.
Summary of related/possibly related AEs in the Western European study
| Treatment | Number of subjects treated (N) | TEAE intensity | Number of times event occurred (e) | Number of subjects experiencing TEAE (n) | % |
|---|---|---|---|---|---|
| Placebo single dose | 10 | Mild | 2 | 2 | 20 |
| 50 mg single dose | 6 | Mild | 1 | 1 | 17 |
| 150 mg single dose | 6 | Mild | 10 | 5 | 83 |
| 300 mg single dose | 6 | N/A | |||
| 600 mg single dose | 6 | Mild | 3 | 2 | 33 |
| 1000 mg single dose | 6 | Mild | 1 | 1 | 17 |
| Placebo multiple dose | 8 | Mild | 1 | 1 | 13 |
| 10 mg multiple dose | 6 | N/A | |||
| 50 mg multiple dose | 6 | Mild | 3 | 1 | 17 |
| 150 mg multiple dose | 6 | Mild | 3 | 3 | 50 |
| 450 mg multiple dose | 6 | Mild | 1 | 1 | 17 |
N/A = not applicable; % = percentage of subjects who experienced the TEAE per treatment = (n/N) × 100.
Discussion
Inflammation plays an important role in a variety of respiratory, cardiovascular and oncological diseases and the pro‐inflammatory leukotrienes have been shown to have profound pathological effects in animal models of asthma, atherosclerosis and other inflammatory diseases [b19]. Clinical trials have shown that several FLAP inhibitors are efficacious and safe in subjects with asthma, yet there are no approved drugs that fully inhibit the production of LTs [10,11,20,21,22]. Given the evidence for the involvement of both LTB4 and the cysLTs in asthma and cardiovascular disease, a FLAP inhibitor may have the potential to be more efficacious than CysLT1 receptor antagonists in the treatment of asthma and other inflammatory diseases in which LTB4 or other CysLT receptors are strongly implicated.
In these two phase I studies, GSK2190915 demonstrated a good pharmacokinetic profile. In healthy Western European subjects, GSK2190915 showed rapid increases in plasma concentrations after drug administration, with maximum mean plasma concentrations being reached at ∼2 h post‐dose. Thereafter, an initial rapid decrease in plasma GSK2190915 concentrations was followed by a slower terminal elimination phase in the majority of subjects. There appeared to be a prolongation of the t1/2 after multiple dosing, with mean t1/2 values ranging from 23 to 34 h. An approximate 2‐fold accumulation after multiple days dosing was demonstrated in Western European subjects. The AUC/dose appears lower for the 300 mg single dose cohort relative to the other cohorts and the Cmax/dose appears to increase with increasing dose. However when exploratory statistical analysis of dose‐proportionality on logarithmically transformed Cmax and AUC values was performed there was no obvious deviation in dose proportionality. Instead, these differences may be the result high inter‐subject variability combined with small numbers of subjects. Although the reason for the inter‐subject variability is unclear, we note that we have also observed significant variability in the pharmacokinetic studies in rats and in the phase II studies after oral dosing with a tablet.
The t1/2 for Japanese subjects was comparable with Western European subjects and ranged from 16 h to 23 h. However, differences were noted in the pharmacokinetics of the Japanese study as compared with the Western European study. In Japanese subjects, maximum concentrations of GSK2190915 were reached at 4–6 h post‐dose and plasma exposures were slightly higher as compared with the Western European subjects. An exploratory assessment of dose proportionality was performed on both AUC(0,∞) and Cmax using the power model. There was no suggestion of a deviation from dose proportionality with AUC(0,∞), however, a statistically significant departure from dose proportionality was observed with Cmax, with increasing does of GSK2190915 yielding slightly larger than dose proportional increases in Cmax. These pharmacokinetic differences should, however, be interpreted with caution. These are two independent studies performed at different times and at different sites. In addition, these studies were not designed or powered to detect pharmacokinetic equivalence between the two subject populations. It is likely that inter‐study design differences combined with high inter‐subject variability and a small number of subjects are contributing factors to the differences between the Western European and Japanese subjects. We do not believe that the minor formulation change significantly affected the pharmacokinetics as the ethanol containing formulation has been used in our phase 2a allergen challenge study enrolling predominately Western European subjects and has shown comparable pharmacokinetics with the Lutrol containing formulation.
Single and multiple doses of GSK2190915 markedly inhibited ex vivo calcium ionophore stimulated blood LTB4 formation with a median maximum inhibition of ∼60% to 100% across the dose ranges studied. GSK2190915 showed near identical EC50 values for inhibition of blood LTB4 production in the Western European and Japanese studies (85 nm and 89 nm), thus demonstrating similar pharmacodynamics between the two populations. These EC50 values are in good agreement with the IC50 of 76 nm determined for the in vitro inhibition of LTB4 production by GSK2190915 in human blood [b17]. Therefore, as expected from the preclinical PK/PD, there is good alignment between the in vitro and in vivo potencies in blood LTB4 inhibition. However, inhibition of urinary LTE4 production is likely more relevant to clinical efficacy in mild asthmatics since urinary LTE4 is derived from cysteinyl leukotriene production within the lung. FLAP inhibitors which have shown clinical activity vs. early and late phase lung volume decreases after allergen challenge also show inhibition of urinary LTE4 that outlasts the inhibition of ionophore‐challenged blood LTB4 production [b23]. In contrast, two 5‐LO inhibitors (Zileuton and ZD2138) were relatively ineffective against early and late phase allergen‐induced responses [b10,b24]. In preclinical studies, the 5‐LO inhibitors were much less effective in inhibiting cysteinyl leukotriene production in ionophore‐challenged lung vs. ionophore‐challenged blood (Lorrain, unpublished data). Therefore, inhibition of leukotriene production within the lung, which is reflected in urinary LTE4 concentrations, is likely an important contributor to clinical efficacy. Here we show that single and multiple doses of GSK2190915 showed marked inhibition of urinary LTE4 formation. Repeat dosing with ≥ 50 mg GSK2190915 showed near full inhibition of urinary LTE4 for 24 h whereas only partial inhibition of urinary LTE4 was seen with repeat dosing of 10 mg. To begin to evaluate the relationship between target engagement and clinical efficacy, doses of 10, 50 and 100 mg GSK2190915 were evaluated in a phase IIa allergen challenge study in patients with mild asthma. After 3 days dosing with 50 mg or 100 mg GSK2190915 there was an approximately 60% attenuation of the early asthmatic response compared with the placebo group [b25]. This magnitude of effect is comparable with that seen with montelukast and is the expected maximal effect of a FLAP inhibitor given that the response is driven by cysteinyl leukotriene production without involvement of LTB4 [b26]. On the other hand, 3 days dosing with 10 mg GSK2190915 showed sub‐maximal efficacy with an approximately 21% attenuation of the placebo response to inhaled allergen [b25]. Based on these data, the dose related inhibition of urinary LTE4 in healthy volunteers correlates well with dose related effects on clinical endpoints in mild asthmatic patients.
GSK2190915 demonstrated a favourable clinical safety and tolerability profile in healthy subjects. Treatment with a single oral dose of GSK2190915 ranging from 10 mg to 1000 mg, and treatment with multiple oral doses once daily for 11 days ranging from 10 mg to 450 mg, was safe and well‐tolerated. In both studies, there was no apparent dose relationship between the number of adverse events or the number of subjects reporting adverse events. In addition, there was no clear difference between the adverse events in the placebo vs. drug‐treated subjects. Most adverse events considered possibly related to the study medication occurred in the 150 mg single dose cohort (10 events; all gastrointestinal). Since these events occurred in the same cohort in both placebo and drug‐treated subjects and did not increase in frequency with higher dose or repeat dosing it is more likely that the gastrointestinal events were related to some other cause such as food acquired infection. Despite 24 h inhibition of LT production for at least 12 days (following 450 mg), there were no clinically meaningful findings in adverse events, vital signs, 12‐lead ECG, clinical laboratory parameters or physical examinations. This safety profile is consistent with the lack of LT‐related symptoms in an individual who is unable to make any LTB4 or CysLTs due to a genetic deletion of the cPLA2 gene [b27].
The pharmacokinetic profile of equivalent doses of AM103, another novel FLAP inhibitor, and GSK2190915 were similar with comparable tmax, Cmax and AUC(0,24 h) values [b14]. Both inhibitors were well tolerated at all doses and showed dose‐dependent inhibition of ex vivo calcium ionophore‐challenged blood LTB4 and urinary LTE4. However, GSK2190915 showed an extended mean plasma half‐life as compared with AM103 (t1/2 of 17–27 h and 8–12 h, respectively) and this increase in plasma t1/2 likely contributed to the prolonged inhibition of blood LTB4 synthesis and urinary LTE4 concentrations observed with the low dose of GSK2190915 [b14]. For example, a single 50 mg dose of AM103 showed minimal inhibition of blood LTB4 by 8 h post‐dose, whereas a single 50 mg dose of GSK2190915 showed ∼80% inhibition of blood LTB4 through 12 h post‐dose [b14]. Additionally, a single 50 mg dose of AM103 showed little to no inhibition of urinary LTE4 concentrations at 24 h post‐dose, whereas a 50 mg dose of GSK2190915 maintained almost complete inhibition of urinary LTE4 through 36 h post‐dose [b14]. Because of its inferior pharmacodynamic profile, clinical investigation of AM103 has not proceeded beyond phase I. On the other hand, GSK2190915 appears safe and well‐tolerated and shows an extended pharmacokinetic and pharmacodynamic profile compared with AM103. GSK2190915 has shown efficacy in phase IIa allergen challenge studies and is now being studied in multiple phase IIb asthma trials (NCT0114774, NCT01248975 and NCT01156792)[b27]. In addition, due to its good safety profile and potent inhibition of 5‐LO pathway products GSK2190915 holds promise for therapy in cardiovascular and other inflammatory diseases.
Acknowledgments
Amira Pharmaceuticals, Inc., San Diego, California sponsored the study in Western European subjects and GlaxoSmithKline (GSK) funded the study in Japanese subjects.
We thank all Amira and GSK employees who contributed to these studies and in particular the clinical support of D. Shapiro, M. Moran, K. Kelly and I. De Armond. We also thank G. Milne and the staff at Vanderbilt University for urinary leukotriene measurements and the staff and subjects at PRA and FOCUS who conducted and participated in the studies.
Competing Interests
GB, CDK, KS, MR, AS, NS, JH and JE were formerly employed by Amira and own shares in FLAP, LLC. VN, CA, JHB and MY are employed by GlaxoSmithKline and own Shares in the company.
References
- 1.Mandal AK, Jones PB, Bair AM, Christmas P, Miller D, Yamin TT, Wisniewski D, Menke J, Evans JF, Hyman BT, Bacskai B, Chen M, Lee DM, Nikolic B, Soberman RJ. The nuclear membrane organization of leukotriene synthesis. Proc Natl Acad Sci U S A. 2008;105:20434–20439. doi: 10.1073/pnas.0808211106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller DK, Gillard JW, Vickers PJ, Sadowski S, Leveille C, Mancini JA, Charleson P, Dixon RA, Ford‐Hutchinson AW, Fortin R, Gauthier JY, Rodkey J, Rosen R, Rouzer C, Sigal IS, Strader CD, Evans JF. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- 3.Back M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009;23:41–48. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5‐lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 5.Holloway JW, Barton SJ, Holgate ST, Rose‐Zerilli MJ, Sayers I. The role of LTA4H and ALOX5AP polymorphism in asthma and allergy susceptibility. Allergy. 2008;63:1046–1053. doi: 10.1111/j.1398-9995.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 6.Via M, De Giacomo A, Corvol H, Eng C, Seibold MA, Gillett C, Galanter J, Sen S, Tcheurekdjian H, Chapela R, Rodriguez‐Santana JR, Rodriguez‐Cintron W, Thyne S, Avila PC, Choudhry S, Gonzalez Burchard E. The role of LTA4H and ALOX5AP genes in the risk for asthma in Latinos. Clin Exp Allergy. 2010;40:582–589. doi: 10.1111/j.1365-2222.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlen B, Kumlin M, Ihre E, Zetterstrom O, Dahlen SE. Inhibition of allergen‐induced airway obstruction and leukotriene generation in atopic asthmatic subjects by the leukotriene biosynthesis inhibitor BAYx 1005. Thorax. 1997;52:342–347. doi: 10.1136/thx.52.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamant Z, van der Timmers MC, Veen H, Friedman BS, De Smet M, Depre M, Hilliard D, Bel EH, Sterk PJ. The effect of MK‐0591, a novel 5‐lipoxygenase activating protein inhibitor, on leukotriene biosynthesis and allergen‐induced airway responses in asthmatic subjects in vivo. J Allergy Clin Immunol. 1995;95:42–51. doi: 10.1016/s0091-6749(95)70151-6. [DOI] [PubMed] [Google Scholar]
- 9.Friedman BS, Bel EH, Buntinx A, Tanaka W, Han YH, Shingo S, Spector R, Sterk P. Oral leukotriene inhibitor (MK‐886) blocks allergen‐induced airway responses. Am Rev Respir Dis. 1993;147:839–844. doi: 10.1164/ajrccm/147.4.839. [DOI] [PubMed] [Google Scholar]
- 10.Berger W, De Chandt MT, Cairns CB. Zileuton: clinical implications of 5‐Lipoxygenase inhibition in severe airway disease. Int J Clin Pract. 2007;61:663–676. doi: 10.1111/j.1742-1241.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- 11.Evans JF, Ferguson AD, Mosley RT, Hutchinson JH. What's all the FLAP about? 5‐lipoxygenase‐activating protein inhibitors for inflammatory diseases. Trends Pharmacol Sci. 2008;29:72–78. doi: 10.1016/j.tips.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis and exercise‐induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173–2187. doi: 10.1517/14656566.8.13.2173. [DOI] [PubMed] [Google Scholar]
- 13.Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF, Juniper EF, Ayres JG, Kemp L, Blyth A, Wilson EC, Wolfe S, Freeman D, Mugford HM, Murdoch J, Harvey I. Leukotriene antagonists as first‐line or add‐on asthma‐controller therapy. N Engl J Med. 2011;364:1695–1707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 14.Bain G, King CD, Rewolinski M, Schaab K, Santini AM, Shapiro D, de Moran M, Rooij S, Roffel AF, Schuilenga‐Hut P, Milne GL, Lorrain DS, Li Y, Arruda JM, Hutchinson JH, Prasit P, Evans JF. Pharmacodynamics and pharmacokinetics of AM103, a novel inhibitor of 5‐lipoxygenase‐activating protein (FLAP) Clin Pharmacol Ther. 2010;87:437–444. doi: 10.1038/clpt.2009.301. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson JH, Li Y, Arruda JM, Baccei C, Bain G, Chapman C, Correa L, Darlington J, King CD, Lee C, Lorrain D, Prodanovich P, Rong H, Santini A, Stock N, Prasit P, Evans JF. 5‐lipoxygenase‐activating protein inhibitors: development of 3‐[3‐tert‐butylsulfanyl‐1‐[4‐(6‐methoxy‐pyridin‐3‐yl)‐benzyl]‐5‐(pyridin‐2 ‐ylmethoxy)‐1H‐indol‐2‐yl]‐2,2‐dimethyl‐propionic acid (AM103) J Med Chem. 2009;52:5803–5815. doi: 10.1021/jm900945d. [DOI] [PubMed] [Google Scholar]
- 16.Lorrain DS, Bain G, Correa LD, Chapman C, Broadhead AR, Santini AM, Prodanovich P, Darlington JV, Hutchinson JH, King C, Lee C, Baccei C, Li Y, Arruda JM, Evans JF. Pharmacological characterization of 3‐[3‐tert‐butylsulfanyl‐1‐[4‐(6‐methoxy‐pyridin‐3‐yl)‐benzyl]‐5‐(pyridin‐2 ‐ylmethoxy)‐1H‐indol‐2‐yl]‐2,2‐dimethyl‐propionic acid (AM103), a novel selective 5‐lipoxygenase‐activating protein inhibitor that reduces acute and chronic inflammation. J Pharmacol Exp Ther. 2009;331:1042–1050. doi: 10.1124/jpet.109.158089. [DOI] [PubMed] [Google Scholar]
- 17.Lorrain DS, Bain G, Correa LD, Chapman C, Broadhead AR, Santini AM, Prodanovich PP, Darlington JV, Stock NS, Zunic J, King CD, Lee C, Baccei CS, Stearns B, Roppe J, Hutchinson JH, Prasit P, Evans JF. Pharmacology of AM803, a novel selective five‐lipoxygenase‐activating protein (FLAP) inhibitor in rodent models of acute inflammation. Eur J Pharmacol. 2010;640:211–218. doi: 10.1016/j.ejphar.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Stock N, Baccei C, Bain G, Broadhead A, Chapman C, Darlington J, King C, Lee C, Li Y, Lorrain DS, Prodanovich P, Rong H, Santini A, Zunic J, Evans JF, Hutchinson JH, Prasit P. 5‐Lipoxygenase‐activating protein inhibitors. Part 2: 3‐{5‐((S)‐1‐Acetyl‐2,3‐dihydro‐1H‐indol‐2‐ylmethoxy)‐3‐tert‐butylsulfanyl‐ 1‐[4‐(5‐methoxy‐pyrimidin‐2‐yl)‐benzyl]‐1H‐indol‐2‐yl}‐2,2‐dimethyl‐propio nic acid (AM679)–a potent FLAP inhibitor. Bioorg Med Chem Lett. 2010;20:213–217. doi: 10.1016/j.bmcl.2009.10.131. [DOI] [PubMed] [Google Scholar]
- 19.Sampson AP. FLAP inhibitors for the treatment of inflammatory diseases. Curr Opin Investig Drugs. 2009;10:1163–1172. [PubMed] [Google Scholar]
- 20.Chapman KR, Friedman BS, Shingo S, Heyse J, Relss T, Spector R. The efficacy of an oral inhibitor of leukotriene synthesis (MK‐0591) in asthmatics treated with inhaled steroids (abstract) Am J Respir Crit Care Med. 1994;151:A215. [Google Scholar]
- 21.Storms W, Friedman BS, Zhang J, Santanello N, Allergar N, Appel D, Beaucher W, Bronsky F, Busse W, Chervinsky P, Dockhorn R, Edwards T, Goldstein M, Grossman J, Hendeles L, Kemp J, Memon N, Nooman M, Owens G, Shapiro G, Spirn I, Strek M, Stricker W, Tinkelman D, Townley R, Wanderer A, Weisberg S, Winder J, Woehler T. Treating asthma by blocking the lipoxygenase pathway (abstract) Am J Respir Crit Care Med. 1995;149:A377. [Google Scholar]
- 22.Young RN. Inhibitors of 5‐lipoxygenase: a therapeutic potential yet to be fully realized? Eur J Med Chem. 1999;34:671–685. [Google Scholar]
- 23.Depre M, Friedman B, de Van Hecken A, Lepeleire I, Tanaka W, Dallob A, Shingo S, Porras A, de Lin C, Schepper PJ. Pharmacokinetics and pharmacodynamics of multiple oral doses of MK‐0591, a 5‐lipoxygenase‐activating protein inhibitor. Clin Pharmacol Ther. 1994;56:22–30. doi: 10.1038/clpt.1994.96. [DOI] [PubMed] [Google Scholar]
- 24.Hui KP, Taylor IK, Taylor GW, Rubin P, Kesterson J, Barnes NC, Barnes PJ. Effect of a 5‐lipoxygenase inhibitor on leukotriene generation and airway responses after allergen challenge in asthmatic patients. Thorax. 1991;46:184–189. doi: 10.1136/thx.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent S, Boyce M, Diamant Z, Singh D, O'Connor B, Sagu P, Norris V. The 5‐lipoxygenase activating protein inhibitor, GSK2190915, attenuates the early and late responses to inhaled allergen in mild asthma. Clin Exp Allergy (in press) [DOI] [PubMed] [Google Scholar]
- 26.Singh D, Richards D, Knowles RG, Schwartz S, Woodcock A, Langley S, O'Connor BJ. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–993. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- 27.Adler DH, Cogan JD, Phillips JA, 3rd, Schnetz‐Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]