Abstract
Tight junction (TJ) is recognized as a second barrier of the skin. Altered expression of TJ proteins in various skin diseases characterized by the abnormal permeability barrier such as psoriasis suggests that TJ could be affected by stratum corneum (SC) barrier status. However, the physiological relationship between SC and TJ barrier remains to be investigated. Therefore, we examined the effect of SC barrier disruption on the expression of TJ proteins, claudin (Cldn)-1 and Cldn-4, and TJ barrier function in hairless mouse skin. We also investigated whether the alterations in epidermal Ca2+ affected TJ proteins expression in vivo. Repeated tape-stripping induced a sequential change of the expression and function of TJ. As early as 15-30 minutes after tape-stripping, downregulation of Cldn-1 and Cldn-4 immunoreactivity and protein level without change in mRNA level was found. This was accompanied by the abnormal leakage of lanthanum. However, by 1 hour Cldn-1 and Cldn-4 immunolocalization recovered along with normalized lanthanum permeation pattern. Moreover, the mRNA and protein levels of Cldn-1 and Cldn-4 were increased by 1 to 6 hours after tape-stripping. Inhibition of calcium loss by immersion of barrier-disrupted skin into a high Ca2+ solution prevented the dislocation of Cldn-1 and Cldn-4. Occlusion of barrier-disrupted skin delayed the restoration of Cldn-1 and Cldn-4. Our results suggest that the alteration of epidermal Ca2+ gradient caused by SC barrier perturbation affects the TJ structure and function and the faster recovery of TJ as compared to the SC barrier may imply the protective homeostatic mechanism of skin barrier.
Keywords: Tight junction, claudin-1, claudin-4, calcium gradient, stratum corneum permeability barrier
The functional barrier of mammalian skin is generally accepted to reside mainly in the stratum corneum (SC). Recently, the proteins of tight junction (TJ), which is a major regulator of barrier function in simple epithelia has also been identified in human skin.1 In the inflammatory diseases of the intestine, many cytokines have been reported to affect TJ barrier status. Moreover, there is increasing evidence now that TJ in skin, which localizes in stratum granulosum (SG),2 also contribute to epidermal barrier formation.3-5 Among TJ proteins, claudin (Cldn)-1 and Cldn-4 have been demonstrated to have a role in barrier function of the skin.3-5 Cldn-1 null mutant mice showed normal SC structure, however died shortly after birth with increased transepidermal water loss (TEWL).3 Moreover, mutations in the Cldn-1 gene cause neonatal sclerosing cholangitis with ichthyosis which is associated with impaired barrier function.4 A recent study suggested that Cldn-4 also plays a role in barrier function with observation that the diffusion of 550 Da tracer which was normally stopped at TJ areas was no longer blocked in skin incubated with ochratoxin A that removes Cldn-4 from the TJ structure.5 TJs are highly dynamic structures which transiently open and close in response to numerous stimuli such as pathogens,6 UV irradiation,5 and wounding.7 Because skin is the first barrier to external stimuli, the regulation of TJ in response to external stimuli is important; however, little is known about the physiological relationship between SC barrier and TJ function and the regulatory mechanisms for TJ barrier homeostasis in vivo.
In the present study, we investigated the effect of SC barrier disruption on the expression of TJ proteins, Cldn-1 and Cldn-4, and TJ barrier function in murine epidermis and whether the epidermal calcium gradient change, as a result of SC barrier disruption, may modulate TJ protein expression.
Hairless female mice, aged 8-10 weeks old, were used for this study. The epidermal permeability barrier was disrupted by repeated tape-stripping on the flank with cellophane tape until the TEWL reached 35 mg/cm2/h and skin samples for immunofluorescence staining, real-time polymerase chain reaction (PCR), western blot and lanthanum permeation assay were taken from the treated area at 15 minutes, 30 minutes, 1 hour, 3 hours, and 6 hours after each treatment (n=5 from each group). Then, to modulate epidermal Ca2+ gradient, two independent methods including immersion in solution with calcium ions and occlusion were performed. For the immersion experiment, the barrier-disrupted flank of mice were submerged in phosphate buffered saline solution containing 1.8 mM calcium immediately after tape-stripping for 15 minutes, 30 minutes, 1 hour, 3 hours, and 6 hours. For occlusion study, the hairless mice were wrapped with vapor-impermeable membranes or air-exposed immediately after tape-stripping for 15 minutes, 30 minutes, 1 hour, 3 hours, and 6 hours. After each time point, specimens were taken for immunofluorescence staining.
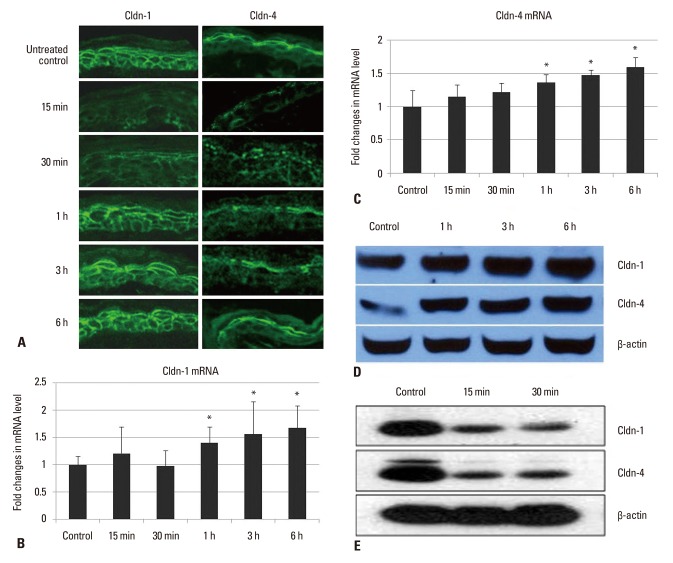
In the untreated murine epidermis, Cldn-1 was expressed in the intercellular spaces throughout all living layers, whereas Cldn-4 was restricted in SG (Fig. 1A). At 15 and 30 minutes after tape-stripping, the reduction of Cldn-1 immunofluorescence from the granular and spinous layers was observed. However, by 1 hour, Cldn-1 expression recovered and by 3-6 hours, Cldn-1 immunoreactivity was slightly increased (Fig. 1A). The immunofluorescence study for Cldn-4 showed a similar pattern of expression change in response to tape-stripping. At 15 and 30 minutes after tape-stripping, the intensity of Cldn-4 immunostaining was slightly decreased (Fig. 1A). By 1-3 hours, Cldn-4 expression recovered and even increased at 6 hours after tape-stripping (Fig. 1A). Cldn-7, occludin and ZO-1 immunoreactivity did not change. To confirm that these staining pattern changes are due to the change of gene and protein levels, we examined Cldn-1 and Cldn-4 mRNA and protein levels by real-time PCR and western blot. We found no statistical differences in Cldn-1 and Cldn-4 mRNA level and downregulation of protein level between untreated and tape-stripped group at 15 and 30 minutes after treatment (Fig. 1B-E). Moreover, by 1-6 hours, Cldn-1 and Cldn-4 mRNA expression was significantly increased in comparison to controls (Fig. 1B and C). The increase in band intensity of Cldn-1 and Cldn-4 from western blot images was also observed at 1, 3, and 6 hours after tape-stripping (Fig. 1D).
Fig. 1.
Effect of acute permeability barrier disruption on the expression and localization of TJ proteins, Cldn-1 and Cldn-4 in murine epidermis. Skin samples were taken at 15 min, 30 min, 1 h, 3 h, and 6 h after tape-stripping. Frozen sections (5 µm) were immunostained with Cldn-1 and Cldn-4 primary antibodies (Zymed Laboratories, San Francisco, CA, USA) and an FITC conjugated donkey anti-rabbit IgG, secondary antibody (Santa Cruz, CA, USA) and examined by confocal microscopy. Magnification ×400 (A). The levels of mRNA for Cldn-1 (B) and Cldn-4 (C) were determined using real-time PCR and normalized to that of β-actin. Cldn-1 and Cldn-4 protein expression was determined by western blot analysis of tape-stripped murine epidermis (D and E). β-actin was used as a loading control. *p<0.05 for Cldn-1 and Cldn-4 mRNA of tape-stripped epidermis compared with that of untreated control. TJ, tight junction; Cldn, claudin. FITC, fluorescein isothiocyanate; PCR, polymerase chain reaction.
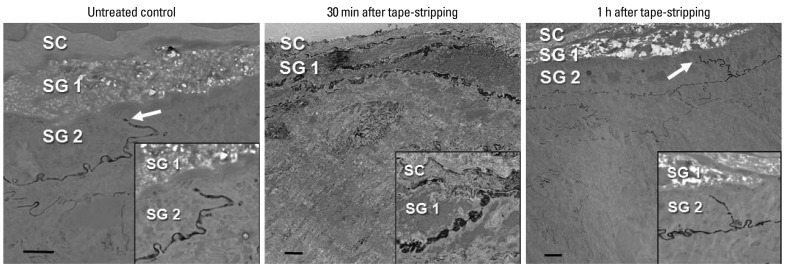
To investigate whether SC barrier disruption-induced changes in the localization or distribution of Cldn-1 and Cldn-4, influence on the TJ barrier function, we next performed the lanthanum penetration assay to assess the inside-out barrier function. TJ has been demonstrated to be located in the SG2 layer.8,9 Consistent with these previous reports, we found a limited lanthanum penetration at SG2 layer in untreated murine epidermis by transmission electron microscope (Fig. 2). In contrast, at 30 minutes after tape-stripping at which time there were distributional changes of Cldn-1 and Cldn-4, an upward diffusion of lanthanum beyond SG2 layer was found, suggesting a defective inside-out barrier function (Fig. 2). However, at 1 hour after barrier disruption, the passage of lanthanum was again restricted at SG2 layer similar to control skin, indicating the restoration of TJ barrier function (Fig. 2). This functional study suggests that the acute SC barrier disruption-induced transient Cldn-1 and Cldn-4 alteration was accompanied by impaired barrier function of TJ and this defect in inside-out barrier function was recovered when Cldn-1 and Cldn-4 expression patterns were normalized.
Fig. 2.
Effect of acute permeability barrier disruption on the inside-out barrier function of TJ in murine epidermis. Skin samples were taken from untreated skin and barrier-disrupted skin at 30 min and 1 h after tape-stripping. Freshly obtained skin biopsies were submerged en bloc in 4% colloidal lanthanum nitrate and post-fixed in osmium tetroxide and examined by transmission electron microscope. Insets show the enlarged view of upper stratum granulosum (SG) and stratum corneum (SC) layer. Bars: 2 µm. TJ, tight junction.
There have been in vitro evidences that extracellular Ca2+ plays a key role in maintaining TJ integrity. In simple epithelial cells, depleting Ca2+ from the culture medium temporarily dislocate TJ accompanied by weakening of TJ barrier, while switch to high Ca2+ reorganized TJ with normal barrier function.10 Recent study has also found that Ca2+ depletion in stratified cultures of human keratinocytes caused an altered expression of TJ proteins with decreased barrier function, which recovered following switch to the high-calcium medium.11 Epidermal permeability barrier homeostasis is tightly regulated by epidermal Ca2+ gradients.12 Upon barrier disruption, extracellular calcium in the upper epidermis rapidly decreases along with increased TEWL.13 From these findings, we postulated that the mechanisms underlying the rapid and transient alteration of Cldn-1 and Cldn-4 is associated with rapid loss of extracellular calcium in the SG where TJ located. To address this issue, we performed two methods to modulate epidermal Ca2+ gradient.
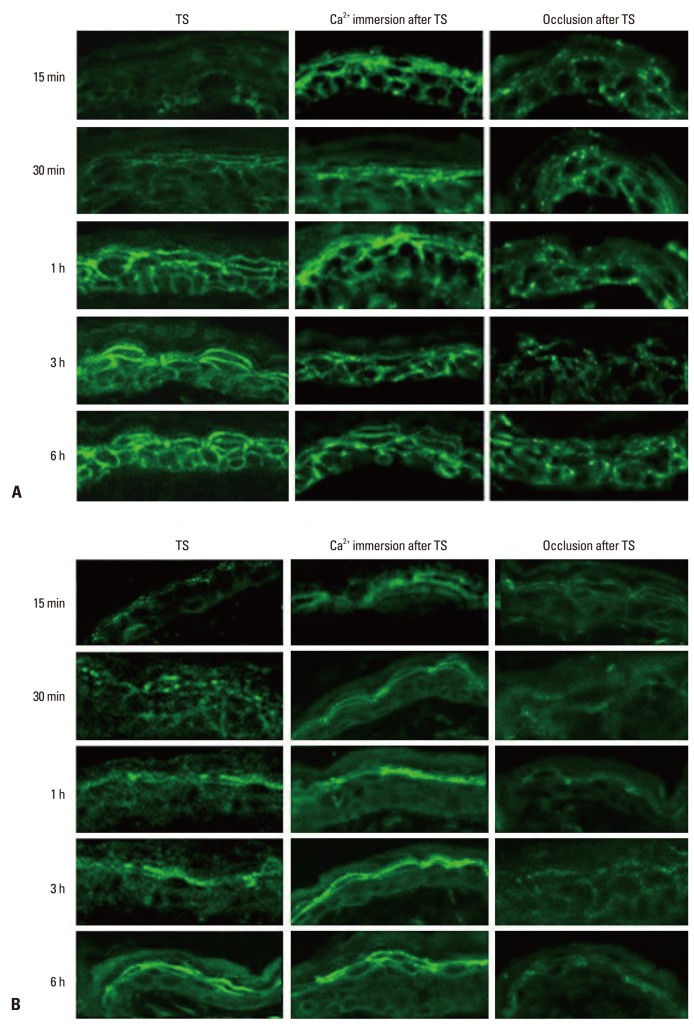
Previous studies demonstrated that immersion of the barrier disrupted skin in high calcium containing solutions delayed barrier repair by preventing the loss of calcium contents from the upper epidermis.14 By the method as previous described, we evaluated the relative changes in Cldn-1 and Cldn-4 expression in the tape-stripped skin which immediately immersed in 1.8 mM Ca2+-containing solution or non-immersed skin. There was no obvious change in Cldn-1 and Cldn-4 expression at 15 and 30 minutes after tape-stripping in high calcium solution-immersed skin, whereas non-immersed skin showed decreased staining intensity and discontinuous staining pattern of Cldn-1 and Cldn-4 compared to control skin (Fig. 3). These results show that prevention of the rapid decrease in the upper epidermal extracellular Ca2+ inhibits the alteration of Cldn-1 and Cldn-4 expression, suggesting that extracellular Ca2+ decrease by acute barrier disruption induces Cldn-1 and Cldn-4 dislocation as early response. We found that the decreased immunoreactivity of Cldn-1 and Cldn-4 following barrier disruption almost recovered their normal expression pattern by 1 hour (Fig. 1A). To investigate whether this subsequent recovery of Cldn-1 and Cldn-4 expression following barrier disruption could be associated with recovery of the calcium gradient, we applied occlusion method to the barrier disrupted animals. It was shown that the rapid artificial restoration of permeability barrier by occlusion with vapor-impermeable membrane inhibited Ca2+ gradient recovery following barrier perturbation and consequently delayed barrier recovery.15 The same occlusion method caused a delay in Cldn-1 and Cldn-4 recovery, even at 6 hours after tape-stripping compared with non-occluded group, indicating that inhibition of Ca2+ gradient recovery prevented Cldn-1 and Cldn-4 restoration (Fig. 3). Taken together, we concluded that acute loss of extracellular Ca2+ in SG layer by tape-stripping induced alteration of Cldn-1 and Cldn-4 expression with impaired TJ barrier function and adequate recovery of Ca2+ gradient stimulated restoration of Cldn-1 and Cldn-4 expression. The regulatory mechanisms of epidermal TJ by epidermal Ca2+ gradient have not been fully elucidated. One possible explanation is that loss of extracellular Ca2+ in the SG might inhibit the signal of E-cadherin, which is important in the formation of functional TJ by a protein kinase C-induced Cldn incorporation into TJ.16
Fig. 3.
Effect of epidermal Ca2+ gradient modulation on the expression and localization of Cldn-1 and Cldn-4 in murine epidermis. Skin samples were taken at 15 min, 30 min, 1 h, 3 h, and 6 h after tape-stripping (TS) with/without immersion in calcium containing solution or occlusion. Frozen sections (5 µm) were immunostained with Cldn-1 (A) and Cldn-4 (B) primary antibodies (Zymed Laboratories, San Francisco, CA, USA) and an FITC conjugated donkey anti-rabbit IgG, secondary antibody (Santa Cruz, CA, USA) and examined by confocal microscopy. Magnification ×400. FITC, fluorescein isothiocyanate; Cldn, claudin.
Regarding the recovery kinetics of the barrier, our results show that the TJ barrier immediately disturbed following SC barrier disruption and then normalized within 1 hour after injury. Moreover, an increase in Cldn-1 and Cldn-4 mRNA and protein started 1 hour after barrier disruption until 6 hours. Based on the recovery kinetic of SC barrier, which includes several steps such as preformed lamellar bodies secretion within 30 minutes,17,18 accelerated lamellar body formation between 2 to 6 hours,17 and increased DNA synthesis within 16-24 hours,19 we found that recovery time of TJ barrier is faster compared with SC barrier. This fast recovery kinetic of TJ in response to barrier disruption might be a compensation mechanism of the skin by enhancing the inside-out permeability barrier and epidermal calcium ion gradient before the full recovery of SC permeability barrier function to maintain homeostasis. These findings of our study are in accordance with previous studies, which observed the enhanced TJ formation in the conditions of abnormal or absent SC barrier such as early stage of wound healing7 and developing fetal skin.20
In summary, we demonstrated the physiological relationship between the TJ and SC barrier during barrier repair following acute SC barrier disruption.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Brandner JM, Proksch E. Epidermal barrier function: role of tight junctions. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor and Francis; 2006. pp. 191–210. [Google Scholar]
- 2.Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, et al. Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol. 2002;81:253–263. doi: 10.1078/0171-9335-00244. [DOI] [PubMed] [Google Scholar]
- 3.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Yuki T, Hachiya A, Kusaka A, Sriwiriyanont P, Visscher MO, Morita K, et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J Invest Dermatol. 2011;131:744–752. doi: 10.1038/jid.2010.385. [DOI] [PubMed] [Google Scholar]
- 6.Ohnemus U, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, et al. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. J Invest Dermatol. 2008;128:906–916. doi: 10.1038/sj.jid.5701070. [DOI] [PubMed] [Google Scholar]
- 7.Malminen M, Koivukangas V, Peltonen J, Karvonen SL, Oikarinen A, Peltonen S. Immunohistological distribution of the tight junction components ZO-1 and occludin in regenerating human epidermis. Br J Dermatol. 2003;149:255–260. doi: 10.1046/j.1365-2133.2003.05438.x. [DOI] [PubMed] [Google Scholar]
- 8.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuruta D, Green KJ, Getsios S, Jones JC. The barrier function of skin: how to keep a tight lid on water loss. Trends Cell Biol. 2002;12:355–357. doi: 10.1016/s0962-8924(02)02316-4. [DOI] [PubMed] [Google Scholar]
- 10.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 11.Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp Dermatol. 2007;16:324–330. doi: 10.1111/j.1600-0625.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–538. doi: 10.1172/JCI115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992;270:503–512. doi: 10.1007/BF00645052. [DOI] [PubMed] [Google Scholar]
- 14.Denda M, Fuziwara S, Inoue K. Influx of calcium and chloride ions into epidermal keratinocytes regulates exocytosis of epidermal lamellar bodies and skin permeability barrier homeostasis. J Invest Dermatol. 2003;121:362–367. doi: 10.1046/j.1523-1747.2003.12367.x. [DOI] [PubMed] [Google Scholar]
- 15.Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002;119:1269–1274. doi: 10.1046/j.1523-1747.2002.19622.x. [DOI] [PubMed] [Google Scholar]
- 16.Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Günzel D, Fromm M, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992;98:279–289. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- 18.Elias PM, Cullander C, Mauro T, Rassner U, Kömüves L, Brown BE, et al. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J Investig Dermatol Symp Proc. 1998;3:87–100. doi: 10.1038/jidsymp.1998.20. [DOI] [PubMed] [Google Scholar]
- 19.Proksch E, Feingold KR, Man MQ, Elias PM. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991;87:1668–1673. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol. 2001;117:1050–1058. doi: 10.1046/j.0022-202x.2001.01493.x. [DOI] [PubMed] [Google Scholar]