Abstract
Purpose
Hypoxia-inducible factor-1α (HIF-1α) increases transcription of the vascular endothelial growth factor (VEGF) gene. Inhibition of VEGF abolishes VEGF mediated induction of HIF-1α. Recent reports suggested that HIF-1α also mediated the induction of class III β-tubulin (TUBB3) in hypoxia. TUBB3 confers resistance to taxanes. Inhibition of VEGF may decrease the expression of HIF-1α and TUBB3. This study was undertaken to investigate the roles of vascular endothelial growth factor receptor (VEGFR) in gastric cancer cell behavior and to identify methods to overcome paclitaxel resistance in vitro.
Materials and Methods
The protein expression levels of HIF-1α and TUBB3 were measured in human gastric cancer cell lines (AGS) under normoxic and hypoxic conditions. The relationship between TUBB3 and paclitaxel resistance was assessed with small interfering TUBB3 RNA. AGS cells were treated with anti-VEGFR-1, anti-VEGFR-2, placental growth factor (PlGF), bevacizuamb, and paclitaxel.
Results
Hypoxia induced paclitaxel resistance was decreased by knockdown of TUBB3. Induction of HIF-1α and TUBB3 in AGS is VEGFR-1 mediated and PlGF dependent. Hypoxia-dependent upregulation of HIF-1α and TUBB3 was reduced in response to paclitaxel treatment. Expressions of HIF-1α and TUBB3 were most decreased when AGS cells were treated with a combination of paclitaxel and anti-VEGFR-1. AGS cell cytotoxicity was most increased in response to paclitaxel, anti-VEGFR-1, and anti-VEGFR-2.
Conclusion
We suggest that blockade of VEGFR-1 and VEGFR-2 enhances paclitaxel sensitivity in TUBB3-expressing gastric cancer cells.
Keywords: Vascular endothelial growth factor, Hypoxia-inducible factor 1 alpha, class III beta-tubulin, paclitaxel
INTRODUCTION
Although the incidence and mortality of gastric cancer have decreased in the Western world, gastric cancer persists as a common malignancy and leading cause of cancer-related death in Asian contries.1,2 Surgery is the only curative treatment for gastric cancer. Chemotherapy achieves favorable results for unresectable advanced and recurrent gastric cancers; however, the prognosis for these cancers is very poor.
Taxanes such as paclitaxel and docetaxel are a class of anticancer agents that bind to the β tubulin subunit of polymerized microtubules and induce hyperstabilization, which causes cell cycle arrest and apoptosis.3 Taxanes are used most commonly for the treatment of breast, lung, ovary, and gastric cancer. The taxanes paclitaxel and docetaxel exhibit similar efficacies in gastric cancer treatment.4-11
There are at least eight mammalian β tubulin isotypes, and the isotype composition in humans varies across tissues.12 Overexpression of class III β tubulin (TUBB3) has been observed in several human cancer cell lines such as prostate, ovarian, breast, and non small cell lung cancer. Cells expressing TUBB3 show resistance to docetaxel and paclitaxel.12-15 A small cohort study of advanced gastric cancer patients who were receiving preoperative docetaxel-based chemotherapy detected a correlation between expression of TUBB3 and poor response to chemotherapy.16
Hypoxia in solid tumors is associated with resistance to chemotherapy, induction of angiogenesis, and poor patient prognosis, and angiogenesis is a hallmark of human malignancies. The induction of vascular endothelial growth factor (VEGF), mediated by interacting genetic and environmental signals, is an essential component of tumor angiogenesis.17
The transcription factor hypoxia-inducible factor-1α (HIF-1α) is a primary regulator of VEGF during hypoxic conditions, and Calvani, et al.18 reported that inhibition of VEGF or the VEGF receptor-2 (VEGFR-2), abolishes VEGF-mediated induction of HIF-1α. That is, HIF-1α increases VEGF induction, and VEGF also induces HIF-1α expression. A recent report suggested that HIF-1α also mediates TUBB3 induction in hypoxia.19-21 Through these results, we postulated that blockade of VEGF in gastric cancer cells would be associated with a decrease in HIF-1α and TUBB3 expression and a concomitant increase in sensitivity to paclitaxel.
The response of gastric cancer cells to anti-VEGF antibodies is of interest because bevacizumab, a humanized monoclonal antibody against VEGF, is used currently as an anti-angiogenic treatment for metastatic colorectal cancer, non-squamous non-small cell lung cancer, and metastatic breast cancer.22-24 Recent the Avastin in Gastric Cancer trial evaluated the efficacy of adding bevacizumab to chemotherapy in the first-line treatment of advanced gastric cancer. Adding bevacizumab to chemotherapy was associated with significant increases in progression free survival and overall response rate, but not associated with significant increases in overall survival.25 To date, we had insufficient evidences for the benefit of adding bevacizumab to chemotherapy in the treatment of gastric cancer.
Placental growth factor (PlGF) was discovered shortly after VEGF. Whereas VEGF binds both VEGFR-1 and VEGFR-2, PlGF binds to VEGFR-1 but not VEGFR-2. The key role of VEGF and its receptor VEGFR-2 in tumor angiogenesis is firmly established, but the contribution of VEGFR-1 remains poorly defined.26 In the present study, we investigated the relationships between VEGF, HIF-1α, and TUBB3 in gastric cancer cells (AGS). We report that blockade of VEGFR-1 and VEGFR-2 with concomitant paclitaxel treatment increase the cell cytotoxicity of TUBB3-expressing gastric cancer cells.
MATERIALS AND METHODS
Drugs and reagents
Paclitaxel (BMS Pharmaceuticals, New York, NY, USA) and bevacizumab (Roche Pharmaceuticals, Basel, Switzerland) were diluted in absolute DMSO. This solution was diluted to 0.1% DMSO on each day of experimentation. The PlGF and VEGFR-1/2 neutralizing monoclonal antibodies were purchased from research and development systems.
Cell culture and hypoxic treatment
The human gastric cancer cell lines, AGS, SNU, and TMK1 were used as in vitro model systems. Cells were cultured in RPMI 1640 with 10% fetal bovine serum. For normoxia, cultures were incubated at 37℃ in a humidified chamber of 5% CO2. For hypoxia, cells were treated with CoCl2. Confirmatory experiments under hypoxic conditions (1% O2/5% CO2/94% N2) were performed using a Modular Incubator Chamber-101. Each hypoxia experiment was conducted under CoCl2-treated and true hypoxic conditions, and the results were equivalent.
siRNA
The TUBB3 siRNA duplex targeted a sequence 531 bases downstream of the TUBB3 start codon (siTUBB3: 5'-CACGGTGGTGGAGCCCTACAA-3'). siRNA duplex oligonucleotides were purchased from QIAGEN (Gaithersburg, MD, USA) and transfected at 100 nM using Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA, USA).
Cell proliferation assay
To estimate viability, 105 cells were seeded in 24-well plates, subjected to normoxia or hypoxia, and exposed to neutralizing antibodies in the culture media for 4 h. Cells then were treated with bevacizuamb and/or paclitaxel for 24 h. Cells were incubated for 24, 48, or 72 h, and EZ-CyTox (Daeil Lab Service, Seoul, Korea) was added to each well. After 3 h, absorbances at 420 nm were measured using a microplate reader (EL800, BioTek, Winooski, VT, USA).
Tubulin polymerization assay
Cells were treated with various concentrations of drugs for 24 h, washed twice with phosphate buffered saline, and lysed for 5 min in 100 µL hypotonic buffer [1 mM MgCl2, 2 mM EGTA, 0.5% NP40, 20 mM Tris-HCL (pH 6.8), protease and phosphatase inhibitors]. Samples then were centrifuged at 16000×g for 10 min at room temperature. Supernatants, containing soluble tubulin, were separated from pellets, containing assembled tubulin. Each fraction was separated by SDS-PAGE. Immunoblots then were performed using anti-pan-α-tubulin rabbit polyclonal antibody (Santa Cruz Biotechonology, Santa Cruz, CA, USA).
Immunoblotting
Cells were lysed in Mammalian Cell Lysis Reagent (Fermentas, Waltham, MA, USA), and approximately 30 µg protein was separated in 8-10% polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The following antibodies were used: anti-HIF-1α and anti-TUBB3 (Abcam PLC, Cambridge, UK), anti-tubulin (Invitrogen, Carlsbad, CA, USA), β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Statistical analysis
The Student's t-test was used to detect significant differences among treatment groups. Statistical significance was assigned at p<0.05.
RESULTS
Induction of HIF-1α, TUBB3 and VEGF in hypoxia
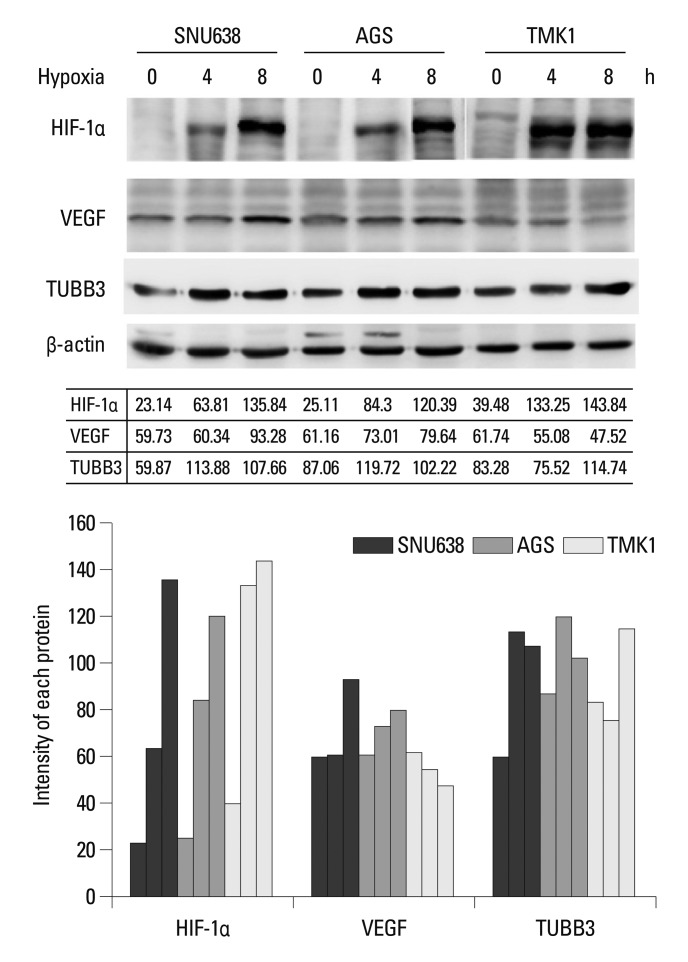
The expression levels of TUBB3 and VEGF were measured during the induction of HIF-1α in hypoxic AGS. AGS exhibited well-defined hypoxic induction of HIF-1α, VEGF, and TUBB3 compared with other cell lines (SNU638, TMK1) (Fig. 1).
Fig. 1.
Induction of HIF-1α and its target genes, VEGF and TUBB3, in human gastric cancer cell lines under hypoxia. HIF-1α, VEGF and TUBB3 expression levels were increased during hypoxia, compared to normoxia. Actin was used as a loading control. Band intensities were quantified by densitometry. HIF-1α, hypoxia inducible factor-1α; VEGF, vascular endothelial growth factor; TUBB3, class III β-tubulin.
TUBB3 confers gastric cancer cells with resistance to paclitaxel
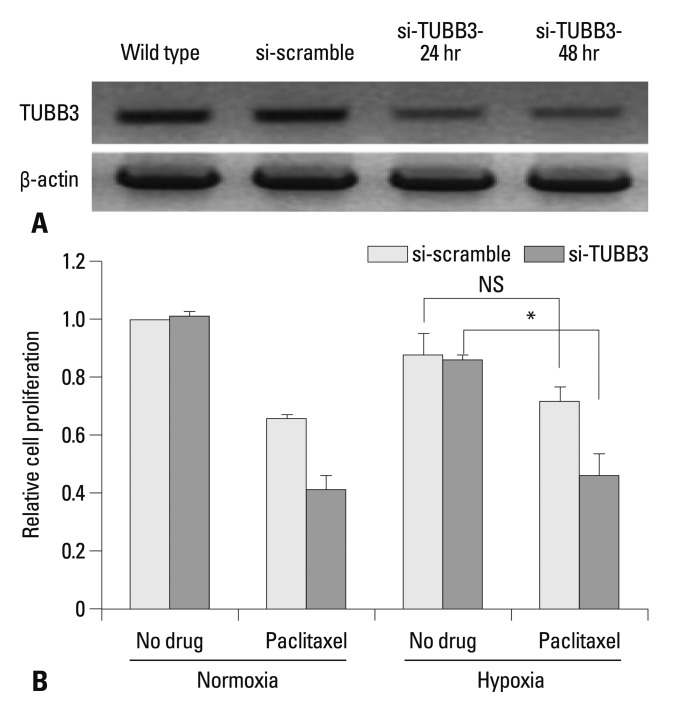
To assess whether TUBB3 expressions is associated with chemotherapeutic drug resistance in AGS, TUBB3 was knocked down with small interfering RNA (siTUBB3), and the effects of paclitaxel on AGS growth under normoxic and hypoxic conditions were evaluated over a 24-48 h period. AGS was strongly resistant to paclitaxel during hypoxia. Knockdown of TUBB3 decreased the resistance to paclitaxel during hypoxia, and cytotoxicity was increased (Fig. 2).
Fig. 2.
TUBB3 knockdown sensitizes gastric cancer cells to paclitaxel. (A) Western blot analysis of TUBB3 in AGS cells without treatment or in the presence of si-scramble control or si-TUBB3 RNAs. Actin was used as a loading control. (B) AGS cells were treated with si-scramble or si-TUBB. Cells were maintained in normoxia or hypoxia for 24 h with paclitaxel. Cell proliferation was normalized to the normoxic control. Values are expressed as means±SEM (n=3). *p<0.05. NS, not significant. TUBB3, class III β-tubulin; SEM, standard error of the mean.
VEGFR-1 signals increase HIF-1α and TUBB3 expressions in AGS cells
After treatment with paclitaxel, cytoskeletal component were isolated from AGS cells, and tubulin polymerization was monitored. Western blots were performed using a pan α-tubulin polyclonal antibody to probe lysates from cells that had been treated with paclitaxel or paclitaxel and bevacizumab under normoxic conditions or after 24 h of hypoxia. Paclitaxel activity was inhibited in hypoxia. Tubulin polymerization was decreased in hypoxia compared with normoxia and it was statistically significant (p<0.01). Hypoxic decrease of polymerization was lessened after treated with bevacizumab (Fig. 3A).
Fig. 3.
VEGFR-1/2 contributes to tubulin polymerization. (A) Western blots using a pan α-tubulin polyclonal antibody to probe cell lysates treated with paclitaxel with or without bevacizumab for 24 h during normoxia or hypoxia. Paclitaxel activity was inhibited in hypoxia. Tubulin polymerization was decreased in hypoxia compared with normoxia and it was statistically significant. Cells were treated with paclitaxel with/without bevacizumab at 100 nM and 100 ug/mL, respectively. Lane 1: no drug, 2: paclitaxel, 3: paclitaxel with bevacizumab. Actin was used as a loading control. Band intensities were quantified by densitometry. (B) Hypoxia-dependent upregulation of HIF-1α and TUBB3 was most reversed in the presence of paclitaxel and anti-VEGFR-1 for 24 h. HIF-1α was measured from nuclear fractions, and TUBB3 was measured from total cell lysates. Band intensities were quantified by densitometry. *p<0.01. HIF-1α, hypoxia inducible factor-1α; TUBB3, class III β-tubulin; VEGFR, vascular endothelial growth factor receptor.
As previously mentioned, we postulated that blockade of VEGF in AGS would be associated with a decrease in HIF-1α and TUBB3 expression and a concomitant increase in sensitivity to paclitaxel. We treated cells with bevacizumab, anti-VEGFR-1 and anti-VEGFR-2 neutralizing antibodies to test this hypothesis. AGS cells were cultured under normoxic and hypoxic conditions for 24 h without exogenous growth factors, in the absence or presence of anti-VEGFR-1, anti-VEGFR-2, or bevacizumab with simultaneous paclitaxel treatment. HIF-1α and TUBB3 were increased in a hypoxic condition. Paclitaxel treatment decreased the hypoxia-dependent increased expression of HIF-1α and TUBB3. When paclitaxel was combined with bevacizumab or anti-VEGFR-2, expression levels of HIF-1α and TUBB3 were further decreased. The greatest decrease in HIF-1α and TUBB3 levels occurred in response to paclitaxel and anti-VEGFR-1 treatment in AGS cells (Fig. 3B). Our results using AGS cells contrast with results using HCT116 cells, in which hypoxia-induced HIF-1α was inhibited via VEGFR-2.18
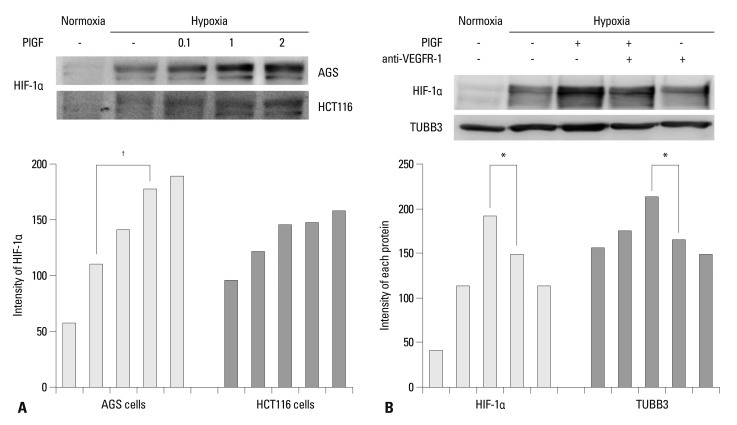
To examine VEGFR-1 function, we investigated whether the VEGFR-1 ligand, PlGF, influences the expression of HIF-1α in AGS and HCT116 cells. Cells were cultured under normoxic or hypoxic conditions in the presence of PlGF (range, 0.1-2.0 ng/mL). HIF-1α expression was increased in hypoxia and was dose dependently more increased according to the concentration of PlGF (Fig. 4A). In contrast with AGS cells, PlGF rarely influenced HIF-1α expression in HCT116 cells. AGS cells were cultured under normoxic and hypoxic conditions in the absence or presence of PlGF and anti-VEGFR-1. Expression levels of HIF-1α and TUBB3 were increased in hypoxia and were more increased in response to PlGF treatment. The increased expressions of HIF-1α and TUBB3 were suppressed when anti-VEGFR-1 antibodies were combined with PlGF treatment (Fig. 4B). We concluded that induction of HIF-1α in AGS is VEGFR-1 mediated and PlGF dependent.
Fig. 4.
PlGF induces HIF-1α. (A) Nuclear HIF-1α protein expression was increased in hypoxia and was dose dependently increased according to the increased concentration of PlGF (range 0.1-2 ng/mL) in AGS. PlGF rarely influenced HIF-1α expression in HCT116 cells. Band intensities were quantified by densitometry. (B) Expression levels of HIF-1α and TUBB3 were increased in hypoxia and were more increased with PlGF treatment (1 ng/mL) in AGS cells. HIF-1α is measured from nuclear fractions, and TUBB3 is measured from total cell lysates. The observed increases in HIF-1α and TUBB3 expression levels were decreased when anti-VEGFR-1 was combined with PlGF treatment. Band intensities were quantified by densitometry. *p<0.05, †p<0.01. PlGF, placental growth factor; HIF-1α, hypoxia inducible factor-1α; TUBB3, class III β-tubulin; VEGFR, vascular endothelial growth factor receptor.
Blockade of VEGFR-1/VEGFR-2 increases sensitivity to paclitaxel in AGS cells
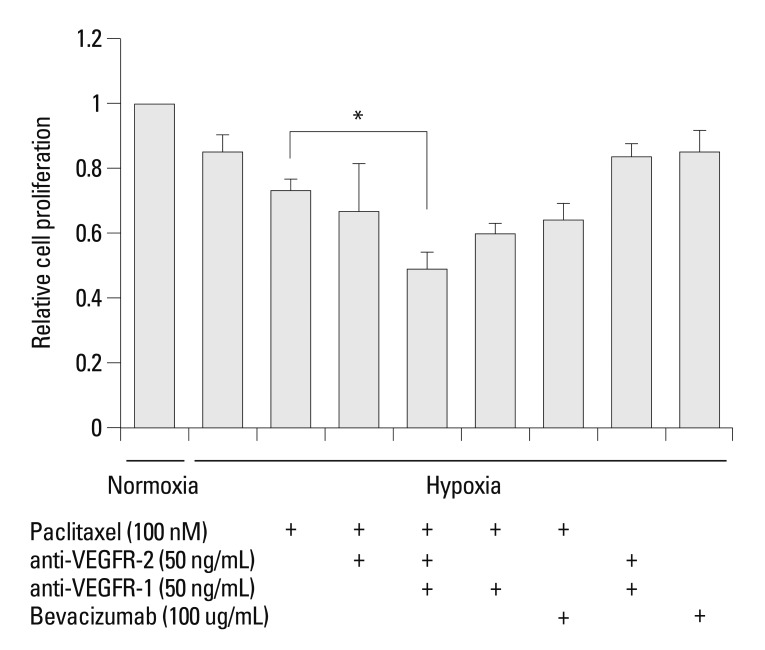
AGS cells were cultured under hypoxic conditions with or without anti-VEGFR-1, anti-VEGFR-2, and bevacizumab with simultaneous paclitaxel exposure. The cell viability assay demonstrated that AGS cell cytotoxicity was increased in response to combined treatment with paclitaxel and bevacizumab compared with paclitaxel treatment alone. Cytotoxicity was increased synergistically in response to simultaneous treatment with paclitaxel, anti-VEGFR-1, and anti-VEGFR-2 (p<0.05) (Fig. 5).
Fig. 5.
Blockade of VEGFR-1 and VEGFR-2 decreases resistance to paclitaxel in AGS cells. The cell viability assay demonstrated that AGS cell cytotoxicity was increased following combined treatment with paclitaxel and bevacizumab compared with treatment with paclitaxel alone. The increase of cytotoxicity was most pronounced when paclitaxel, anti-VEGFR-1, and anti-VEGFR-2 were applied simultaneously. Cells were maintained in normoxia or hypoxia for 24 h with paclitaxel following a 4-h pre-incubation with neutralizing antibody. Cell proliferation was normalized on the normoxic control. Values are expressed as means±SEM (n=3). *p<0.05. VEGFR, vascular endothelial growth factor receptor; SEM, standard error of the mean.
DISCUSSION
Bevacizumab is accepted as the standard treatment for advanced metastatic colorectal cancer, but to date is not considered as the standard treatment of gastric cancer.24 VEGF can bind with the receptor tyrosine kinases, VEGFR-1 and VEGFR-2. The key roles of VEGF and its receptor VEGFR-2 in tumor angiogenesis have been established.26,27 Activation of VEGFR-2 leads to auto-phosphorylation and the activation of downstream signaling pathways, such as Raf/MEK/ERK and PI3K/Akt kinase cascades.25 In contrast, the function of VEGFR-1 remains poorly defined. VEGFR-1 has been associated with tumor growth, tumor cell activation, and metastasis. Treatment with VEGFR-1 inhibitors, such as anti-VEGFR-1 antibody, suppresses tumor growth and metastasis in various models.26 However, the degree of tumor growth inhibition varies. Some studies have reported that VEGFR-1 inhibition is not sufficient to block tumor growth without combined inhibition of VEGFR-2.28,29 In addition, it is unclear if VEGFR-1 inhibitors, by preventing the binding of VEGF to VEGFR-1, increase the concentration of free VEGF that can subsequently activate VEGFR-2 and stimulate angiogenesis by an alternate pathway. The precise mechanisms of VEGFR-1 inhibitor functioning warrant further exploration.26
We demonstrated that HIF-1α expressions increase in association with elevated PlGF. HIF-1α and TUBB3 were upregulated in hypoxia and became more induced in response to PlGF treatment. We also demonstrated that inhibition of VEGFR-1 is associated with decreases in HIF-1α and TUBB3 expression. Calvani, et al.18 reported that VEGFR-2, rather than VEGFR-1, mediates VEGF-dependent induction of HIF-1α in hypoxic HCT116 colon cancer cells, suggesting that functional VEGFR-2, but not functional VEGFR-1, exists in this colon cancer cell line. Cell viability assay results demonstrated that AGS cell cytotoxicity was most pronounced when cells were treated simultaneously with paclitaxel, anti-VEGFR-1, and anti-VEGFR-2. Through these results, we suggest that AGS cells express functional VEGFR-1 and VEGFR-2.
TUBB3 confers chemoresistance to taxanes. Our experiment results of paclitaxel resistance in AGS cells are consistent with other reports suggesting that TUBB3 affects chemoresistance to taxanes in diverse cancer cells.12-16 Raspaglio, et al.21 also reported that TUBB3 is not only a parameter related to the chemoresponsiveness, but also could be a pure prognostic marker. TUBB3 expression levels differ by cell and tissue types. In some tissues, TUBB3 is constitutively expressed and is not inducible upon hypoxia.21 In AGS cells, we observed TUBB3 was constitutively expressed in normoxia and became more induced in hypoxia.
The present study demonstrated that blockade of both VEGFR-1 and VEGFR-2 in conjunction with paclitaxel synergistically depresses induction of HIF-1α and TUBB3 expression, and more effectively increases cytotoxicity in gastric cancer cells.
ACKNOWLEDGEMENTS
This study was supported by a grant (CRI11073-1) Chonnam national university hospital research institute of clinical medicine.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009;41:122–131. doi: 10.4143/crt.2009.41.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruban RH, Yardley JH, Donehower RC, Boitnott JK. Taxol toxicity. Epithelial necrosis in the gastrointestinal tract associated with polymerized microtubule accumulation and mitotic arrest. Cancer. 1989;63:1944–1950. doi: 10.1002/1097-0142(19890515)63:10<1944::aid-cncr2820631013>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660–5667. doi: 10.1200/JCO.2005.17.376. [DOI] [PubMed] [Google Scholar]
- 5.Constenla M, Garcia-Arroyo R, Lorenzo I, Carrete N, Campos B, Palacios P. Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric Cancer. 2002;5:142–147. doi: 10.1007/s101200200025. [DOI] [PubMed] [Google Scholar]
- 6.Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295–301. [PubMed] [Google Scholar]
- 7.Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999;22:580–586. doi: 10.1097/00000421-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, et al. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006;17:225–229. doi: 10.1097/00001813-200602000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, et al. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO) Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol. 2000;11:301–306. doi: 10.1023/a:1008342013224. [DOI] [PubMed] [Google Scholar]
- 10.Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: a randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217–3223. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee A. Increased levels of tyrosinated alpha-, beta(III)-, and beta(IV)-tubulin isotypes in paclitaxel-resistant MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:598–601. doi: 10.1016/S0006-291X(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 13.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer. 1999;80:1020–1025. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranganathan S, Benetatos CA, Colarusso PJ, Dexter DW, Hudes GR. Altered beta-tubulin isotype expression in paclitaxel-resistant human prostate carcinoma cells. Br J Cancer. 1998;77:562–566. doi: 10.1038/bjc.1998.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urano N, Fujiwara Y, Doki Y, Kim SJ, Miyoshi Y, Noguchi S, et al. Clinical significance of class III beta-tubulin expression and its predictive value for resistance to docetaxel-based chemotherapy in gastric cancer. Int J Oncol. 2006;28:375–381. [PubMed] [Google Scholar]
- 17.Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70:93–102. doi: 10.1016/j.critrevonc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Calvani M, Trisciuoglio D, Bergamaschi C, Shoemaker RH, Melillo G. Differential involvement of vascular endothelial growth factor in the survival of hypoxic colon cancer cells. Cancer Res. 2008;68:285–291. doi: 10.1158/0008-5472.CAN-07-5564. [DOI] [PubMed] [Google Scholar]
- 19.Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, et al. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–181. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 20.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 21.Raspaglio G, Filippetti F, Prislei S, Penci R, De Maria I, Cicchillitti L, et al. Hypoxia induces class III beta-tubulin gene expression by HIF-1alpha binding to its 3' flanking region. Gene. 2008;409:100–108. doi: 10.1016/j.gene.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 23.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 26.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 27.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 28.Gille J, Heidenreich R, Pinter A, Schmitz J, Boehme B, Hicklin DJ, et al. Simultaneous blockade of VEGFR-1 and VEGFR-2 activation is necessary to efficiently inhibit experimental melanoma growth and metastasis formation. Int J Cancer. 2007;120:1899–1908. doi: 10.1002/ijc.22531. [DOI] [PubMed] [Google Scholar]
- 29.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]