Abstract
Aim
This study evaluated a presumed gradual decline in cognitive function in nursing home residents when the anticholinergic drug scale (ADS) score increased above 3.
Method
The study population was recruited from 21 nursing homes in Norway. Criteria for inclusion were ADS score ≥ 3 and no severe dementia, defined as Clinical Dementia Rating (CDR) score < 3. Primary cognitive end points were CERAD 10‐word lists for recall and Mini Mental State Examination (MMSE). Secondary end points were activity of daily living (ADL), mouth dryness and serum anticholinergic activity (SAA). The patients were stratified into subgroups according to ADS score, i.e. a reference group with score 3 and test groups with scores 4, 5 or ≥6. End points were compared by analyses of covariance (ancova).
Results
Overall, 230 of the 1101 screened nursing home residents (21%) had an ADS score ≥3. After exclusion 101 residents were recruited and among these, 87 managed to participate in the study. No significant differences were detected in cognitive function or ADL when ADS increased above 3 (P > 0.10), but in vivo (mouth dryness) and in vitro (SAA) measures of peripheral anticholinergic activity were significantly higher in patients with an ADS score ≥6 (P < 0.01).
Conclusion
The present study does not support a progressive decline in cognitive function with ADS score above 3. This might indicate that the ADS score model has limited potential to predict the clinical risk of central anticholinergic side effects in frail elderly patients receiving multiple anticholinergic drugs.
Keywords: anticholinergic drugs, cognitive function, elderly, nursing homes, saliva
What is Already Known about this Subject
Drugs with anticholinergic properties are frequently used in older people despite their high potential of precipitating central and peripheral adverse effects.
Many institutionalized older persons use several drugs with potential anticholinergic effects concurrently.
Observational studies have reported that patients with a high anticholinergic burden, i.e. a score of 3 or higher on the anticholinergic drug scale (ADS), have increased risk of cognitive impairment compared with non‐users of anticholinergics (ADS score ‘0’).
What this Paper Adds
About one fifth of nursing home residents had an ADS score of 3 or higher.
Residents with dementia had lower ADS scores than those without dementia.
After adjustment for clinical dementia rate, there was no evidence of a progressive decline in cognitive function when ADS scores increased above 3 in frail nursing home residents.
Introduction
Many commonly prescribed drugs have shown affinity to muscarinic receptors in vitro and may cause central and peripheral antimuscarinic symptoms in vivo [1]. Aged people, especially those who are cognitively impaired, have increased sensitivity to central antimuscarinic adverse effects due to reduction in cholinergic neurotransmission, and use of antimuscarinic drugs (in the following referred to as ‘anticholinergic’) has been associated with reduced cognitive and physical function in the elderly [2–4]. Still, anticholinergic drugs are frequently prescribed to elderly people and previous studies have shown that nursing home patients often use several anticholinergic drugs simultaneously [5,6].
It is believed that drug‐induced anticholinergic activity (AA) is additive and that the overall anticholinergic burden determines the risk of adverse effects [4,7,8,9]. In 2006, Carnahan and colleagues published the Anticholinergic Drug Scale (ADS) score model for estimation of the overall anticholinergic burden. The ADS has four score levels for each included drug, ranging from level ‘0’ (‘no known AA’) to level ‘3’ (‘markedly AA’) [8]. Summation of each medication's ADS score reflects the total anticholinergic burden of a subject.
Large observational studies using the ADS score model have previously reported that a high anticholinergic burden increases the risk for peripheral and central side effects when comparing patients with ADS score ‘0’ (non‐users of anticholinergics) with those using anticholinergics [4,9,10,11]. However, the proposed additive properties of the ADS inventory have not previously been evaluated. Thus, it is not known whether cognitive function gradually declines in aged people who are exposed to an increasing number of anticholinergic medications. The aim of the present study was, therefore, to investigate the cognitive function when the ADS scores increased above 3 in frail nursing home residents.
Methods
Study population
The participants were long term nursing home residents recruited from 21 institutions in two different Norwegian counties during 2008–2009. Anonymized medical records were screened for anticholinergic drugs by a clinical pharmacist (HK) and a trained study nurse. Anticholinergic drug score was assessed using the ADS score model published by Carnahan et al. in 2006, with some modifications based on a more recent, comprehensive in vitro screening of anticholinergic activities at therapeutic serum concentrations published by Chew et al. in 2008 [1,8]. Patients with overall ADS score ≥3 were considered for inclusion by a local caregiver who evaluated their physical and mental eligibility to participate. Patients with blindness, deafness, aphasia, delirium or severe dementia, i.e. score 3 on the Clinical Dementia Rating scale (CDR) were excluded [b12,b13].
Outcome measures
Cognitive function
One study nurse, blinded for the patients' ADS scores, performed the cognitive tests on all the participants. The cognitive test battery included the Norwegian translated version of the 10 words tests of immediate recall, delayed recall and recognition, from the Consortium to Establish a Registry for Alzheimer Disease (CERAD) neuropsychological test battery [b14] and the Mini Mental State Examiation (MMSE), revised for use in Norwegian nursing homes [b15]. The CERAD subtests were chosen as they can sensitively differentiate between cognitive impairments of different severity [b16].
Self‐care capacity
The patients' self‐care capacity was assessed using the Barthel's Index of Activity of Daily Living (ADL) [b17]. The ADL scores of the patients were determined by a nurse or auxiliary nurse at each nursing home included.
Mouth dryness
A swab technique was used to measure the resting whole mouth saliva flow rate. The test was performed by first placing two pre‐weighted dental cotton rolls in the patients' lower jowl for 3 min and then in the upper vestibules at the opening of the parotid gland ducts for 3 min. The weight difference of the cotton rolls was used to determine the salivary flow. This test has been shown to be reliable and practicable in cognitively impaired elderly adults [b18].
Serum anticholinergic activity
Blood was sampled from the patients for measurement of serum AA (SAA) using a modified version of the radio receptor assay published by Tune & Coyle in 1980 [b19]. In the modified assay, samples of 20 μl were applied in 96 well plates for high throughput analyses of SAA [b20]. A standard curve with atropine (0.05 to 100 nm) was used as reference for anticholinergic activity. Standard curves were fitted to a one site competitive binding model using GraphPad Prism version 5.01 (GraphPad Software Inc, CA).
Covariates
Information about age, gender, educational level, smoking habits, time since last meal and medication intake, and use of dental prostheses was collected from the patients' nursing home records. Further, information about other possible confounders, such as diagnoses, neuropsychiatric symptoms, serum creatinine, the use of cholinesterase inhibitors and the total number of drugs used was recorded. Co‐morbidity was assessed by the Charlson co‐morbidity Index [b21]. The frequency (F) and intensity (I) of neuropsychiatric symptoms were rated by use of the Norwegian version of the neuropsychiatric inventory for nursing homes (NPI). Each symptom with an item score ≥ 4 (F x I) was assessed to be of clinical relevance [b22]. Glomerular filtration rate (GFR) was calculated according to the Modification of Diet in Renal Disease (MDRD) study equation [b23].
Statistical analyses
Prior to the statistical analyses, the patients were stratified into four subgroups according to overall ADS scores; i.e. a reference group with score 3 and three test groups with scores 4, 5 and 6–10, respectively. Distribution of the covariates across the four strata was compared by the Kruskal–Wallis test. All the outcome measures were explored by distribution plots and descriptive analyses. Log10 transformations of SAA and saliva production were conducted to attain normal distribution. Two‐tailed Spearman's rank correlations between the covariates and the outcome measures were inspected to identify possible confounders and collinearity between the covariates. Analyses of covariance were performed to compare the mean difference in each outcome measures between the test groups (ADS score = 4, 5 and ≥ 6) and the reference group (ADS score = 3). We adjusted for the possible effects of the imbalanced covariates with significance level ≤0.1. In addition, analyses of cognitively intact patients and patients with mild to moderate dementia were performed separately in each ADS subgroup. We included the 45 patients with severe dementia in a Mann–Whitney test performed to compare the ADS scores in patients with dementia (CDR 1, 2 and 3) vs. patients without dementia (CDR = 0). All statistical analyses were performed using PASW Statistics for Windows version 19 (SPSS Inc., Chicago, USA).
Ethics
We obtained written informed consent from all participants. For participants with substantial cognitive impairment, written informed consent was collected from the closest relative, in accordance with Norwegian legal regulation. The study was approved by the Regional Committee for Medical Research Ethics, the Norwegian Directorate of Health and the Data Protection Officer at Oslo University Hospital.
Results
Of the 1101 screened residents, 230 (21%) had an ADS sum score ≥3. After exclusion based on predefined criteria, 101 nursing home residents were recruited. Among these 87 managed to participate and comprised the final included study population. Figure 1 shows the sample selection and the reasons for exclusion. The nursing home staff considered 84 of the patients with ADS score ≥ 3 to be incapable of executing the tests; 44 due to severe dementia (CDR = 3) and 40 because of physical impairments such as aphasia, loss of hearing or sight. The 87 patients finally included were allocated to the ADS subgroups (score 3, n = 35; score 4, n = 22; score 5, n = 16; and score ≥6, n = 13).
Figure 1.
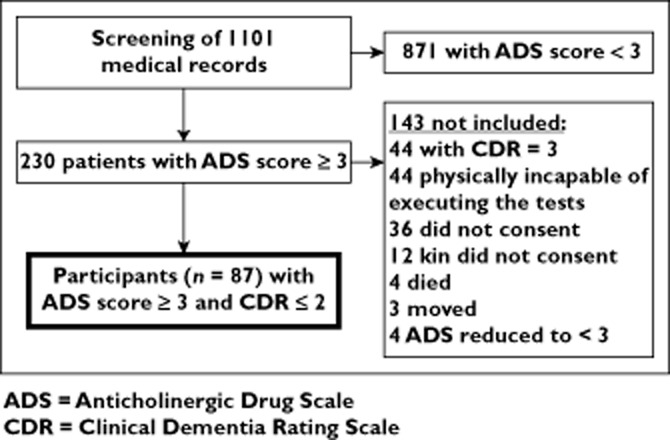
Selection of study population. ADS, anticholinergic drug scale; CDR, clinical dementia rating scale
Clinical characteristics of the study participants stratified by their ADS score are presented in Table 1. The study population (n = 87) comprised 69 women and 18 men, all aged above 73 years, women being older (median age 87 years, interquartile range (IQR) 84–92) than men (median age 81 years, IQR 79–85). Their median Charlson co‐morbidity score was 4 (IQR 3–5), almost 70% of the included patients had mild to moderate dementia (CDR 1–2) and four patients were recorded with clinical significant symptoms of depression (NPI item score ≥ 4). The median number of drugs used on a regular basis was 9 (IQR 7–12), and four patients were treated with cholinesterase inhibitors. The distribution of number of scheduled medications and degree of dementia (CDR) was significantly different across the categories of ADS score, (P = 0.004 and P = 0.007 respectively, Kruskal–Wallis test). Patients with ADS score ≥6 had less dementia and used more drugs on a regular basis than the other groups and none of the patients with moderate dementia (CDR = 2) had an ADS score ≥6. The distribution of patients with dementia (CDR = 1, 2 or 3) vs. patients without dementia (CDR = 0), was significantly asymmetrical across the ADS categories (P = 0.011), and the patients with dementia had significantly lower ADS scores than the patients without dementia (P = 0.024).
Table 1.
Characteristics of the study cohort represented by the four strata with different anticholinergic drug scale (ADS) scores
| Characteristics | ADS = 3 | ADS = 4 | ADS = 5 | ADS ≥ 6 |
|---|---|---|---|---|
| n = 36 | n = 22 | n = 16 | n = 13 | |
| Age (years) | 87 (84–93) | 85 (83–87) | 84 (81–91) | 83 (81–90) |
| 73–99 | 77–93 | 74–96 | 77–93 | |
| Female gender | 29 (81%) | 16 (73%) | 13 (81%) | 11 (85%) |
| Education >12 years | 4 (11%) | 2 (9%) | 1 (6%) | 0 |
| Daily smoking | 5 (14%) | 3 (14%) | 1 (6%) | 4 (31%) |
| Number of dental prostheses | 16 (44%) | 11 (50%) | 7 (44%) | 4 (31%) |
| CDR* | ||||
| 0 = no dementia, n (%) | 8 (22%) | 5 (23%) | 5 (31%) | 9 (69%) |
| 1 = mild dementia, n (%) | 17 (47%) | 8 (36%) | 7 (44%) | 4 (31%) |
| 2 = moderate dementia, n (%) | 11 (31%) | 9 (41%) | 4 (25%) | 0 |
| Scheduled drugs* | 8 (6–10) | 9 (7–10) | 10.5 (8–13) | 12 (9.5‐5.5) |
| 1–18 | 4–16 | 5–14 | 7–17 | |
| †Glomerular filtration rate | 69 (54–84.5) | 72 (46–87) | 72 (82.5–87) | 55 (41–77) |
| 33–207 | 18–125 | 38–147 | 24–129 | |
| Charlson co‐morbidity score | 3.5 (3–4) | 4 (2–6) | 3 (2.5‐5) | 3 (3–5) |
| 1–6 | 1–9 | 1–9 | 2–7 | |
| Number of participants using cholinesterase inhibitors | 1 | 1 | 1 | 1 |
| Prevalence of clinical significant symptoms of depression (Fx I) ≥4 in neuropsychiatric inventory (NPI) | 1 | 2 | 1 | |
Data represent median, interquartile range (IQR) and range or frequency and percentages within the stratum; n gives the valid data for each characteristics;
Significant different distribution across the ADS subgroups, P < 0.05, Kruskal–Wallis test.
Calculated by the Modification of Diet in Renal Disease (MDRD) formula; CDR = clinical dementia rate.
The 31 different anticholinergic drugs used by the participants are listed in Table 2. Fifty‐nine patients used one drug with an ADS score of 3 (hydroxyzine, chlorprothixene, and alimemazine most frequently, being used by 15%), while three patients used two drugs in this category. The most frequently used anticholinergic drug regardless of score was furosemide (ADS score = 1). Psychotropic drugs with anticholinergic properties were common in the nursing home patients. Overall, 50% used an antidepressant and 27% used an antipsychotic listed in the ADS score model. Two patients used five different anticholinergic drugs and five patients had a total ADS score ≥7. Altogether, the 87 patients used 204 prescribed anticholinergic medications on a regular basis.
Table 2.
Drugs with anticholinergic properties ranked by modified anticholinergic drug scale (ADS) within the study population, n = 87
| Therapeutic drug group | Frequency (% of n) | Drug name | ||
|---|---|---|---|---|
| ADS = 3 | ADS = 2 | ADS = 1 | ||
| 62 (71.3%)* | 10 (10.3%)* | 67 (77.0%)* | ||
| Antidepressants | 44 (50.6) | Amitriptyline. trimipramine | Noritriptyline, paroxetine | Escitalopram, citalopram, mirtazapine, fluoxetine |
| Antipsychotics | 24 (276) | Chlorprothixene, levomepromazine | Olanzapine | Quetiapine, zuclopenthixol |
| High‐ceiling diuretics | 27 (31.0) | Furosemide | ||
| Antihistamines for systemic use | 31 (35.6) | Hydroxyzine, alimemazine, dexchlorpheninamine, promethazine | ||
| Opioids | 14 (16.1) | Fentanyl oxycodone | ||
| Glucocorticoids | 14 (16.1) | Prednisolone | ||
| Drugs for obstructive airway disease, inhalant and systemic use | 14 (16.1) | Theophylline, ipatropium bromide | ||
| Cardiac glycosides | 13 (14.9) | Digitoxin | ||
| Urinary spasmolytics | 12 (13.8) | Tolterodine, solifenacine | ||
| Anxiolytics | 3 (3.4) | Diazepam | ||
| Antiepileptics | 3 (3.4) | Oxcarbazepine | ||
| H2‐receptor antagonists | 2 (2.3) | Ranitidine | ||
| ACE inhibitors | 2 (2.3) | Captopril | ||
| Lincosamides | 1 (1.1) | Clindamycin | ||
| Total drug prevalence | 204 | 65 | 10 | 129 |
Frequency represent the number of participants exposed to drugs in present therapeutic group;
Number of participants using at least one drug in present ADS category. (Several patients used more than one drug in each category).
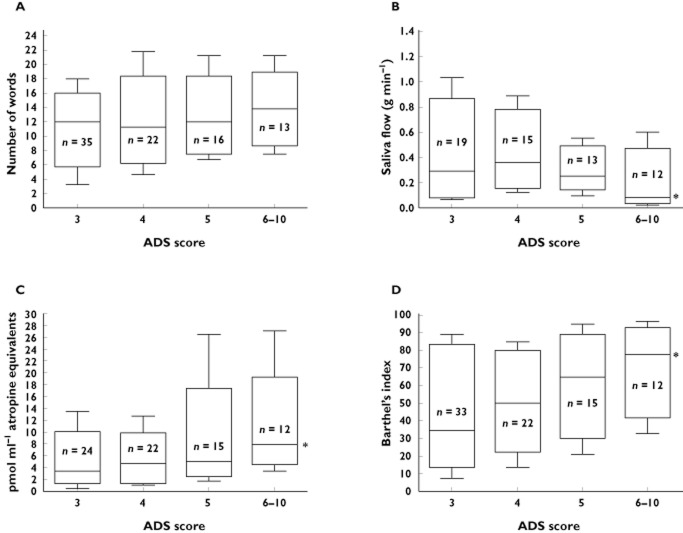
The median values of immediate recall, ADL, salivary flow and SAA in each ADS subgroup are illustrated in Figure 2. Table 3 shows unadjusted and adjusted mean differences in end points between the ADS subgroups with reference to ADS = 3. CDR and number of scheduled drugs were significantly imbalanced between the ADS subgroups, but only CDR was identified as a covariate with sign level P ≤ 0.1 and thus adjusted for in the multivariate model. In the unadjusted and adjusted models, no significant differences in cognitive end points were detected between patients with increasing ADS scores (P > 0.11). Separate analyses of cognitively intact patients (n = 27) and patients with dementia (n = 60) did not show any significant cognitive decline with increasing ADS scores in any of the subgroups (P > 0.45 and P > 0.65, respectively).
Figure 2.
Median, IQR and range (10–90 percentiles) for CERADs word list for immediate recall (A), whole mouth resting saliva flow (B), serum anticholinergic activity (C), and Activity of daily living (D) in nursing home patients with anticholinergic drug scale (ADS) score of 3 (control group), 4, 5 and 6–10 (test groups). Lines indicate median values. *Significantly different distribution across the four ADS categories, P < 0.05, Kruskal–Wallis test
Table 3.
Analysis of covariance to compare the mean differences in anticholinergic end points between the different subgroups of ADS score with reference to ADS score = 3
| End points | ADS = 3 reference | ADS = 4 | ADS = 5 | ADS ≥ 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | B (95% CI) | n | B (95% CI) | n | B (95% CI) | n | ||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||
| CERAD verbal immediate recall, number of words | 10.9 (4.9) | 35 | 1.0 (−1.7, 3.8) | 1.3 (−1.1, 3.8) | 22 | 1.5 (−1.6, 4.5) | 1.0 (−1.8, 3,7) | 16 | 2.7 (−0.69, 6.0) | 0.2 (−2.9, 3.4) | 13 |
| CERAD verbal delayed recall, number of words | 2.5 (2.5) | 34 | 0.5 (−1.4, 1.5) | 0.2 (−1.1, 1.7) | 22 | 0.3 (−1.5, 1.8) | 0.1 (−1.4, 1.6) | 16 | 1.0 (−0.9, 2.7) | −0.1 (−1.9, 1.6) | 13 |
| CERAD verbal recognition, number of words | 6.4 (3.2) | 26 | −1.4 (−3.2, 0.5) | −1.2 (−3.1, 0.7) | 17 | −0.6 (−2.6, 1.5) | −0.6 (−2.7, 1.4) | 13 | 0.6 (−1.5, 2.6) | 0.1 (−2.0, 2.3) | 13 |
| Mini mental state examination (MMSE) | 19.5 (5.1) | 31 | 0.6 (−2.2, 3.4) | 1.1 (−2.9, 2.8) | 22 | 0.5 (−3.1, 3.2) | 0.1 (−2.9, 2.8) | 15 | 2.1 (−1.2, 5.5) | −0.1 (−3.4, 3.1) | 13 |
| Whole mouth resting salivary flow (g min−1)† | 0.3 (0.1, 0.7)‡ | 26 | 0.0 (−0.2, 0.3) | 0.0 (−0.3, 0.3) | 17 | −0.1 (−0.3, 0.2) | −0.1 (−0.3, 0.2) | 14 | −0.5** (−0.7, −0.2) | −0.4** (−0.7, 0.1) | 12 |
| Serum anticholinergic activity (SAA) (pmol ml−1 atropine equivalents)† | 3.6 (2.0, 6.5)‡ | 34 | 0.1 (−0.2, 0.3) | 0.1 (−0.2, 0.3) | 22 | 0.2 (−0.1, 0.4) | 0.2 (−0.1, 0.4) | 15 | 0.4** (0.1, 0.6) | 0.3* (0.1, 0.6) | 12 |
| Barthel's index of activity of daily living (ADL) | 46.8 (35.9, 57.7) | 33 | 3.6 (−11.42, 18.7) | 4.5 (−10.5, 19.6) | 22 | 15.8 (−1.2, 32.9) | 15.67 (−1.3, 32.6) | 15 | 23.18* (4.7, 41.6) | 19.11 (−0.2, 30.4) | 12 |
Controlled for CDR in the adjusted models; B, estimates of mean difference with reference to ADS score = 3;
Analysis of covariance performed with log‐transformed data;
Data given as median and interquartile range; CI confidence interval; SD, standard deviation;
< 0.02;
<0.01; only significant P‐values shown; ADS, anticholinergic drug score; n, number of subjects in the subgroup completing each test.
The self‐care capacity increased significantly with increasing ADS scores (P = 0.05), and the participants with ADS score ≥ 6 had approximately 23% better self‐care capacity than the participants in with ADS score = 3, P = 0.014 (Table 3). After adjusting for the imbalance in CDR the difference in ADL was not statistically significant (P = 0.13).
The measure of peripheral circulating anticholinergic activity (SAA), and the peripheral clinical measure mouth dryness, were both significantly increased when participants with ADS score ≥6 were compared with participants with ADS score = 3 (P < 0.01) (Table 3). No differences in peripheral activity measures were observed between test groups with ADS score of 4 or 5 compared with score 3 (P > 0.15). The significant increase in SAA and mouth dryness in patients with an ADS score ≥6 persisted after controlling for differences in CDR (P < 0.02).
Discussion
We found that one fifth of nursing home residents had an ADS score ≥ 3, while around 10% had ADS scores 4–10. This implies that a substantial proportion of the patients used at least two anticholinergic drugs concurrently. Large observational studies have reported that a high ADS score is a significant predictor of cognitive impairment, but the dose–response relationship with an ADS score ≥ 3 has not previously been investigated [2,4,8,9,10,11]. As no significant differences in cognitive outcomes were observed between the patient subgroups, our results do not support a gradual decrease in cognitive function when the ADS score increases above 3. However, the number of nursing homes patients eligible for inclusion was limited, and larger studies would be desirable to confirm the present findings.
The lack of association between ADS score and cognitive function in the current study could be explained by several factors. Firstly, the ADS score model has a rather simple concept which does not take into account systemic drug exposure, distribution to the brain or pharmacodynamic interactions. The cognitive decline is dependent on the AA in the brain which is previously reported to be dose‐dependent, especially in people with dementia [b24]. Moreover, the pharmacodynamic brain effects of multiple anticholinergic drugs are probably not additive in a linear pattern that can be predicted by the ADS score model. Secondly, since ageing and Alzheimer's dementia (AD) has previously been associated with hypersensitivity to anticholinergic drugs due to loss of cholinergic neurotransmission, it is possible that a saturation of the receptors might be reached by excessive anticholinergic activity (ADS scores ≥ 3) [b25,b26]. As a consequence, a further anticholinergic increase cannot displace more acetylcholine from the muscarinic receptors. Finally, the present variability in anticholinergic drug sensitivity related to advanced age, multi‐morbidity, different degrees of dementia and the multiple comedications might affect the results. Thus, we adjusted for the differences in dementia between the ADS subgroups, but the adjusted models did not show significant decline in any of the cognitive test scores when the ADS score increased above 3. In addition, we controlled for the influence of comorbid depression and concurrent use of cholinesterase inhibitors due to the potential importance of these covariates on cognitive test scores, but this did not alter the results. However, the NPI recordings might have underestimated the prevalence of depression (<5%) which is supported by the fact that 50% of the residents were treated with antidepressants.
Furthermore, the high prevalence of cognitive impairment in our study population might have reduced the sensitivity of the anticholinergic drug burden on the cognitive test scores. This is consistent with two previous studies reporting no cognitive impact of high anticholinergic burden in old patients with dementia [b6,b27] However, the separate analyses of cognitively intact participants (CDR = 0, n = 27) in the present study did not show a greater decline in cognitive test performances than observed in the patients with dementia (CDR = 1–2, n = 60). Unfortunately, the small number of participants in each ADS subgroup limits the interpretation of these data. Nevertheless, among nursing home residents with no, mild or moderate dementia, the ADS score model appears to have a limited potential to predict the clinical risk of central anticholinergic side effects.
In addition to the cognitive tests included, we decided to measure activity of daily living (ADL). The inclusion of ADL was based on a small previous study reporting greater impairment in ADL in demented patients with high vs. low anticholinergic burden [b28]. Unfortunately, the validity of the current ADL registration is considered to be reduced because the registration forms for Barthel's Index were filled in by 21 different caregivers with variable knowledge about the patients. However, we observed a significant increase in ADL with increasing ADS score above 3, but the increase in ADL was not statistically significant after adjusting for the imbalance in CDR which indicates that the observed increase in ADL is explained by the absence of patients with moderate dementia in the group with the highest ADS scores.
In the present study, nursing home residents with dementia had significantly lower ADS scores than those without dementia. This observation might indicate that patients with dementia are prescribed less anticholinergic drugs than others, which is appropriate from a pharmacological point of view. However, whether the lower ADS score in patients with dementia was due to rational medical decisions, or simply reflected a generally restrictive prescription policy in this patient subgroup, is unclear. Nevertheless, as the pathophysiological changes in cholinergic brain transmission in Alzheimer's dementia increase the sensitivity to temporary cholinergic blockade [b24], it would be favourable to avoid or limit use of anticholinergic drugs in people with dementia.
Interestingly, we observed that the overall ADS score was significantly associated with peripheral anticholinergic end‐points in terms of a 1.2‐fold higher serum anticholinergic activity and 0.7‐fold lower saliva production in subjects with ADS scores ≥ 6 compared with those with an ADS score of 3. The significant increase in SAA and in peripheral, but not central adverse effects demonstrated for subjects with ADS ≥ 6, might be understood in terms of how the ADS score model was developed. The potential anticholinergic effects of many drugs included in the model were characterized by in vitro activity to muscarinic receptors measured by the same bio‐assay as used for determination of SAA. The drugs were further graded as mild (ADS score = 1), moderate (ADS score = 2), or markedly anticholinergic (ADS score = 3) based upon a consensus of clinical experience, previously reported adverse effects and knowledge of the drugs' properties. As symptoms of central anticholinergic side‐effects may be subtle in patients with cognitive disorders (e.g. mild alterations in verbal short time memory and attention), it is possible that the model was primarily based upon symptoms of peripheral anticholinergic activity.
The interpretation of the present results is restricted by the cross‐sectional design and the relatively low and imbalanced number of patients in each ADS category. A randomized, controlled study investigating the potential improvement in cognitive function after an interventional reduction in ADS score would be more conclusive to clarify the clinical utility of the risk score model for evaluation of adverse drug effects. Similarly with the validity restrictions of all prescription risk tools in the elderly, the external validity of the present results is limited by the great variability in drug response associated with advanced age, multi‐morbidity and polypharmacy. On the contrary, the prospective design and the consistent findings within the cognitive and peripheral end points strengthen the validity. The results are further strengthened by the fact that all cognitive measurements were performed by one study nurse who was blinded to the participants' ADS scores.
In conclusion, the current study does not support a progressive decline in cognitive function with ADS score above 3. Despite the relatively low number of participants included and restrictions in the external validity, the findings might indicate that the ADS score model has limited potential to predict the clinical risk of central anticholinergic side effects in frail elderly patients receiving multiple anticholinergic drugs.
Acknowledgments
We appreciate the excellent assistance of the study nurse, Inga Kristin Tolo, who performed all the clinical tests. We are thankful to the staff of the 21 different nursing homes and all the nursing home residents participating in this study. Finally, we thank Eva Skovlund and Haavard Aakre for their helpful assistance with the statistical data analysis.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare HK had support from The South‐Eastern Norway Regional Health and The Norwegian Directorate of Health Authority and no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years.
References
- 1.Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, Kirshner MA, Sorisio DA, Bries RR, Gharabawi G. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56:1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 2.Campbell N, Boustani M, Limbil T, Ott H, Fox C, Maidment I, Scubert CC, Munger S, Flick D, Miller D, Gulati R. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnjidic D, Cumming RG, Le Couteur DG, Handelsman DJ, Naganathan V, Abernethy DR, Hilmer SN. Drug burden index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68:97–105. doi: 10.1111/j.1365-2125.2009.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc. 2008;56:2203–2210. doi: 10.1111/j.1532-5415.2008.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters NL. Snipping the thread of life. Antimuscarinic side effects of medications in the elderly. Arch Intern Med. 1989;149:2414–2420. [PubMed] [Google Scholar]
- 6.Kolanowski A, Fick DM, Campbell J, Litaker M, Boustani M. A preliminary study of anticholinergic burden and relationship to a quality of life indicator, engagement in activities, in nursing home residents with dementia. J Am Med Dir Assoc. 2009;10:252–257. doi: 10.1016/j.jamda.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tune LE, Strauss ME, Lew MF, Breitlingeer E, Coyle JT. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry. 1982;139:1460–1462. doi: 10.1176/ajp.139.11.1460. [DOI] [PubMed] [Google Scholar]
- 8.Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The anticholinergic drug scale as a measure of drug‐related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508–513. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- 10.Lechevallier‐Michel N, Molimard M, Dartigues JF, Fabrigoule C, Fourrier‐Reglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol. 2005;59:143–151. doi: 10.1111/j.1365-2125.2004.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non‐degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–459. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marin DB, Flynn S, Mare M, Lantz M, Hsu MA, Laurans M, Paredes M, Shreve T, Zaklad GR, Mohs RC. Reliability and validity of a chronic care facility adaptation of the clinical dementia rating scale. Int J Geriatr Psychiatry. 2001;16:745–750. doi: 10.1002/gps.385. [DOI] [PubMed] [Google Scholar]
- 13.Williams MM, Roe CM, Morris JC. Stability of the clinical dementia rating, 1979–2007. Arch Neurol. 2009;66:773–777. doi: 10.1001/archneurol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillenbaum GG, van BG, Morris JC, Mohns RC, Mirra SS, Davis PC, Tariot PN, Silverman JM, Clark CM, Welsh‐Bohmer KA, Heyman A. Consortium to establish a registry for Alzheimer's disease (CERAD): the first twenty years. Alzheimers Dement. 2008;4:96–109. doi: 10.1016/j.jalz.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braekhus A, Laake K, Engedal K. The mini‐mental state examination: identifying the most efficient variables for detecting cognitive impairment in the elderly. J Am Geriatr Soc. 1992;40:1139–1145. doi: 10.1111/j.1532-5415.1992.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 16.Karrasch M, Sinerva E, Gronholm P, Rinne J, Laine M. CERAD test performances in amnestic mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2005;111:172–179. doi: 10.1111/j.1600-0404.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61:1158–1162. doi: 10.1177/00220345820610100901. [DOI] [PubMed] [Google Scholar]
- 19.Tune L, Coyle JT. Serum levels of anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry. 1980;37:293–297. doi: 10.1001/archpsyc.1980.01780160063007. [DOI] [PubMed] [Google Scholar]
- 20.Jakobsen SM, Kersten H, Molden E. Evaluation of brain anticholinergic activities of urinary spasmolytic drugs using a high‐throughput radio receptor bioassay. J Am Geriatr Soc. 2011;59:501–505. doi: 10.1111/j.1532-5415.2010.03307.x. [DOI] [PubMed] [Google Scholar]
- 21.van Doorn C, Bogardus ST, Williams CS, Concato J, Towle VR, Inouye SK. Risk adjustment for older hospitalized persons: a comparison of two methods of data collection for the Charlson index. J Clin Epidemiol. 2001;54:694–701. doi: 10.1016/s0895-4356(00)00367-x. [DOI] [PubMed] [Google Scholar]
- 22.Selbaek G, Kirkevold O, Sommer OH. The reliability and validity of the Norwegian version of the Neuropsychiatric Inventory, nursing home version (NPI‐NH) Int Psychogeriatr. 2008;20:375–382. doi: 10.1017/S1041610207005601. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney Foundation. Calculators for health care professionals. Available at http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm (last accessed 27 August 2012).
- 24.Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age‐matched controls. A dose‐response study. Arch Gen Psychiatry. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 25.Little JT, Broocks A, Martin A, Hill JL, Tune LE, Mack C, Cantillon M, Molchan S, Murphy DL. Serotonergic modulation of anticholinergic effects on cognition and behavior in elderly humans. Psychopharmacology (Berl) 1995;120:280–288. doi: 10.1007/BF02311175. [DOI] [PubMed] [Google Scholar]
- 26.Ray PG, Meador KJ, Loring DW, Zamrini EW, Yang XH, Buccafusco JJ. Central anticholinergic hypersensitivity in aging. J Geriatr Psychiatry Neurol. 1992;5:72–77. doi: 10.1177/002383099200500203. [DOI] [PubMed] [Google Scholar]
- 27.Fox C, Livingston G, Maidment ID, Coulton S, Smithard DG, Brustani M, Katona C. The impact of anticholinergic burden in Alzheimer's dementia‐the LASER‐AD study. Age Ageing. 2011;40:730–735. doi: 10.1093/ageing/afr102. [DOI] [PubMed] [Google Scholar]
- 28.Rovner BW, David A, Lucas‐Blaustein MJ, Conklin B, Filipp L, Tune L. Self‐care capacity and anticholinergic drug levels in nursing home patients. Am J Psychiatry. 1988;145:107–109. doi: 10.1176/ajp.145.1.107. [DOI] [PubMed] [Google Scholar]