Abstract
Aims
Angiotensin‐II receptor 1 antagonists (AT1‐antagonists) may cause severe and even lethal fetopathy in late pregnancy. However, exposure still occurs in spite of warnings in package leaflets. This study aimed to assess the risk of fetopathy, the sensitive time window, and possible new symptoms in prospective as well as retrospective cases with AT1‐antagonist treatment during the second or third trimester of pregnancy.
Methods
Patients were enrolled by the Berlin Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy between 1999 and 2011 through risk consultation. Symptoms defined as indicative of AT1‐antagonist fetopathy were: oligo‐/anhydramnios, renal insufficiency, lung hypoplasia, joint contractures, skull hypoplasia and fetal/neonatal death.
Results
In 5/29 (17%) prospectively enrolled cases with AT1‐antagonist exposure beyond the first trimester oligo‐/anhydramnios was diagnosed. Two infants showed additional symptoms of fetopathy. The risk is more than 30% if treatment continues beyond the 20th week of pregnancy. Oligo‐/anhydramnios was reversible after AT1‐antagonist withdrawal. Among 16 retrospective case reports, three infants presented with a thrombosis of the inferior vena cava in the vicinity of the renal veins. Four out of 13 live births did not survive.
Conclusions
Our survey suggests that the risk increases with duration of AT1‐antagonist treatment into late pregnancy and oligo‐/anhydramnios may be reversible after AT1‐antagonist discontinuation. Thrombosis of inferior vena cava may be a new feature of AT1‐antagonist fetopathy. AT1‐antagonist medication during pregnancy constitutes a considerable risk and must be discontinued immediately. In case of indicative diagnostic findings in either the fetus or newborn, previous maternal AT1‐antagonist exposure should be considered.
Keywords: AT1‐antagonist, drug safety, pregnancy outcomes, teratology
What is already known about this subject
Similar to ACE inhibitors, AT1‐receptor antagonists are known to cause fetotoxicity, if taken during late pregnancy. AT1‐receptor antagonist fetopathy may include oligo‐/anhydramnios, renal insufficiency, lung hypoplasia, joint contractures, skull hypoplasia and fetal/neonatal death. So far, experience is limited to retrospective case reports and small case series.
What this study adds
This study is the first to evaluate prospectively the fetotoxic risk of AT1‐receptor antagonist treatment in late pregnancy. Oligohydramnios was observed in about 30% of pregnancies exposed beyond gestational week 20. Furthermore, there is some evidence that thrombosis of the inferior vena cava might be an additional feature of fetopathy.
Introduction
Angiotensin‐II receptor 1 antagonists (‘sartans’, AT1‐antagonists) are commonly used for treatment of essential hypertension and other cardiovascular conditions. They act via inhibition of the angiotensin II/AT1‐receptor interaction, thereby decreasing the activity of angiotensin II, the main effector of the renin‐angiotensin‐aldosterone system (RAS). Thus, the mechanism is similar to that of angiotensin converting enzyme (ACE) inhibitors, which suppress RAS activity by blocking of angiotensin activation. A fetotoxic potential of ACE inhibitors has clearly been established. In cases of maternal therapy within the second or third trimester of pregnancy, the effect is believed to be a result of decreased RAS tonus in the fetal circulation system. Fetal renal function and urine production commence at the end of the first trimester. The decreased renal vascular tonus inhibits urine formation to the extent necessary to maintain amniotic fluid. Fetal renal dysfunction subsequently results in oligohydramnios or anhydramnios, skull hypoplasia, lung hypoplasia and fetal or neonatal death [1]. The importance of a sufficient RAS tonus for adequate tubular differentiation and maintaining of the renal function is underlined by the evidence that genetic defects of key RAS proteins are causally related to autosomal recessive renal tubular dysgenesis [2].
It is expected that AT1‐antagonists exhibit a potential for fetopathy equal to that of ACE inhibitors. Although the risk is well known and therapeutic alternatives are available, still a considerable number of pregnant women receive AT1‐antagonists during their second or third trimester [3]. As of today, data are limited to case series and reports. In 2001, Saji et al. were the first to report fetopathy caused by losartan [4]. Until today more than 30 cases with AT1‐antagonist therapy in the second or third trimester have been published [3,5–12]. They reveal a pattern of anomalies corresponding to those that are caused by ACE inhibitors [b13,b14]. However, due to the nature of retrospective evaluation, the incidence of fetopathy has not yet been established.
Methods
We present all cases with AT1‐antagonist treatment in late pregnancy that were reported to the Berlin Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy (henceforth referred to as the Institute). The Institute offers patients and physicians individual risk characterizations in case of drug and chemical exposure during pregnancy or lactation. Over 12 000 requests per year are received from throughout Germany, Switzerland and Austria. Most consultations take place during pregnancy when a particular drug exposure raises the question of developmental toxicity, or when physicians or patients ask about a drug of choice to treat a particular disease. These pregnancies are regarded as prospective cases if the outcome, including prenatal diagnostics, is not known at the first contact. Consultation is performed by an interdisciplinary team of physicians, pharmacists and nurses. Prescription and non‐prescription drug use, including dosage, treatment indications and exposure time are recorded, as well as demographic characteristics, obstetric history, family history, maternal chronic diseases and other risks or exposures. Gestational age is calculated by ultrasound and/or the first day of the last menstrual period.
Eight weeks after the expected date of birth, all women eligible for follow‐up are contacted by mail or telephone in consideration of the requirements of the local data protection committee. The obtained information includes complications during pregnancy (e.g. oligo‐/anhydramnios, gestational diabetes, pre‐eclampsia), results of prenatal diagnostics, the outcome of pregnancy, mode of delivery, gestational age at birth, sex, birth weight, length, head circumference, umbilical artery pH and Apgar scores. Particular focus is on congenital anomalies and postnatal disorders. The infant's hospital discharge summary is also obtained, if available. For further details on the methodology and adaptation of the STROBE statement to the needs of pregnancy outcome studies see Schaefer et al. [b15]. The study protocol was approved by the ethics committee at the Charité‐Universitätsmedizin Berlin. Data analysis was performed entirely by the Institute.
We included all cases with confirmed AT1‐antagonist exposure after the first trimester, i.e. after week 13, irrespective of the beginning of drug therapy. Currently approved AT1‐antagonists in Germany are candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan and valsartan. The following conditions were defined as indicative for AT1‐antagonist‐induced fetopathy: oligo‐/anhydramnios, neonatal renal insufficiency or failure, lung hypoplasia, joint contractures, skull hypoplasia and fetal/neonatal death. Respiratory distress, neonatal respiratory distress syndrome, apnoea or respiratory acidosis were not considered to be indicative for lung hypoplasia unless more details were available. Clubfoot was regarded as joint contracture in cases with present oligohydramnios. In addition to the prospective database, the Institute collects retrospective cases that are spontaneously reported after birth. In these cases, a complete history is obtained accordingly. Among retrospectively reported cases, children with developmental defects are commonly over‐represented. Although it is not possible to calculate frequencies of events from retrospective reports, they yield further insights into the pattern of anomalies and the critical exposure time window.
Results
Prospective cases
The total number of prospective and retrospective cases with AT1‐antagonist treatment after the first trimester per year is presented in Figure 1. Altogether, 31 reports fulfilled the criteria of prospective cases. Of these, one case was lost to follow‐up and in another case follow‐up could not be initiated. Thus, we could include 29 prospective cases with completed follow‐up reported between 1999 and 2011 including one twin pregnancy (Figure 2, number 25). Hospital discharge summaries were available in nine cases. All patients were treated for hypertension. In most of the cases the treatment was started before pregnancy and continued after the first trimester, due to late pregnancy diagnosis or unawareness of risk. The maternal age ranged between 24 and 43 years (mean 36.8 years). Concomitant exposures included other antihypertensive, antibiotic, antidiabetic, antithrombotic and gastroenterological drugs (each in at least two cases). Other risks implied smoking (n = 5), overweight/obesity (body mass index > 27 kg m–2 or reported obesity, n = 16), and diabetes mellitus (n = 5 plus three gestational diabetes).
Figure 1.
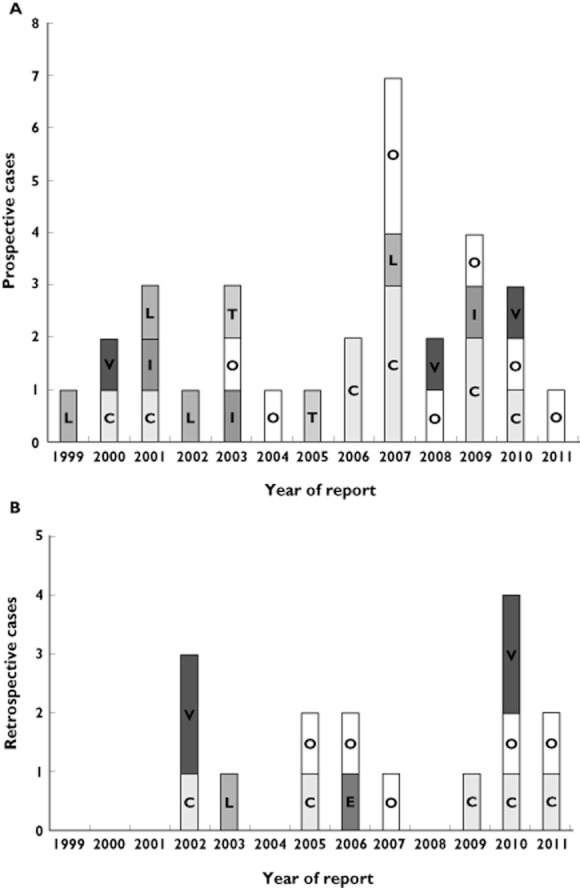
Numbers of (A) prospective and (B) retrospective cases reported to the Institute with AT1‐antagonist treatment during the second or third trimester of pregnancy. The particular substance is indicated for each year and case. C: candesartan, E: eprosartan, I: irbesartan, L: losartan, O: olmesartan, T: telmisartan, V: valsartan. All prospective cases reported to the Institute are counted irrespective of follow‐up status
Figure 2.
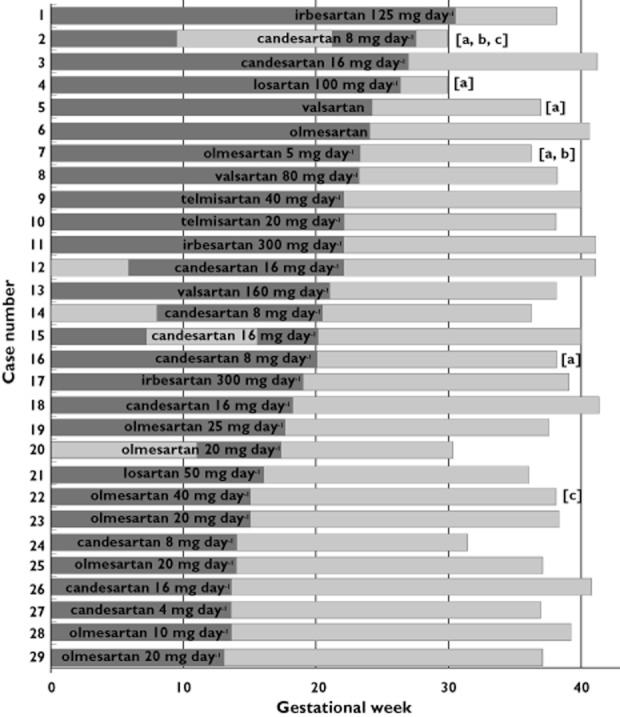
Prospectively evaluated pregnancies with AT1‐antagonist treatment during the second or third trimester in the order of duration of treatment (as indicated by the dark bar within the overall pregnancy duration). Specification of AT1‐antagonist and latest reported dose before treatment discontinuation are given in each case. Pregnancies with symptoms of fetopathy are marked: (a) oligo‐ or anhydramnios was detected but reversible after AT1‐antagonist discontinuation. (b) The infant presented with symptoms of AT1‐antagonist‐induced fetopathy at birth. (c) Symptoms or congenital anomalies not indicative for AT1‐antagonist fetopathy (see text for case description)
Infants with symptoms of fetopathy were born between completed week 29 and 38, and those without between week 30 and 42. In five of the 29 cases (17.2%), oligo‐/anhydramnios was observed, but reversible after AT1‐antagonist withdrawal (numbers 2, 4, 5, 7 and 16 in Figure 2). In all of these pregnancies, the AT1‐antagonist was used at least until gestational week 20. A slight reduction of amniotic fluid at the end of the pregnancy in case number 19 was not interpreted as pathologic by the reporting gynaecologist. Olmesartan was discontinued already 20 weeks earlier in this case.
Despite reversibility after discontinuation of candesartan in week 28, one of the infants presented with hyperechogenic kidneys and joint contractures (number 2 in Figure 2). The mother was also treated with the ACE inhibitor ramipril in the first trimester. She was additionally exposed to hydrochlorothiazide, torasemide, prednisone and esomeprazole during the first trimester, and to mycophenolate and cyclophosphamide until 3 months before conception for systemic lupus erythematosus. The boy was delivered by Caesarean section in week 30 and had post‐partum creatinine concentrations of 1.8 mg dl−1, decreasing to 0.54 mg dl−1 within approximately 2 months. Initial arterial hypotonia was treated with dopamine for 1 day. Neonatal diuresis was normal. Additional congenital anomalies were a small ventricular septal defect, patent foramen ovale, patent ductus arteriosus (spontaneous closure on the third day of life), mitral insufficiency, bilateral hernia inguinalis, hydrocele, respiratory distress syndrome and further post‐natal disorders partly attributable to prematurity.
In case number 7, anhydramnios was diagnosed after 22 weeks. At this time, ultrasound showed no kidney anomalies, but the urinary bladder could not be displayed. Olmesartan (5 mg day−1) was replaced by methyldopa, and the amount of amniotic fluid was normal in week 27. In addition, the mother was treated with simvastatin until week 28 and with low dose acetylsalicylic acid. A girl was delivered at 36 weeks by Caesarean section due to preterm labour and breech presentation. Kidney parenchyma was hyperechogenic, a slight renal pelvis dilation was detectable, but diuresis was inconspicuous. Creatinine was 0.9 mg dl−1 on the third day of life.
Altogether, the two cases with postnatal fetopathy symptoms account for a 2/29 or 6.9% risk. The infants of three remaining pregnancies with reversible oligohydramnios did not show any signs of AT1‐antagonist fetopathy post‐natally. In these cases, the mothers had taken valsartan until week 24 (dosage unknown), candesartan until week 20 (8 mg day−1) and losartan until week 26 (100 mg day−1, reported previously [b16]). The latter child was born preterm in week 30 and presented with respiratory distress syndrome.
In one case (number 22), olmesartan was taken until week 15, plus enalapril, lercanidipine, metoprolol, nifedipine, methyldopa and antiretroviral medication for HIV. A male full term child was delivered by Caesarean section, presenting with an accessory nipple, normally functioning duplex kidneys on both sides, a choroid plexus cyst and an unilateral hearing disorder, but no anomalies complying with AT1‐antagonist‐induced fetopathy.
It is worth mentioning a child (number 1) who was born with diabetic macrosomia and a transient cardiac septal hypertrophy. In this case, the mother was obese, had been treated with irbesartan and ramipril until week 30 and with methyldopa and insulin for diabetes type I throughout her pregnancy.
Retrospective cases
Sixteen cases fulfilled the inclusion criteria for retrospective reports (Table 1). Of the 16 retrospective cases, five (R3, R4, R5, R8 and R14) were reported previously [b3,b16]. In two of these cases, the AT1‐antagonist was started after week 28, and in all but five, treatment was maintained until birth. Additional exposures included antihypertensive, anti‐asthmatic, antibiotic, antidiabetic drugs and smoking. Symptoms included oligo‐/anhydramnios, neonatal renal insufficiency or failure, lung hypoplasia, joint contractures, skull hypoplasia and fetal or neonatal death. Also large and/or hyperechogenic kidneys, polycystic kidneys and dilation of tubules were observed and regarded as fetotoxic effects. It is noteworthy that large or polycystic kidneys are unusual in other conditions of secondary and primary renal tubular dysgenesis. In four of the 16 retrospective cases oligohydramnios was reversible (R10, R13, R14, R16) after drug discontinuation. There were two stillbirths (R9, R15) in the retrospective group, one elective termination of pregnancy (R12) and four of 13 liveborns did not survive (R5, R6, R7, R11). An external ear anomaly (R1) after candesartan exposure throughout pregnancy was not considered indicative for AT1‐antagonist fetopathy. Hospital discharge summaries were available in all retrospective cases except R4, R8, R9, R13, R15, where detailed descriptions were obtained through the patient and/or the involved physician.
Table 1.
Retrospective cases with AT1‐antagonist treatment in the second or third trimester
| Case | Maternal age (years) | AT1‐antagonist (mg day−1) | Pregnancy week treatment | Sex/week of delivery | Oligo‐/anhydram‐nios | Renal structural anomaliesa) | Renal dysfunction/failure, anuria | Joint contrac‐tures | Skull hypoplasia | Lung hypoplasia | Other outcomes: |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | 29 | Candesartan (32) | 0–39+6 | Male 39+6 |
|
||||||
| R2 | 26 | Olmesartan (20) | 30–36+3 | Male 36+3 | + | + |
|
||||
| R3* | 38 | Valsartan (80) | Approx. 29–36 | Female 36 | + (suspected) | + | + | + | + |
|
|
| R4* | 41 | Valsartan | 0–34 | Male 34 | + | (See other outcomes) | + |
|
|||
| R5* | ? | Candesartan (16) | 0–33 | Female 33 | + | + | + | + | + |
|
|
| R6 | 25 | Olmesartan (40) | 0–32+4 | Male 32+4 | + | + | + | +b) |
|
||
| R7 | 42 | Valsartan (80) | 11+3 – 31+6 | Male 31+6 | + | + | + | + |
|
||
| R8* | 24 | Losartan (50) | 0–31 | Female 31 | + | + | + | + |
|
||
| R9 | 37 | Olmesartan (20) | 0–30+4 | Male 30+4 | + | + | + |
|
|||
| R10 | 37 | Candesartan (16) | 0–27+1 | Male 35+6 | +c) | + | + | + | + |
|
|
| R11 | 36 | Eprosartan (600) | 0–26+4 | Female 27+2 | + | +d) | + |
|
|||
| R12 | 29 | Valsartan (40) | 0–25+5 | male 25+5 | + | + | + |
|
|||
| R13 | 39 | Olmesartan (20) | 0–24+0 | Male 38+1 | +c) |
|
|||||
| R14† | 41 | Candesartan | 0–24 | Female 34+1 | +c) | + | + | + |
|
||
| R15 | ? | Candesartan (32) | Full | Female approx. 32 | Unknown |
|
|||||
| R16 | 34 | Olmesartan (5) | 0–25+6 | Female 39+1 | +c) | + | + |
|
Pregnancy weeks are represented as completed weeks + days. If only weeks were reported they were regarded as full weeks.
Case is presented in Schaefer [b16].
Case was reported to the Institute for by the Drug Commission of the German Medical Association and is apparently presented in Hünseler et al. [3].
Includes: renal dysplasia/hyperechogenicity, renal tubular dysgenesis, polycystic kidneys.
Hyaline membrane disease untypical for gestational week was suspected to be caused by lung hypoplasia.
Anhydramnios/oligohydramnios improved after termination of AT1‐antagonist treatment.
Microcystic tubular dilation.
In three retrospective cases, thrombosis of the fetal inferior vena cava was reported, a possible new feature of AT1‐antagonist fetopathy. In case R3, a partial thrombosis of the inferior vena cava was diagnosed by umbilical venous catheterization. Hereditary thrombophilia was suspected because of single nucleotide polymorphisms in the factor II, factor V and MTHFR gene, and the child was treated with dalteparin. An autopsy in case R6 revealed thrombosis of the vena cava of 12 mm length in the vicinity of the renal vein outlets with intrusions into the renal veins, particularly the left one was almost completely obstructed. Microscopic analysis revealed calcification of the thrombosis which speaks against recent occlusion. The kidneys were considerably blood‐filled. Similarly, there was a thrombosis measuring 4 × 1 cm obstructing both renal veins, and additional renal thromboses in the stillborn child of case R9. Noteworthy is a hypoplasia of the inferior vena cava in case R2 that was reported at 2 years of age. Figure 1B gives an overview of all retrospective cases with treatment within the second or third trimester.
Discussion
To our knowledge, no prospective evaluations of AT1‐antagonist‐induced fetopathy have been published so far. Our prospective cohort was not selected by outcome and therefore allows an estimation of the risk of fetopathy. The proportion of cases with fetopathy is much higher in the retrospective cohort, since such cases are selectively reported after the detection of an adverse event. Therefore, these cases do not allow the estimation of relative risks. However, both prospective and retrospective cases allow the sensitive period to be specified and additional features of the fetopathy to be explored. The inferior vena cava thrombosis observed in three cases can possibly be considered as a new AT1‐antagonist effect.
The RAS is active during renal development in the fetus. Especially, it is involved in tubular genesis, as shown in human genetic studies with cases of autosomal recessive (non‐drug associated) renal tubular dysgenesis [b2,b17]. The AT2‐receptor dominates during early embryogenesis, while the AT1‐receptor is of importance during the later stages of fetal development and expressed at higher levels in more mature renal tissues [b13]. Both receptors seem to play a crucial role in kidney development [b18]. Blocking the RAS in differentiating areas may lead to the observed tubular dysgenesis. In the more mature tissues the arterial resistance and thereby the renal blood flow are mediated by AT1‐receptors. An appropriate RAS activity is critical in the fetus to maintain an adequate arteriolar constriction and, thereby, glomerular filtration [b19]. Inhibition of angiotensin II activity decreases fetal filtration and urine output, which leads to oligohydramnios resulting in lung hypoplasia, joint contractures and fetal or neonatal death. Skull hypoplasia is discussed as a result of decreased calvaria perfusion in addition to the renal effects [b13].
Our survey suggests that the risk for fetopathy increases with duration of AT1‐antagonist treatment into late pregnancy. Irrespective of the duration of therapy, in none of the cases with fetopathy (either prospective or retrospective), AT1‐antagonist treatment was terminated before week 20, suggesting that the critical time period for developing oligohydramnios starts around week 20. Including all pregnant women exposed after week 13, the risk of developing oligohydramnios was 5/29 or 17.2%, in the prospective cohort. Limiting the cohort to pregnancies with treatment until week 20 or longer the risk increases to 5/16 or 31.1%. Within the prospective cohort, all reported oligo‐/anhydramnios were reversible and after birth three children had no symptoms of fetopathy at all. Thus, the risk for developing severe symptoms seems to be significantly lower.
Limitations of our study are the small numbers and the fact that our cases might not be representative for all pregnant women exposed to AT1‐antagonists. Factors such as higher educational status, anxiety, etc., may be over‐represented among women contacting a teratology information service [b20,b21]. However, other authors could not demonstrate a significant influence on the outcome [b22]. Furthermore, a postulated lower risk of adverse pregnancy outcome in a population of higher education would suggest the effects would even be greater in the general population.
Since we advise the discontinuation of AT1‐antagonist intake for the remaining pregnancy time, the prospective cohort cannot be regarded as intervention‐free. Without this consultation, more cases would have been treated longer and thereby have been at higher risk, such as those reported retrospectively to us. Our cases demonstrate that oligo‐/anhydramnios improves after AT1‐antagonist discontinuation. This suggests that the fetal renal injury is reversible and stresses the causal relationship.
In addition to the known symptoms of AT1‐antagonist fetopathy, further established by our data, we observed three children with the rare thrombosis of the inferior vena cava. This observation is staggering, since it has not been described before in association with AT1‐antagonist or ACE inhibitor exposure. There is only one case with a similar observation after maternal losartan treatment until week 27 [b23]. The authors attributed this to an antithrombotic effect of losartan mediated by an angiotensin‐II metabolite [b24], and the contrary effect after losartan withdrawal. However, in our study, the AT1‐antagonist was applied until birth in all three cases. In case R6, the thrombosis was reported to be older and already calcified, so at least in this case a formation after delivery or AT1‐antagonist withdrawal can be ruled out. It was proposed that a decreased renal blood flow may cause prenatal renal vein thrombosis [b25,b26] which may outweigh a possible antithrombotic effect of the AT1‐antagonist. Our cases strongly suggest that prenatal AT1‐antagonist exposure constitutes such a condition. This is further substantiated by the evidence that RAS inhibition may lead to increased risk of renal vein thrombosis which was observed in two out of 29 cases with autosomal recessive renal tubular dysgenesis and in three further cases of earlier studies on this topic [b17]. A profound hypotension induced by lack of RAS activity was discussed as a relevant factor. However, additional genetic or acquired risk factors like primary thrombophilia or vein hypoplasia could also contribute to the risk for developing a thrombosis.
Altogether, we found AT1‐antagonist‐induced fetopathy in association with five of the seven available ‘sartans’ independently of the applied dose. This confirms the fetopathy to be a group effect rather than attributable to individual substances. We did not observe fetopathy in cases with telmisartan or irbesartan, but this is probably due to the limited number of exposed pregnancies (two and three cases, respectively). Also, we did not observe a specifically higher risk for candesartan, which was discussed before because of its stronger binding to the receptor [b27,b28].
It was suggested that RAS inhibition in the first trimester may lead to increased incidences of cardiovascular and central nervous system malformations [b29]. However, according to other reports [b16,b30], our data are not suggestive of a specific pattern of birth defects after first trimester exposure. Although the risk of fetopathy warrants contraindication during pregnancy AT1‐antagonists as well as ACE inhibitors are still used as reported before [b3,b31]. Therefore, additional efforts are required to inform prescribing physicians that in particular during the second or third trimester AT1‐antagonist medication is strongly contraindicated and in case of inadvertent exposure treatment must be discontinued immediately, and amniotic fluid should be monitored by ultrasound. Hypertensive women who conceive or are planning to conceive and are treated with an RAS‐inhibitor should be switched immediately. Both obstetricians and paediatricians should consider AT1‐antagonist exposure as a causal factor in any case of indicative clinical or diagnostic findings.
Acknowledgments
We would like to thank Professor Reinhard Meister of the Beuth Hochschule Berlin, University of Applied Sciences and his biometry working group for their support, and the entire Institute staff for the consultation and documentation.
Competing Interests
This study was supported by the German Federal Institute for Drugs and Medical Devices (BfArM), and the German Federal Ministry of Health (BMG). All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any other organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Barr M., Jr Teratogen update: angiotensin‐converting enzyme inhibitors. Teratology. 1994;50:399–409. doi: 10.1002/tera.1420500606. [DOI] [PubMed] [Google Scholar]
- 2.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben AH, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon‐Roman E, Roume J, Joubert M, Antignac C, Gubler MC. Mutations in genes in the renin‐angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 3.Hunseler C, Paneitz A, Friedrich D, Lindner U, Oberthuer A, Korber F, Schmitt K, Welzing L, Muller A, Herkenrath P, Hoppe B, Gortner L, Roth B, Kattner E, Schaible T. Angiotensin II receptor blocker induced fetopathy: 7 Cases. Klin Padiatr. 2011;223:10–14. doi: 10.1055/s-0030-1269895. [DOI] [PubMed] [Google Scholar]
- 4.Saji H, Yamanaka M, Hagiwara A, Ijiri R. Losartan and fetal toxic effects. Lancet. 2001;357:363. doi: 10.1016/S0140-6736(00)03648-5. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Okuda M, Ishikawa H, Takahashi T, Hirahara F. Oligohydramnios and pulmonary hypoplasia: a case in which involvement of an angiotensin II receptor antagonist was suspected. J Obstet Gynaecol Res. 2008;34:242–246. doi: 10.1111/j.1447-0756.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinelli MT, Cattarelli D, Cortinovis S, Maroccolo D, Chirico G. Severe neonatal renal failure after maternal use of angiotensin II type I receptor antagonists. Pediatr Med Chir. 2008;30:306–308. [PubMed] [Google Scholar]
- 7.Trakadis Y, Blaser S, Hahn CD, Yoon G. A case report of prenatal exposure to rosuvastatin and telmisartan. Paediatr Child Health. 2009;14:450–452. [PMC free article] [PubMed] [Google Scholar]
- 8.Gersak K, Cvijic M, Cerar LK. Angiotensin II receptor blockers in pregnancy: a report of five cases. Reprod Toxicol. 2009;28:109–112. doi: 10.1016/j.reprotox.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Miura K, Sekine T, Iida A, Takahashi K, Igarashi T. Salt‐losing nephrogenic diabetes insipidus caused by fetal exposure to angiotensin receptor blocker. Pediatr Nephrol. 2009;24:1235–1238. doi: 10.1007/s00467-008-1091-8. [DOI] [PubMed] [Google Scholar]
- 10.Borthen C, Oglaend B, Eggeboe T, Ellingsen CL, Schjott J. Warnings against candesartan in pregnancy are not implemented in physicians’ practice. Eur J Obstet Gynecol Reprod Biol. 2009;146:235. doi: 10.1016/j.ejogrb.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Munk PS, von Brandis P, Larsen AI. Reversible fetal renal failure after maternal treatment with candesartan: a case report. Reprod Toxicol. 2010;29:381–382. doi: 10.1016/j.reprotox.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Haaland K. Angiotensin II receptor antagonists against migraine in pregnancy: fatal outcome. J Headache Pain. 2010;11:167–169. doi: 10.1007/s10194-009-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alwan S, Polifka JE, Friedman JM. Angiotensin II receptor antagonist treatment during pregnancy. Birth Defects Res A Clin Mol Teratol. 2005;73:123–130. doi: 10.1002/bdra.20102. [DOI] [PubMed] [Google Scholar]
- 14.Quan A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum Dev. 2006;82:23–28. doi: 10.1016/j.earlhumdev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer C, Ornoy A, Clementi M, Meister R, Weber‐Schoendorfer C. Using observational cohort data for studying drug effects on pregnancy outcome – methodological considerations. Reprod Toxicol. 2008;26:36–41. doi: 10.1016/j.reprotox.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer C. Angiotensin II‐receptor‐antagonists: further evidence of fetotoxicity but not teratogenicity. Birth Defects Res A Clin Mol Teratol. 2003;67:591–594. doi: 10.1002/bdra.10081. [DOI] [PubMed] [Google Scholar]
- 17.Lacoste M, Cai Y, Guicharnaud L, Mounier F, Dumez Y, Bouvier R, Dijoud F, Gonzales M, Chatten J, Delezoide AL, Daniel L, Joubert M, Laurent N, Aziza J, Sellami T, Amar HB, Jarnet C, Frances AM, ikha‐Dahmane F, Coulomb A, Neuhaus TJ, Foliguet B, Chenal P, Marcorelles P, Gasc JM, Corvol P, Gubler MC. Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: role of the renin‐angiotensin system. J Am Soc Nephrol. 2006;17:2253–2263. doi: 10.1681/ASN.2005121303. [DOI] [PubMed] [Google Scholar]
- 18.Sequeira Lopez ML, Gomez RA. The role of angiotensin II in kidney embryogenesis and kidney abnormalities. Curr Opin Nephrol Hypertens. 2004;13:117–122. doi: 10.1097/00041552-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Tabacova SA, Kimmel CA. Enalapril: pharmacokinetic/dynamic inferences for comparative developmental toxicity. A review. Reprod Toxicol. 2001;15:467–478. doi: 10.1016/s0890-6238(01)00161-7. [DOI] [PubMed] [Google Scholar]
- 20.Martinez‐Frias ML, Rodriguez‐Pinilla E. Problems of using data from Teratology Information Services (TIS) to identify putative teratogens. Teratology. 1999;60:54–55. doi: 10.1002/(SICI)1096-9926(199908)60:2<54::AID-TERA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Ornoy A, Mastroiacovo P. More on data from teratogen information systems (TIS) Teratology. 2000;61:327–328. doi: 10.1002/(SICI)1096-9926(200005)61:5<327::AID-TERA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KA, Weber PA, Jones KL, Chambers CD. Selection bias in Teratology Information Service pregnancy outcome studies. Teratology. 2001;64:79–82. doi: 10.1002/tera.1048. [DOI] [PubMed] [Google Scholar]
- 23.Bakkum JN, Brost BC, Johansen KL, Johnston BW, Watson WJ. In utero losartan withdrawal and subsequent development of fetal inferior vena cava thrombosis. Obstet Gynecol. 2006;108:739–740. doi: 10.1097/01.AOG.0000187942.09107.08. [DOI] [PubMed] [Google Scholar]
- 24.Kucharewicz I, Pawlak R, Matys T, Pawlak D, Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin‐(1‐7) Hypertension. 2002;40:774–779. doi: 10.1161/01.hyp.0000035396.27909.40. [DOI] [PubMed] [Google Scholar]
- 25.Andrew ME, Monagle P, deVeber G, Chan AK. Thromboembolic disease and antithrombotic therapy in newborns. Hematology Am Soc Hematol Educ Program. 2001;2001:358–374. doi: 10.1182/asheducation-2001.1.358. [DOI] [PubMed] [Google Scholar]
- 26.Smorgick N, Herman A, Wiener Y, Halperin R, Sherman D. Prenatal thrombosis of the inferior vena cava and the renal veins. Prenat Diagn. 2007;27:603–607. doi: 10.1002/pd.1739. [DOI] [PubMed] [Google Scholar]
- 27.Cox RM, Anderson JM, Cox P. Defective embryogenesis with angiotensin II receptor antagonists in pregnancy. BJOG. 2003;110:1038–1040. [PubMed] [Google Scholar]
- 28.Gradman AH. AT(1)‐receptor blockers: differences that matter. J Hum Hypertens. 2002;16(Suppl. 3):S9–S16. doi: 10.1038/sj.jhh.1001434. [DOI] [PubMed] [Google Scholar]
- 29.Cooper WO, Hernandez‐Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 30.Diav‐Citrin O, Shechtman S, Halberstadt Y, Finkel‐Pekarsky V, Wajnberg R, Arnon J, Di GE, Clementi M, Ornoy A. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011;31:540–545. doi: 10.1016/j.reprotox.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Bowen ME, Ray WA, Arbogast PG, Ding H, Cooper WO. Increasing exposure to angiotensin‐converting enzyme inhibitors in pregnancy. Am J Obstet Gynecol. 2008;198:291–295. doi: 10.1016/j.ajog.2007.09.009. [DOI] [PubMed] [Google Scholar]


